Introduction
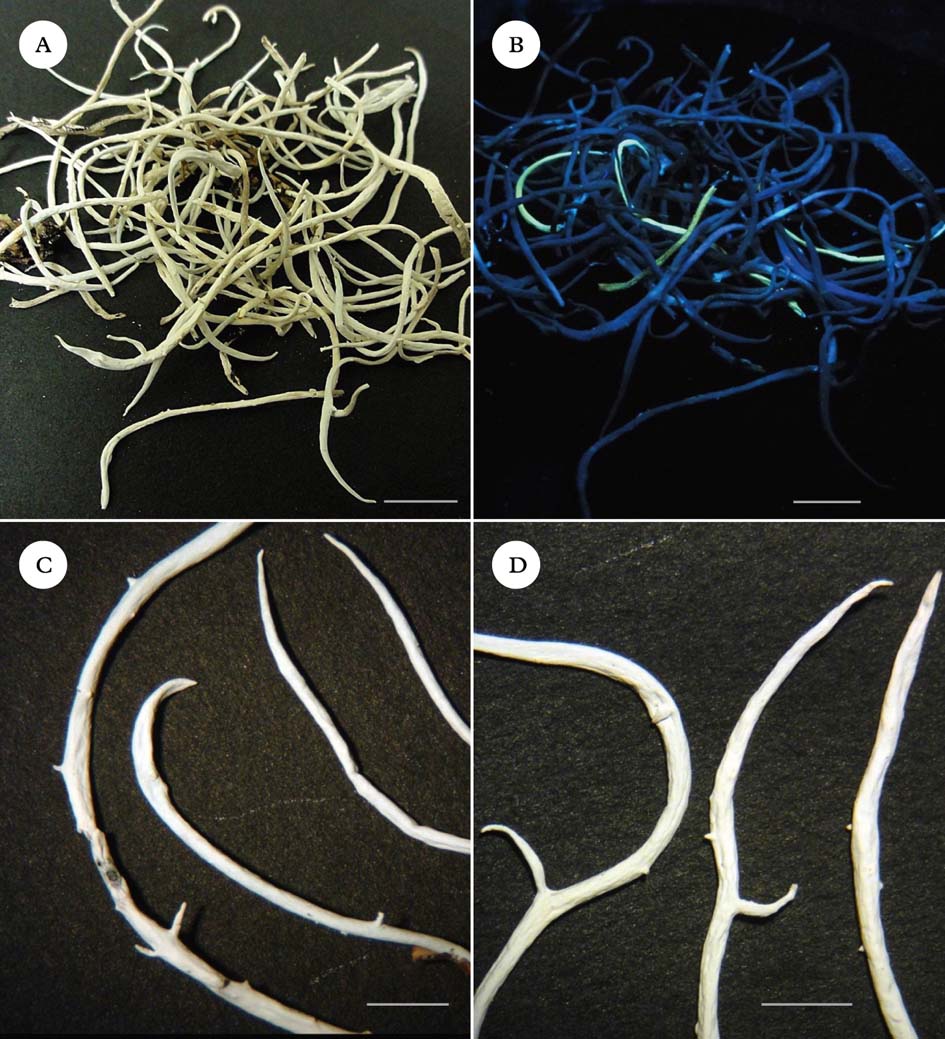
The lichen Thamnolia vermicularis (Sw.) Ach. ex Schaer. is widely distributed in alpine areas throughout the world, and is easily identified by its usually 10–40 mm long, white tubular thalli. It possesses two chemotypes, one that produces squamatic and baeomycesic acids in the thallus and fluoresces yellow under ultraviolet (365 nm) light (henceforth called UV+), and the other that produces thamnolic acid and does not fluoresce (UV−) under ultraviolet light. The UV+ chemotype has previously been recognized as T. subuliformis or T. vermicularis subsp. subuliformis (Culberson Reference Culberson1963; Sato Reference Sato1965; Platt & Spatafora Reference Platt and Spatafora2000). Cassie & Piercey-Normore (Reference Cassie and Piercey-Normore2008) called the two different chemotypes ‘chemospecies’. Molecular evidence has placed Thamnolia in the Icmadophilaceae (Platt & Spatafora Reference Platt and Spatafora2000; Stenroos et al. Reference Stenroos, Myllys, Thell and Hyvönen2002; Grube & Kantvilas Reference Grube and Kantvilas2006). Other studies have questioned the distinctiveness of the two chemotypes within Thamnolia vermicularis, as molecular analysis has failed to demonstrate monophyly of chemotypes (Nelsen & Gargas Reference Nelsen and Gargas2009 a). Here we follow Kärnefelt & Thell (Reference Kärnefelt and Thell1995) and Galloway (Reference Galloway2007), treating the two chemotypes as belonging to the same species, T. vermicularis. Both chemotypes are found worldwide, but the UV+ chemotype dominates in the Northern Hemisphere, whereas the UV− chemotype dominates in the Southern Hemisphere (Sato Reference Sato1965, Reference Sato1968). Because the entire genus Thamnolia is thought to be sterile (Vobis & Hawksworth Reference Vobis, Hawksworth, Cole and Kendrick1981; Kärnefelt & Thell Reference Kärnefelt and Thell1995), its global distribution and the biogeography of chemotypes have provoked much speculation (Sheard Reference Sheard1977; Purvis et al. Reference Purvis, Coppins, Hawksworth, James and Moore1992; Galloway & Aptroot Reference Galloway and Aptroot1995; Kärnefelt & Thell Reference Kärnefelt and Thell1995; Malcolm & Malcolm Reference Malcolm and Malcolm2000). Wind-dispersal of thallus fragments has been suggested as the principal means by which this lichen disperses (Galloway Reference Galloway, Nimis and Crovello1991; Pérez Reference Pérez1991, Reference Pérez1994; Kärnefelt & Thell Reference Kärnefelt and Thell1995; Cassie & Piercey-Normore Reference Cassie and Piercey-Normore2008). However, fragmentation alone seems an inadequate explanation, not only for the global distribution of chemotypes but also for the fact that where both chemotypes are present, such as in New Zealand, Alaska and Peru, they frequently occur as intermixed single strands rather than patches of purely one chemotype (Fig. 1A & B). Furthermore, although growth of individual strands can be rapid (J. M. Lord & A. Knight, unpublished data), the apical pattern of growth and generally low degree of branching of Thamnolia in many habitats provide limited opportunities for the production of viable fragments (Cassie & Piercey-Normore Reference Cassie and Piercey-Normore2008).
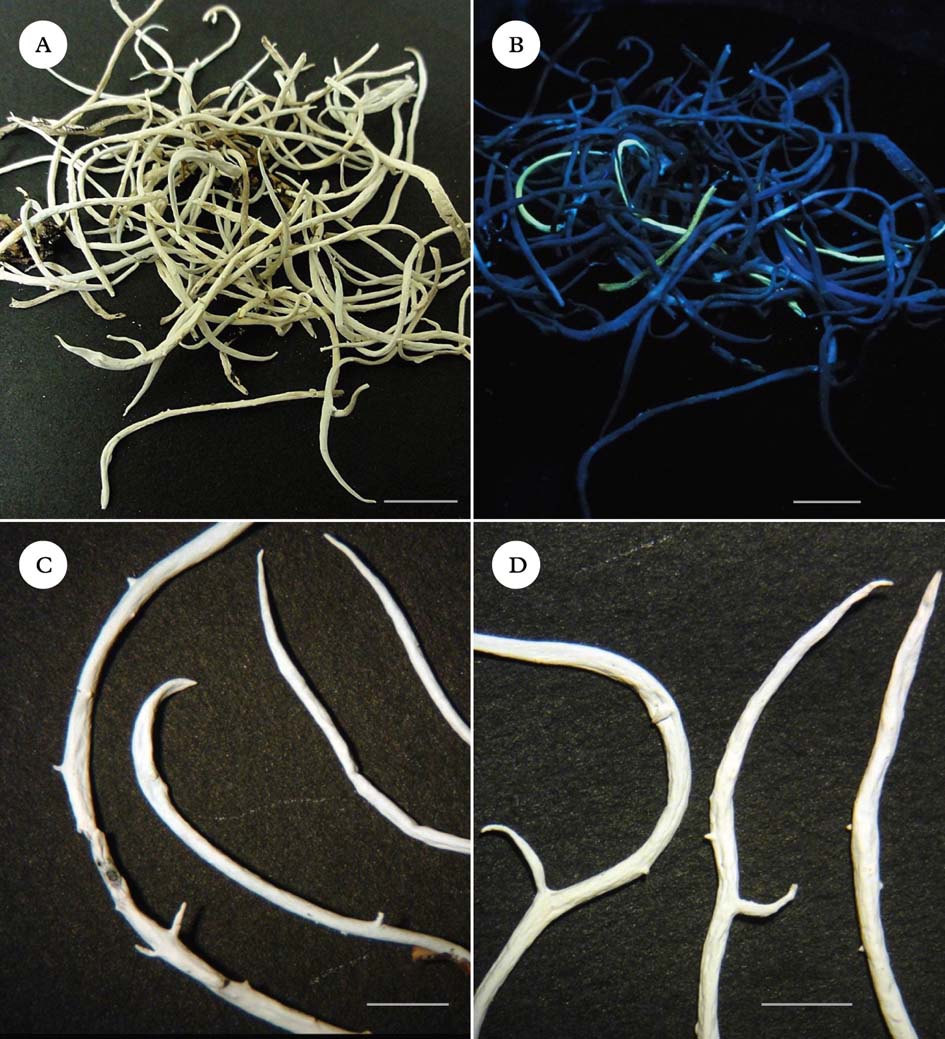
Fig. 1. Thamnolia. A & B, unsorted collection from the Rock and Pillar Range, Otago, New Zealand, showing intermixing of chemotypes; A, under incandescent light; B, under UV light (365 nm). C & D, pycnidial protrusions and branching patterns; C, UV+ thalli; D, UV− thalli. Scales: A & B=10 mm; C & D=5 mm. In colour online.
While examining New Zealand collections of Thamnolia, we noticed what appeared to be pycnidial conidiomata (pycnidia) (Figs 1C & D, 2A–D). Pycnidial conidiomata contain haploid asexual spores called conidia that are derived from one fungal parent and are thought to function as spermatia, capable of fertilizing other individuals (Honegger & Scherrer Reference Honegger, Scherrer and Nash2008). These conidia are also thought to be capable of functioning as propagules via re-lichenization after germination near free-living algae (Henssen & Jahns Reference Henssen and Jahns1974; Hawksworth Reference Hawksworth and Galun1988; Brodo et al. Reference Brodo, Duran Sharnoff and Sharnoff2001). Vobis (Reference Vobis1977) germinated lichen conidia and grew isolated mycelia from them. Experimental evidence for the capability of lichen conidia to re-lichenize was provided by Stocker-Wörgötter (Reference Stocker-Wörgötter, Marcelli and Seaward1998), who successfully performed a resynthesis of Cladonia fissidens from conidia-derived mycelium. Thus even in the absence of apothecia and sexual reproduction, pycnidial conidiomata are able to produce highly vagile conidia that can establish new thalli.
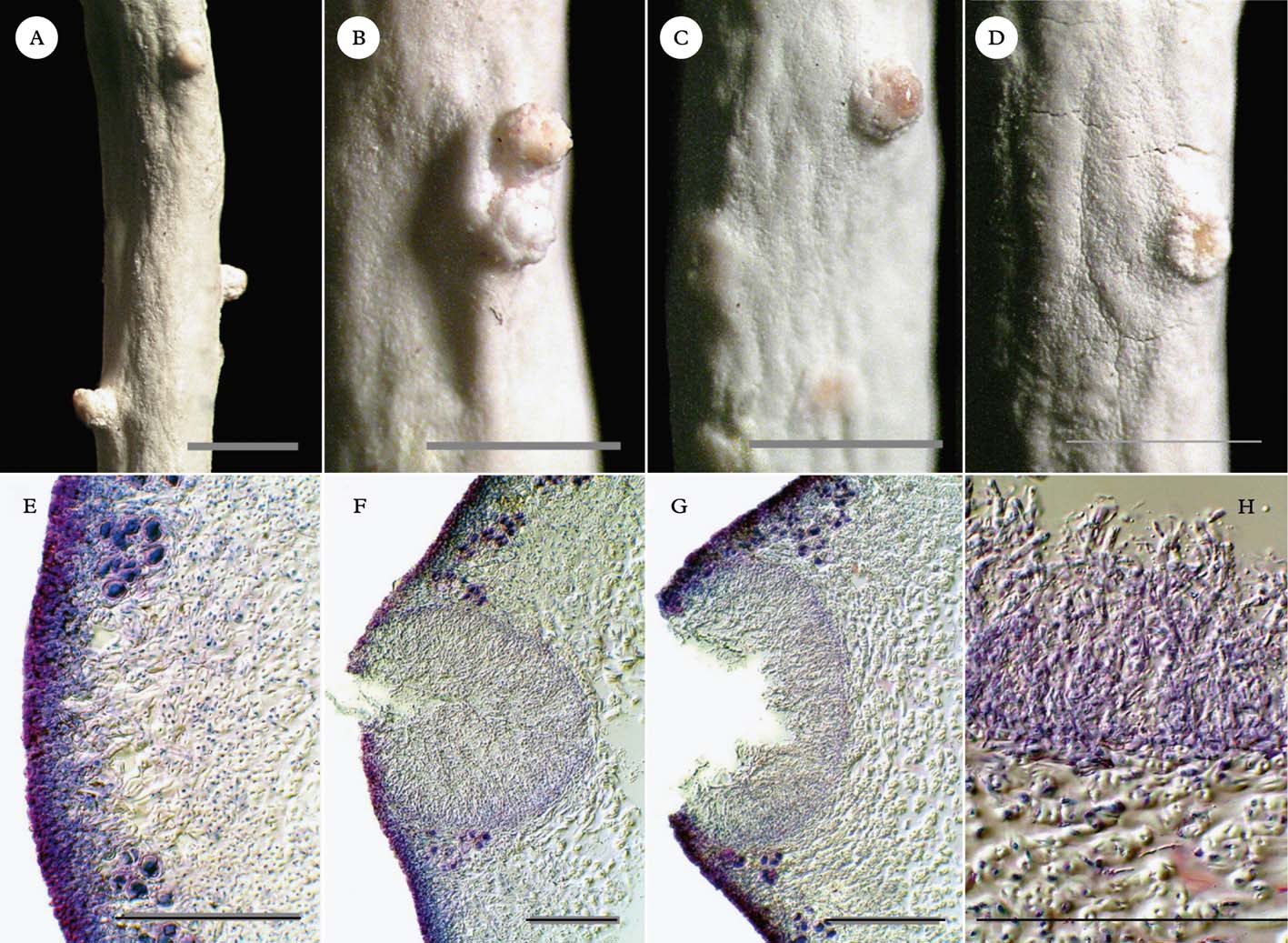
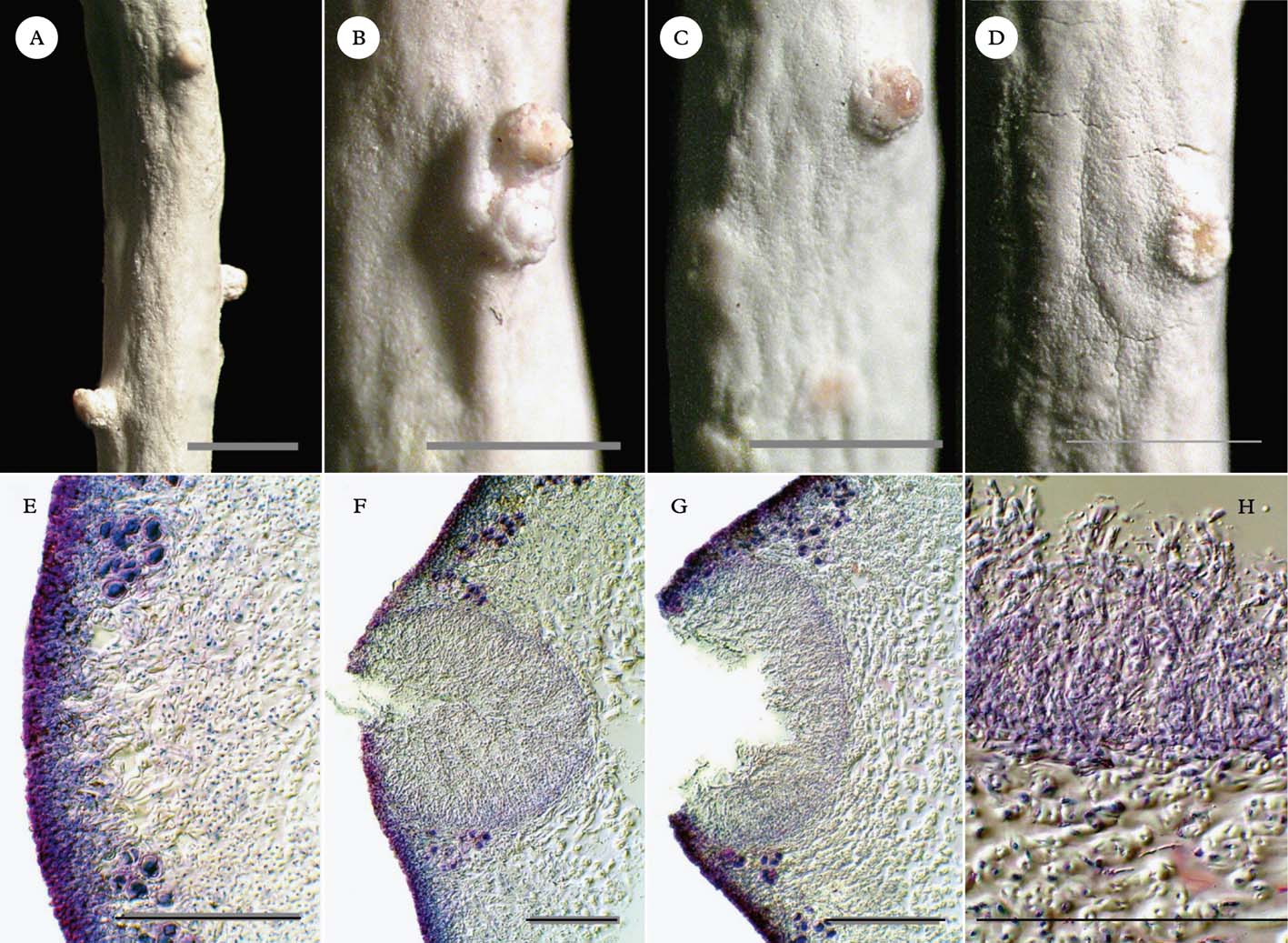
Fig. 2. Thamnolia vermicularis, pycnidial conidiomata from thallus tip downwards. A–D, external views; A, near thallus tip; B & C, middle of thallus; D, mature to over-mature pycnidial conidiomata near base of thallus. E–H, transverse sections; E, immature, closed operculum; F, releasing conidia; G, open, over-mature; H, mature conidia. Scales: A–D=1 mm; E–H=100 µm. In colour online.
Pycnidia, conidiogenous cells or conidia were reported for Thamnolia as early as the 1850s (e.g., Nylander Reference Nylander1856; Lindsay Reference Lindsay1859), but information on these structures has disappeared from the modern literature. The aim of this study was to determine conclusively whether the structures we observed were indeed pycnidial conidiomata. We also trace the history of descriptions of pycnidia in Thamnolia in order to determine how and why information on their presence was lost from the literature. Finally, we discuss the implications of the rediscovery of pycnidia in T. vermicularis for understanding the species' distribution and the pattern of occurrence of chemotypes.
Materials and Methods
Samples used in this study
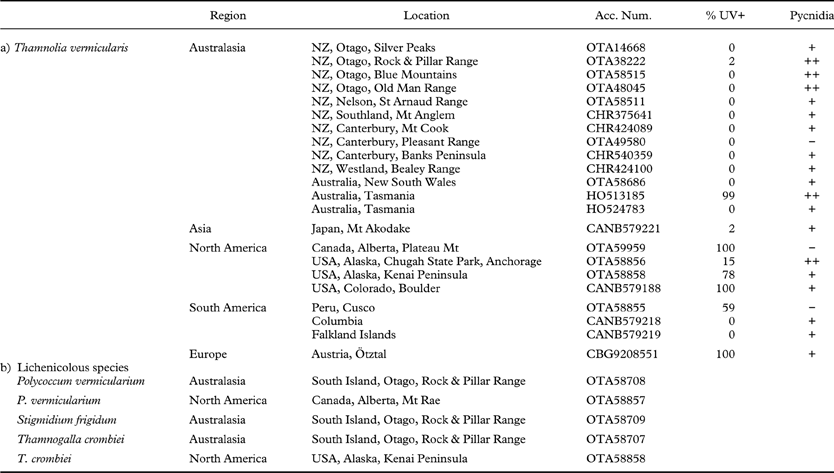
Thalli in collections from New Zealand, Australia, Asia, North and South America and Europe were examined under long-wave UV light (365 nm) to determine the frequency and pattern of intermixing of chemotypes. Each collection was a single unsorted sample, made over the smallest area needed to fill a packet. Packets usually contained over 100 thalli (range 58–296, av. 143 thalli/packet). The intertwined thalli were separated and counted to determine the proportion of UV+ thalli. Herbarium accession numbers are given in Table 1.
Table 1. Representative collections of: (a) Thamnolia vermicularis examined for pycnidia and chemotype mixing (b) lichenicolous fungi associated with Thamnolia in New Zealand. Pycnidia observed per packet: (−) none; (+) 1–10; (++)>10

Morphological investigations
Thamnolia vermicularis thalli of both UV− (thamnolic acid) and UV+ (baeomycesic acid and squamatic acid) chemotypes from the above collections were examined dry, and the presence and abundance of pycnidia noted. Typical thalli were rehydrated and photographed under a dissecting microscope. Thin sections were hand-cut transversely through putative ostioles using a razor blade, and stained in lactophenol cotton blue for light microscopy and photography. Pycnidial conidiomata were examined carefully for conidia and for any sign of foreign hyphae. Lichenicolous fungi were identified and noted. Branching pattern was observed, and branchlets on UV+ and UV− thalli from New Zealand were photographed. No specimens of other species in the genus Thamnolia were examined.
DNA analysis and sequencing
The internal transcribed spacer (ITS) region of nuclear ribosomal DNA was sequenced to confirm the identity of pycnidial conidiomata tissue, investigate monophyly of chemotypes and to determine relationships among collections of Thamnolia vermicularis from several localities around the world. To confirm the identity of pycnidial tissue, single thalli and their associated pycnidial conidiomata were carefully separated under a dissecting microscope from one thallus of each chemotype lacking visible signs of lichenicolous fungi. Monophyly of chemotypes was investigated using single thalli from a further eight OTA accessions and three HO accessions (Table 1). For the two thallus-pycnidia pairs and the eight other OTA accessions, DNA was extracted following the methods described in Bagley & Orlovich (Reference Bagley and Orlovich2004). DNA from the three Tasmanian (HO) collections was extracted using the DNeasy Plant Mini Kit (Qiagen) following the manufacturer's instructions. The polymerase chain reaction (PCR) was carried out using either AmpliTaq Gold PCR Master Mix (Applied Biosystems) or ReddyMix PCR Master Mix (ABGene), and 8–10 pmol of each primer per 20 µl reaction. The ITS region was amplified using the primers PN3 and PN10 (Viaud et al. Reference Viaud, Pasquier and Brygoo2000), or ITS1f (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) and an annealing temperature of 50°C. Where amplification of the entire ITS regions was unsuccessful, the ITS2 region was amplified using the primers ITS3 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) and ITS4. In cases where PCR amplification resulted in two bands, bands were carefully excised under ultraviolet light, incubated in 500 µl purified water at 28°C overnight and centrifuged for 30 s at maximum speed. A 1 µl aliquot of the supernatant was used for reamplification. The PCR protocol was 95°C for 5 min, then 30 cycles of 94°C for 1 min, 55°C for 1·5 min, 72°C for 1 min, and finally 10 min at 72°C. PCR products were purified using a spin column purification kit (Qiagen QiaQuick PCR product purification kit), and were quantified using a spectrophotometer (Nanodrop ND-1000). Sequencing reactions were performed by the Allan Wilson Centre for Molecular Ecology and Evolution Genome Service at Massey University or the Genetics Analysis Service, Department of Anatomy and Structural Biology, University of Otago, using dye-terminator sequencing (Applied Biosystems Big Dye Terminator v. 3.1). Other DNA sequences used by Platt & Spatafora (Reference Platt and Spatafora2000) and Nelsen & Gargas (Reference Nelsen and Gargas2009a ) were obtained from GenBank (http://www.ncbi.nih.nlm.gov/). The three outgroup taxa used were Dibaeis baeomyces (DQ782844), Siphula ceratites (HQ650642) and Icmadophila japonica (AB623070). Alignment of sequences was done using ClustalX v. 2.1 (Larkin et al. Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm and Lopez2007) within Geneious Pro v. 5.5.6 (Drummond et al. Reference Drummond, Ashton, Buxton, Cheung, Cooper, Heled, Kearse, Moir, Stones-Havas and Sturrock2011). Alignment of the ITS sequences produced a data matrix of 1016 characters. A Bayesian analysis of phylogeny was done using the program MrBayes, version 3.2 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). An intron sequenced in six collections (positions 1–431 in the alignment) was excluded from the analysis because it was difficult to align with the outgroup taxon Siphula ceratites, and this region was not available for the majority of ingroup sequences. The remaining data were divided into 5 partitions: 18S (positions 432–462), ITS1 (positions 463–616), 5.8S (positions 617–774), ITS2 (positions 775–951) and 28S (positions 952–1016). All partitions were unlinked. A generalized time reversible (GTR)+Γ+I model was applied to all partitions, as this was also used by Nelsen & Gargas (Reference Nelsen and Gargas2009 b). Two independent runs of four Markov chains were run for 6×106 generations, sampling every 500 generations. Convergence was determined by assessing the average standard deviation of split frequencies, where a value less than 0·01 indicated the runs had converged. A bivariate plot of the split frequencies of the two runs was computed using the compare function of AWTY online (http://king2.scs.fsu.edu/CEBProjects/awty/awty_start.php, Nylander et al. Reference Nylander, Wilgenbusch, Warren and Swofford2007). A high correlation with the line Y/X=1 was considered to be consistent with convergence of the two runs. A Bayesian inference 50% majority-rule tree and posterior probability values were estimated from the samples after discarding the first 25% (3000) of the trees sampled.
Results
Morphological investigations
Close examination of numerous T. vermicularis thalli frequently revealed the protruding colourless ostioles of pycnidial conidiomata (Fig. 2A–D). Some of the protruding ostioles could be mistaken for ordinary developing branchlets, except that when mature they exhibited a pinkish secretion (Fig. 2C). Higher magnification revealed that this secretion was a mucilaginous mass of conidia (Fig. 2G & H). The conidia were bacilliform, measuring 3–5×1–2 µm and tapering slightly at one end. Transverse sections through the ostioles consistently revealed immersed pycnidial conidiomata that were flask-shaped to broadly globose at first, enlarging to fabate in section, 150–200 µm deep×195–350 µm wide when young, unilocular or rarely double, and densely filled with long, parallel conidiophores (Fig. 1F–H). The conidiophores can be confidently classified as type VI (sensu Vobis Reference Vobis1980 and Vobis & Hawksworth Reference Vobis, Hawksworth, Cole and Kendrick1981) or the Parmelia-type (sensu Glück Reference Glück1899), comprising erect chains of intercalary conidiogenous cells with bayonet-like processes near the upper cell septum and occasional anastomosing of the apical cells.
Near the tip of the thallus, young pycnidia still full of conidia were covered by cortical tissue (Fig. 2E & F). The conidiomata matured toward the base of the thallus, resembling the developmental stages of pycnidia illustrated in Vobis & Hawksworth (Reference Vobis, Hawksworth, Cole and Kendrick1981), and emptied their conidia as their ostioles gaped open (Fig. 2G). Towards the base of the thallus, because the conidiomata widened and eroded until just the rim rose proud of the surface, they could at first glance easily be mistaken for broken-off branchlets, or possibly disintegrating or malformed discoid apothecia, especially after they had shed most of their conidiophores and conidia (Fig. 2D). Stained sections showed clearly that the pycnidial conidiomata were contiguous with the cortex of the thallus, with no evidence of any lichenicolous fungal hyphae (Fig. 2E–H). Branchlets appeared to emerge from some of the pycnidia (Fig. 1C & D).
Chemotype co-occurrence
Inspection of Thamnolia vermicularis collections from around the world under UV (365 nm) light showed that the percentage of UV+ thalli ranged from near 100% in sites from Canada, Colorado (Boulder), Austria (Ötztal), and one site in Tasmania, to 0% in a second Tasmanian site, Colombia, the Falkland Islands and most of New Zealand (Table 1). A mixture of chemotypes was present in two collections from Alaska and one from Peru, and in every case the UV+ and UV− thalli were closely intertwined. Likewise where the UV+ chemotype was found in New Zealand, it almost always occurred as single strands within clumps of the dominant UV− chemotype and not as distinct pure patches (Fig. 1B). However, in Tasmania, the chemotypes more often occur as pure clumps or swards than intermixed (G. Kantvilas, pers. comm.).
DNA analysis and sequencing
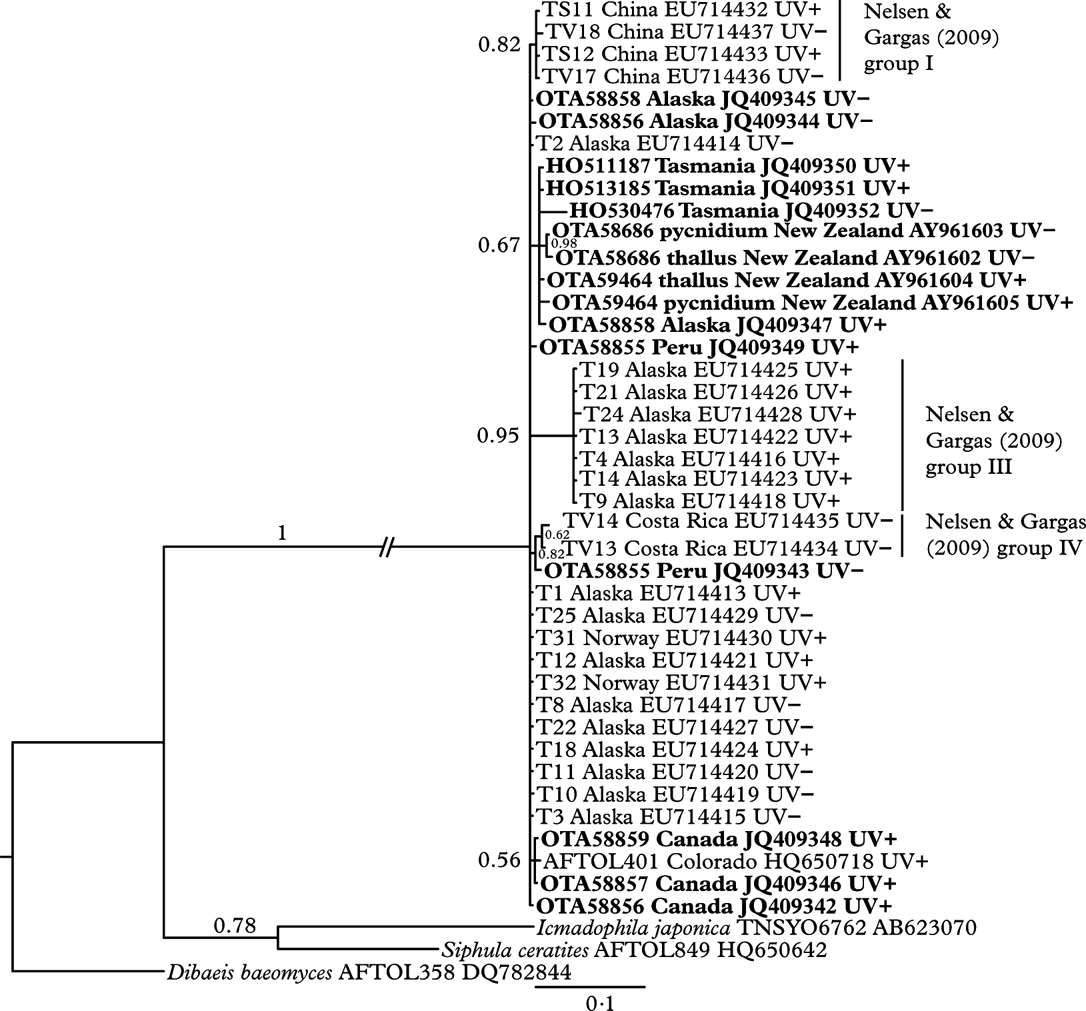
Amplification of the ITS region using the primers ITS1F and ITS4 yielded more than one amplification product. We successfully excised and sequenced a band of c. 1000 bp in length in the collections OTA58855 (UV−), OTA58856 (UV+ and UV−), OTA58857 (UV+) and OTA58858 (UV−). One full-length sequence (971 bp after removal of the primer sequences) was obtained for OTA58855 (UV−). Analysis of this sequence (GenBank JQ409343) indicated the presence of an intron of length 416 bp close to the 3′ end of the 18S gene, inserted in a position corresponding to position 1506 of Gargas et al. (Reference Gargas, DePriest and Taylor1995). The quality of the 5′ and 3′ ends of other sequences was poor, due to low signal-to-noise ratio at the ends of each chromatogram, so only truncated sequences were used in the analysis. Amplification of the ITS region using primers PN3 and PN10 yielded single bands of size c. 560 bp. The ITS2 region was successfully sequenced using the primers ITS3 and ITS4 for the collections HO511187, HO513185 and HO530476. The sequences are deposited in GenBank with accession numbers JQ409342–JQ409352. At the conclusion of the Bayesian analysis (6×106 generations), the standard deviation of split frequencies was 0·005199, which we considered to indicate convergence among tree samples. A bivariate plot indicated a high correlation between the split frequencies of the two runs (data not shown), again indicative of convergence (Nylander et al. Reference Nylander, Wilgenbusch, Warren and Swofford2007).
There is very little genetic variation amongst collections of Thamnolia vermicularis, indicated by the polytomous nature of the phylogenetic tree (Fig. 3). One clade of sequences from Alaska, corresponding to group III from Nelsen & Gargas (Reference Nelsen and Gargas2009 a), was supported by moderate branch support (posterior probability 0·95). All of the New Zealand and Australian sequences formed a clade (with an Alaskan collection OTA58858 UV+), but this was only weakly supported by the Bayesian analysis (posterior probability 0·67). With the exception of Nelsen & Gargas' group III (which were all UV+), there was no pattern to the distribution of UV chemotypes (UV+ or UV−) supported by the Bayesian analysis (posterior probability>0·9).
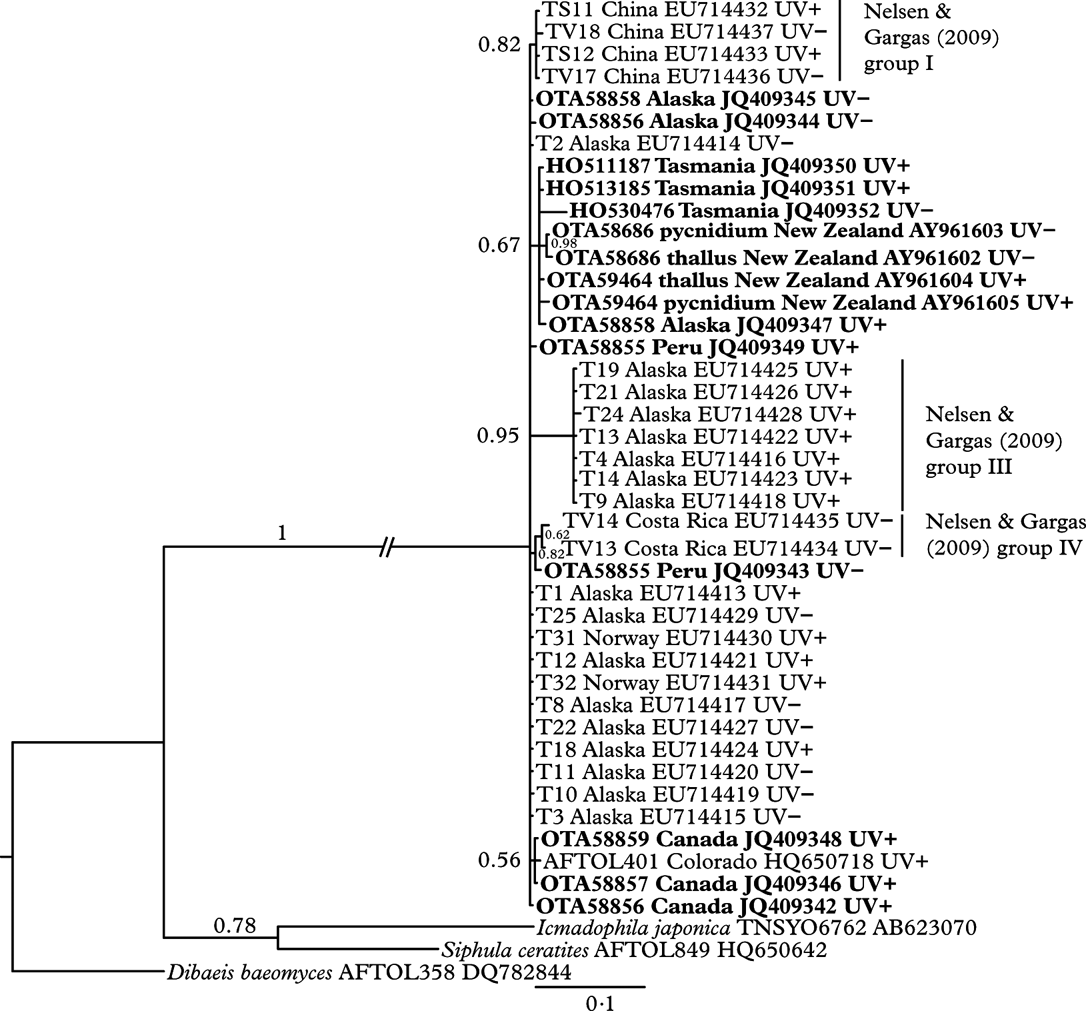
Fig. 3. Bayesian inference tree from the analysis of the internal transcribed spacer region of nuclear ribosomal DNA, with Bayesian posterior probability values above the branches. Samples in bold were sequenced for this study. GenBank accession numbers are included in sample labels between country of origin and chemotype. Pairs of thalli and their associated pycnidia have the same OTA herbarium number and are denoted “thallus” and “pycnidium” respectively.
Sequences of the ITS region showed no differences between excised pycnidial conidiomata and sterile thallus tips of Thamnolia for the UV− thallus, and a single base-pair difference between excised pycnidial conidiomata and thallus tip for the UV+ thallus. We interpret this single base-pair difference as intragenomic variation resulting from the multicopy nature of the ITS region.
The ITS alignment and Bayesian consensus tree are available on TreeBase: http://purl.org/phylo/treebase/phylows/study/TB2:S12800.
Review of historic literature on Thamnolia vermicularis
A number of early descriptions of Thamnolia vermicularis were found to include notes on both sexual and asexual structures. Massalongo (Reference Massalongo1856) and Minks (Reference Minks1874) described what they thought to be apothecia of Thamnolia, but Stein (Reference Stein and Cohn1879) and Rehm (Reference Rehm1896) believed the same structures resembled parasitic fungi. Mudd (Reference Mudd1865) and Arnold (Reference Arnold1874) independently described the same ascomata as a lichenicolous fungus which is now known as Thamnogalla crombiei (Hawksworth Reference Hawksworth1980). Later, Räsänen (Reference Räsänen1932) also thought that he found Thamnolia apothecia, but Keissler (Reference Keissler1960) doubted this observation, instead assuming a parasite was responsible.
Several independent reports of pycnidia, conidiogenous cells or conidia in Thamnolia also date back to the 1850s, and persisted in the literature until the 1970s (Nylander Reference Nylander1856, Reference Nylander1857, Reference Nylander1860, Reference Nylander1861; Lindsay Reference Lindsay1859; Fries Reference Fries1860, Reference Fries1867; Müller Argoviensis Reference Müller Argoviensis1862; Minks Reference Minks1874; Leighton Reference Leighton1879; Stein Reference Stein and Cohn1879; Tuckerman Reference Tuckerman1882; Crombie Reference Crombie1894; Glück Reference Glück1899; Zahlbruckner Reference Zahlbruckner, Engler and Prantl1907; Jatta Reference Jatta1911; Smith Reference Smith1918; Lindau Reference Lindau1923; Anders Reference Anders1928; Migula Reference Migula1929; Räsänen Reference Räsänen1932; Abbayes Reference Abbayes1951; Choisy Reference Choisy1955; Keissler Reference Keissler1960; Ozenda & Clauzade Reference Ozenda and Clauzade1970; Henssen & Jahns Reference Henssen and Jahns1974). Unlike the putative apothecia of Massalongo (Reference Massalongo1856) and Minks (Reference Minks1874), evidence for the parasitic nature of the supposed Thamnolia pycnidia has, to our knowledge, never been presented. Kärnefelt & Thell (Reference Kärnefelt and Thell1995) write “parasites were mistaken for the ascomata or pycnidia of the lichen host” and cite Massalongo (Reference Massalongo1856), Minks (Reference Minks1874), Räsänen (Reference Räsänen1932), Choisy (Reference Choisy1955), and Vareschi (Reference Vareschi1970). However, these references support Kärnefelt & Thell's statement with regard to apothecia only, not pycnidia. Ihlen (Reference Ihlen1995) writes “the species never develops ascomata or pycnidia” and cites Culberson (Reference Culberson1963), Poelt (Reference Poelt1969), Sheard (Reference Sheard1977), Purvis et al. (Reference Purvis, Coppins, Hawksworth, James and Moore1992) and Krog et al. (Reference Krog, Østhagen and Tønsberg1994); however, again, these authors present no disproof of previous pycnidia reports. Hawksworth (Reference Hawksworth1980) merely assumes that the drawings of conidiogenous cells in Minks (Reference Minks1874) represent an “unknown coelomycete”.
The current prevalence of the opinion that Thamnolia is completely sterile dates from the 1960s, and since then has dominated the English-language literature in particular (Motyka Reference Motyka1960; Culberson Reference Culberson1963; Poelt Reference Poelt1963, Reference Poelt1969; Vareschi Reference Vareschi1970; Filson Reference Filson1972; Sheard Reference Sheard1977; Golubkova et al. Reference Golubkova, Savicz and Trass1978; Hale Reference Hale1979; Wirth 1980, 1987, Reference Wirth1995a ,Reference Wirth b ; Vobis & Hawksworth Reference Vobis, Hawksworth, Cole and Kendrick1981; Thomson Reference Thomson1984; Galloway Reference Galloway1985, Reference Galloway and Nash1996, Reference Galloway2007; Purvis et al. Reference Purvis, Coppins, Hawksworth, James and Moore1992; Purvis Reference Purvis2000; Rogers Reference Rogers and George1992; Krog et al. Reference Krog, Østhagen and Tønsberg1994; Kärnefelt & Thell Reference Kärnefelt and Thell1995; Ihlen Reference Ihlen1995; St. Clair Reference St. Clair1999; Brodo et al. Reference Brodo, Duran Sharnoff and Sharnoff2001; Lambley & Purvis Reference Lambley, Purvis, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009; Dobson Reference Dobson2011). Most of these works do not even mention pycnidia in the context of Thamnolia, and those that do, claim them to be absent or unknown. After 1920, all works maintaining the presence of pycnidia were published in languages other than English (Lindau Reference Lindau1923; Anders Reference Anders1928; Migula Reference Migula1929; Räsänen Reference Räsänen1932; Abbayes Reference Abbayes1951; Choisy Reference Choisy1955; Keissler Reference Keissler1960; Ozenda & Clauzade Reference Ozenda and Clauzade1970; Henssen & Jahns Reference Henssen and Jahns1974). Thompson (Reference Thomson1984) is the only late 20th century English written work which indicates the possibility that Thamnolia might produce pycnidia by carefully stating “Apothecia and pycnidia not known with certainty, reports seem to be based on lichen parasites”. Nelsen & Gargas (Reference Nelsen and Gargas2009a , Reference Nelsen and Gargas b ) mention pycnidia [citing pers. comm. with one of the authors of the present paper and Ozenda & Clauzade (Reference Ozenda and Clauzade1970)], but they do not discuss the form of the pycnidia in any detail and did not themselves actually find pycnidia. More recently, the Lichens of Finland by Stenroos et al. (Reference Stenroos, Ahti, Lohtander and Myllys2011) briefly mentions that pycnidia have been reported, based on Nelson & Gargas' (Reference Nelsen and Gargas2009a , Reference Nelsen and Gargas b ) comments, although this reference was not mentioned and Finnish material had not been examined for pycnidia (S. Stenroos, pers. comm.).
Culberson (Reference Culberson1963) greatly influenced all subsequent English-language treatments, but cited relatively few references. The earliest, Koerber (Reference Koerber1855), predates the discovery of pycnidia in the genus Thamnolia. Asahina (Reference Asahina1937), whom Culberson (Reference Culberson1963) also cited, was aware of the work of Minks (Reference Minks1874) and Räsänen (Reference Räsänen1932), as he cited these references with regard to apothecia being parasitic in origin. However, although both Minks (Reference Minks1874) and Räsänen (Reference Räsänen1932) describe pycnidia in Thamnolia, pycnidia were not mentioned in either Asahina's or Culberson's description of the genus. Furthermore, Culberson (Reference Culberson1963) is the sole reference for the Thamnolia treatment of Poelt (Reference Poelt1969), which then became a standard international reference for the following decades.
Discussion
Thamnolia is widely regarded as sterile, the standard definition of which is the absence of spores or a sporocarp (Galloway Reference Galloway1985, Reference Galloway2007). Vobis & Hawksworth (Reference Vobis, Hawksworth, Cole and Kendrick1981) listed it as one of 11 genera of ‘strictly sterile’ lichens, Platt & Spatafora (Reference Platt and Spatafora2000) described it as ‘nonsexual and completely sterile’ and Grube & Kantvilas (Reference Grube and Kantvilas2006) as ‘strictly sterile’. Thus its widespread distribution, from arctic regions to mountain tops around the world, has always been something of a puzzle (Sheard Reference Sheard1977; Purvis et al. Reference Purvis, Coppins, Hawksworth, James and Moore1992; Galloway & Aptroot Reference Galloway and Aptroot1995; Kärnefelt & Thell Reference Kärnefelt and Thell1995; Malcolm & Malcolm Reference Malcolm and Malcolm2000). Colonization by thallus fragments over distances of metres to a kilometre or more may be possible in particularly windy areas (Pérez Reference Pérez1991, Reference Pérez1994); however, the species' global distribution and the patterns of genetic relatedness shown in Fig. 3 and in other phylogenetic studies (e.g. Nelsen & Gargas Reference Nelsen and Gargas2009a ) are more consistent with long-distance dispersal (Galloway Reference Galloway, Nimis and Crovello1991). Furthermore, the lack of monophyly of chemotypes and their occurrence in both hemispheres (but at different frequencies, Sato Reference Sato1968) is even harder to explain without sexual reproduction or readily dispersed micro-propagules (Nelsen & Gargas Reference Nelsen and Gargas2009a ).
This study presents clear evidence that Thamnolia vermicularis produces pycnidial conidiomata; thus the species can no longer be described as strictly sterile, but rather as asexual, as the conidia produced by pycnidia are asexual spores (Galloway Reference Galloway1985, Reference Galloway2007). Our findings are consistent with the descriptions of pycnidial conidiomata, conidiophores and conidia previously reported for other members of Icmadophilaceae in the genera Dibaeis, Icmadophila and Pseudobaeomyces (Gierl & Kalb Reference Gierl and Kalb1993; Rambold et al. Reference Rambold, Triebel and Hertel1993; Ludwig Reference Ludwig2011). Furthermore, the earliest historic reports of pycnidia in Thamnolia more than 150 years ago (e.g., Nylander Reference Nylander1856, Reference Nylander1857, Reference Nylander1860; Lindsay Reference Lindsay1859) describe the same anatomical details, and old illustrations of the conidiophores (Lindsay Reference Lindsay1859; Nylander Reference Nylander1860; Minks Reference Minks1874; Crombie Reference Crombie1894; Zahlbruckner Reference Zahlbruckner, Engler and Prantl1907; Smith Reference Smith1918; Keissler Reference Keissler1960) bear a strong resemblance to our observations. Moreover, although unaware of the phylogenetic affiliations known today, some of the 19th century authors (Lindsay Reference Lindsay1859; Nylander Reference Nylander1860; Crombie Reference Crombie1894) noticed the similarity between the pycnidia or conidiophores of Thamnolia and those of “Baeomyces”, apparently referring to members of modern-day Icmadophilaceae rather than unrelated Baeomyces spp. (Baeomycetaceae) in the modern sense.
It seems very likely that at least some, if not all, previous reports of Thamnolia pycnidia refer to the same structures observed by us. This view is supported by the outcome of our literature review, indicating the absence of any publications demonstrating a parasitic origin for the pycnidia reported for Thamnolia in the past. Minks (Reference Minks1874), in particular, made an interesting observation that supports the supposition that the pycnidia he saw belonged to Thamnolia, rather than to a parasite. He noted that the pycnidia are considerably more abundant on sterile compared with apothecia-bearing thalli. As the apothecia (actually perithecia) he saw represent the parasitic fungus Thamnogalla, his observation is not surprising, as a reduced reproductive capacity of the host is an expected consequence of a parasite infection, in this case a decrease in pycnidial development in the lichen host Thamnolia.
The pale ostioles of the immersed pycnidial conidiomata we have observed in Thamnolia vermicularis make them much less conspicuous and more difficult to detect than typical dark ostioles, being more similar to the pycnidial conidiomata with pale ostioles described for other pale lichens such as Ramalina siliquosa (Fletcher Reference Fletcher1975) and several species of Ramalina from New Zealand (J. M. Bannister, unpublished data). This could explain in part why any apparent means of sexual or asexual reproduction found on Thamnolia have thus far been attributed to infection by lichenicolous fungi (Kärnefelt & Thell Reference Kärnefelt and Thell1995). However, lichenicolous fungi with similar conidia known to infect Thamnolia (e.g., Sphaerellothecium thamnoliae, Polycoccum vermicularium, Phoma thamnoliae) differ in pigmentation of their pycnidia or mycelium, or in anatomical details of the conidiophores (Zhurbenko Reference Zhurbenko2012), and cannot explain the structures we document here (M. Zhurbenko, pers. comm.). It is noteworthy in this context that another type of lichen conidiomata, the bisymmetric campylidia found in many foliicolous lichens, have been mistaken for parasitic fungi in the past (Serusiaux Reference Serusiaux1986; Lücking & Santesson Reference Lücking and Santesson2002). DNA sequencing further supports our claim of pycnidial conidiomata in Thamnolia. ITS sequencing found either no difference or only a single base-pair difference among extracts from thallus tips and excised pycnidia contents from either UV+ or UV− chemotypes of Thamnolia vermicularis, indicating that the observed structures cannot be attributed to lichenicolous fungi. Likewise, sections through the ostioles showed no evidence of any foreign hyphae. A few thalli did support lichenicolous fungi, among them Thamnogalla crombiei, Stigmidium frigidum and Polycoccum vermicularium (Table 1). However, within New Zealand at least, the distribution of pycnidial conidiomata on Thamnolia vermicularis was much wider than the known geographical ranges of the three lichenicolous fungi that have been identified on Thamnolia in New Zealand (Galloway Reference Galloway2007).
Based on our inspections of thalli, pycnidial conidiomata are not only present in Thamnolia from around New Zealand, but also in recent collections from Australia, Alaska, Canada and Peru (Table 1). Furthermore, assuming the historical reports are correct, pycnidia have also been observed on specimens from the British Isles, Scandinavia, and Central Europe (Lindsay Reference Lindsay1859; Nylander Reference Nylander1860), indicating that this means of reproduction is likely a feature of the species as a whole. Four factors might have contributed to the loss of this formerly established knowledge from the modern literature: 1) language barriers, because most 20th century accounts mentioning pycnidia are not written in English; 2) difficult access to rare old, or expensive new, lichenological literature, as mentioned, for instance, in the foreword of Poelt (Reference Poelt1963); 3) the assumption that, because putative apothecia were shown to be parasitic in origin, then reported pycnidia were also due to parasitic fungi; 4) the uncritical citation of the ‘established opinion’ perpetuated the myth that Thamnolia is completely sterile, and lack of reference to pycnidia in earlier English works became an assumption of their absence in later works.
In a similar situation to Thamnolia, pycnidia have been observed in Siphula ceratites (Wahlenb.) Fr. by 19th century lichenologists and were later confirmed or cited far into the 20th century (Sommerfelt Reference Sommerfelt1826; Fries Reference Fries1860; Krempelhuber Reference Krempelhuber1869; Minks Reference Minks1874; Vainio Reference Vainio1921; Räsänen Reference Räsänen1937; Keissler Reference Keissler1960; Dodge Reference Dodge1973), but are now absent from almost all modern Siphula treatments (e.g. Kantvilas Reference Kantvilas2002). Only Kantvilas (Reference Kantvilas1998) briefly mentions that “pycnidia are known in the Northern Hemisphere species, S. ceratites (R. Santesson, in litt.)”. However, it remains unclear if this refers to observations made by Santesson, or if Santesson described the older literature mentioned above. The anatomical details given for the putative pycnidia of Siphula ceratites are at least superficially reminiscent of the pycnidia in Thamnolia and other Icmadophilaceae, as they are described as immersed in the thallus, containing articulated conidiophores with bacilliform conidia (cf. Vainio Reference Vainio1921). Whether or not these putative pycnidia of Siphula do really exist or merely represent a lichenicolous fungus is the subject of an ongoing investigation.
The rediscovery of pycnidial conidiomata in Thamnolia vermicularis also necessitates a reconsideration of hypotheses concerning the species' global distribution. Sheard (Reference Sheard1977) proposed that the current distribution of Thamnolia reflected a palaeogeography dating from the Permian-Triassic; however, he also recognized that long-distance dispersal must have occurred, for example between Australia and New Zealand. The absence of Thamnolia from active volcanoes has also been highlighted as evidence for its limited dispersal capabilities (Thomson Reference Thomson1984); however, as Galloway (Reference Galloway, Nimis and Crovello1991) pointed out, it is abundant on tectonically young mountains in New Zealand. Our rediscovery provides a mechanism for long-distance dispersal, because microscopic conidia are much more plausible as wind-borne agents of dispersal, than are macro-fragments.
We also suggest that dispersal via conidia may account for smaller scale patterns of genetic variation in Thamnolia. Stocker-Wörgötter (Reference Stocker-Wörgötter, Marcelli and Seaward1998) presents evidence that lichen conidia, like ascospores, are capable of germinating and forming new thalli if a suitable photobiont is encountered. The findings of Cassie & Piercey-Normore (Reference Cassie and Piercey-Normore2008), of small-scale population subdivision in Thamnolia despite few opportunities for fragmentation, can be easily explained by individual thalli producing multitudes of conidia via mitosis that then disperse and re-lichenize in the same general area. The considerable genetic variability among photobionts associated with the same fungal genotype of Thamnolia discovered recently by Nelsen & Gargas (Reference Nelsen and Gargas2009b ) is a logical outcome of conidial dispersal followed by re-lichenization with genetically diverse photobionts. A similar pattern of high genetic variability of the photobiont but low variability of the mycobiont has been observed in the rarely fruiting Lasallia pustulata (Sadowska-Des et al. Reference Sadowska-Des, Otte and Schmitt2012), which could also be explained by dispersal and re-lichenization of conidia. Branching in Thamnolia could also be a product of conidial germination from immersed pycnidia rather than from thallus division (Fig. 2C & D). A similar mechanism is found in another fruticose species, Cetraria islandica, where new thalline lobes grow from pycnidia at the thallus edge (Jahns & Schuster Reference Jahns and Schuster1981; Jahns Reference Jahns and Galun1988). Examination of Thamnolia branch development and secondary chemistry could be worthwhile.
Until recently, the two chemotypes within T. vermicularis were regarded as separate species or at least distinct subspecies within T. vermicularis. This study, like those of Platt & Spatafora (Reference Platt and Spatafora2000) and Nelsen & Gargas (Reference Nelsen and Gargas2009a ), failed to find consistent genetic differences between the two chemotypes. Instead we found that different chemotypes from New Zealand were more similar to each other than they were to matching chemotypes from North America and China. This pattern of genetic relatedness could arise if chemotype ‘switching’ was possible. The difference between chemotypes involves a relatively small change in a biochemical pathway (Elix & Gaul Reference Elix and Gaul1986; Nelsen & Gargas Reference Nelsen and Gargas2009a ). Thamnolic, squamatic and baeomycesic acids are all β-orcinol depsides, and para-depsides (baeomycesic and squamatic acids) are thought to serve as precursors to the production of meta-depsides (thamnolic acid) (Elix & Gaul Reference Elix and Gaul1986; Elix et al. Reference Elix, Jenie and Parker1987; Culberson & Elix Reference Culberson, Elix, Dey and Harborne1989; Elix Reference Elix and Nash1996). While environmental factors have been found to correlate with lichen depside chemistry under natural conditions (Armaleo et al. Reference Armaleo, Zhang and Cheung2008) and can affect the production of mycobiont secondary compounds in culture (Stocker-Wörgötter Reference Stocker-Wörgötter2001), the intermixing of Thamnolia chemotypes at some locations is much more consistent with a genotypic difference than phenotypic adaptation. A loss of function mutation in a single dispersed conidium disrupting the pathway from para-depside precursors to thamnolic acid could explain a unidirectional change in thallus chemistry (Nelsen & Gargas Reference Nelsen and Gargas2009a ), but it is unlikely that such a mutation would be completely reversible, so this cannot explain larger scale patterns of chemotype distribution and genetic relatedness. Comparative analyses of lichen-forming fungi have revealed a very high frequency of introns in ribosomal DNA (Gargas et al. Reference Gargas, DePriest and Taylor1995; Grube et al. Reference Grube, Gargas and DePriest1996), but it is not yet known whether this is also the case for other components of the fungal genome. If introns were also a feature of genes involved in the depside pathway of Thamnolia, alternative splicing (Nilsen & Graveley Reference Nilsen and Graveley2010) could provide a mechanism for reversible switching between related compounds, leading to intermixing and non-monophyly of chemotypes.
We are grateful to the Alpine Ecosystems Research Group, Otago University, and a University of Otago Research Grant for supporting some of this work, to the late Professor Peter Bannister and Professor Ulli Ullman for translating old German text, Dr Pernille Bronken Eidesen, Dr Stefan Halloy, Dr Regine Stordeur and Emeritus Professors Alan Mark and Jack Elix for collecting foreign Thamnolia samples and/or obtaining literature, to Dr David Galloway for help with identifying lichenicolous fungi, to Dr Paul Diederich and Associate Professor Paul Guy, Professor Elfie Stocker-Wörgötter, Dr Mikhail Zhurbenko and Anna Sadowska-Deś for helpful comments or discussions, to Dr Haseeb Randhawa, Bjørn Sollie, Professor Soili Stenroos, and Yulia Kiseleva for translating French, Norwegian, Finnish and Russian texts, to herbarium staff at CANB, CHR and HO for providing loans of Thamnolia specimens, and to Dr Gintaras Kantvilas for permission to extract DNA from HO specimens. The helpful suggestions of Dr Alan Fryday and two referees have greatly enhanced this paper.