Introduction
Currently there are three valid species in the tapeworm genus Atractolytocestus Anthony, Reference Anthony1958 (Caryophyllidea: Caryophyllaeidae): Atractolytocestushuronensis Anthony, Reference Anthony1958; Atractolytocestus sagittatus (Kulakovskaya & Akhmerov, Reference Kulakovskaya and Akhmerov1965); and Atractolytocestus tenuicollis (Li, Reference Li1964). All three parasitize exclusively in the intestine of common carp (Cyprinus carpio L.) (Králová-Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). The cosmopolitan A. huronensis was originally described in the River Huron (Michigan, USA) (Anthony, Reference Anthony1958), and then it was recorded in Europe (Oros et al., Reference Oros, Hanzelová and Scholz2004; Kappe et al., Reference Kappe, Seifert, El-Nobi and Bräuer2006; Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová and Štefka2011), South Africa (Scholz et al., Reference Scholz, Tavakol, Halajian and Luus-Powell2015) and Asia (Li et al., Reference Li, Zhang and Boyce2017; Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová, Xi and Štefka2018). Atractolytocestus sagittatus was originally described as Markevitschia sagittata from the common carp in Russia (Kulakovskaya & Akhmerov, Reference Kulakovskaya and Akhmerov1965), and then found in Japan and the People's Republic of China (PRC) (Scholz et al., Reference Scholz, Shimazu, Olson and Nagasawa2001; Xi et al., Reference Xi, Wang, Wu and Nie2009). In contrast, A. tenuicollis was only recorded in the PRC (Li, Reference Li1964; Králová-Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). Atractolytocestus species were recently surveyed in common carp from Central China. During this new investigation, A. huronensis has been found in Taibai Lake (Li et al., Reference Li, Zhang and Boyce2017), and A. tenuicollis in Donghu and Niushan lakes (unpublished data). Atractolytocestus sagittatus was not found in this survey, but tapeworms whose internal transcribed spacers (ITS) sequences differed markedly from all three species of the genus were found in the Danjiangkou Reservoir. In the present paper, these tapeworms were characterized morphologically and genetically. We conclude that they belong to a new Atractolytocestus species.
Materials and methods
Tapeworm collection and morphological identification
Common carp specimens were collected in April 2018 from the Danjiangkou Reservoir (32°36′–33°48′N, 110°59′–111°49′E), Hubei Province, the PRC. Tapeworms, obtained from the intestine of the fish, were gently rinsed in 0.65% sodium chloride solution, and then fixed with hot 70% ethanol. Tapeworm specimens (n = 3) were cut into two parts from the neck. Each scolex segment was preserved in absolute ethanol and stored at 4°C for extraction of the genomic DNA. The remaining part and other complete tapeworms were preserved in 70% ethanol for morphological identification. Specimens for whole mounts (n = 13, including the remaining part of the above three specimens) were stained with iron hydrochloric carmin, dehydrated in a graded ethanol series, cleared in xylene and mounted in Canada balsam as permanent preservation (Fu et al., Reference Fu, Li and Zou2019). Seven individuals were used to perform histological sections using the standard protocols as follows: embedding the samples in paraplast; sectioning by microtome; staining with haematoxylin and eosin; and mounting in Canada balsam (Xi et al., Reference Xi, Barčák, Oros, Chen and Xie2016).
DNA isolation, ribosomal internal transcribed spacers 2 (ITS2), cytochrome c oxidase (cox1) and NADH dehydrogenase subunit 3 (nad3) gene amplification and sequencing
Genomic DNA of the three tapeworm scolex parts was extracted using the Tissue Cell Genome Kit (Beijing, PRC) according to the manufacturer's instructions. Complete ITS2 sequence was amplified using primers 5.8S–2 (5′–GTC GAT GAA GAG CGC AGC–3′) and ITS2 (5′–AGG AGG CGA ATC ACT AT–3′) (Králová-Hromadová et al., Reference Králová-Hromadová, Štefka and Špakulová2010). Polymerase chain reaction (PCR) amplification of ITS2 was conducted using LA Taq polymerase as follows: 5 min at 94°C as the initial step; then 35 cycles of 30 s at 94°C, 30 s at 50 °C, 1 min at 72°C. The final step was 7 min at 72°C. A fragment of the cox1 gene was amplified with primers CFCYT2 (5′–ACT AAG TGT TTT CAA AA–3′) and CRCYT2 (5′–CCA AAA AAC CAA AAC AT–3′) (Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová, Štefka and Scholz2012). Conditions of cox1 gene PCR amplification were the same as that of ITS2, but the annealing temperature was 42°C. The primers NAD3F (5′–AAC GTA GCT AGT TAA GTG CTG AAT TCT–3′) and NAD3R (5′–CTT TTA ATT ATT AGC AGT AAC CGA TCT C–3′), designed with software Primer 6, were used to amplify the complete sequence of the nad3 gene. Except for the 51°C annealing temperature, the PCR amplification of nad3 was also the same as that of ITS2. All PCR products were loaded on a 1.5% agarose gel. After purification, PCR products were sequenced with the PCR primers described above, and the sequences were assembled manually with the software ContigExpress (except for ITS2 sequences). The purified PCR products of ITS2, amplified from three individuals, were cloned into the pGEM®–T Easy vector (Promega) following the manufacturer's protocol. Five recombinant clones were selected from each individual (DJ1/1–5, DJ2/1–5, DJ3/1–5) for sequencing. The boundaries of ITS2 were determined according to the sequences of A. huronensis. The ITS2 sequences were compared with ITS2 variants of A. huronensis, A. tenuicollis and A. sagittatus (table 1). Newly obtained cox1 sequences were compared with previously published cox1 haplotypes of A. huronensis, A. tenuicollis and A. sagittatus, including haplotype 1 (Ha1) from continental Europe (EU), in particular Slovakia (SK), Hungary (HU), Croatia (CR) and Romania (RO), Ha2 from the United Kingdom (UK), the United States of America (USA), the Danjiangkou Reservoir and Poyang Lake, the PRC (CH–D, CH–P), Ha3 from Japan (JP), Ha4 from the PRC (CH), Ha5 from South Africa (SA), Ha6 from the Danjiangkou Reservoir (CH–D), and Ha7/8/9 from Poyang Lake (CH–P) (see table 2 and Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová, Xi and Štefka2018).
Table 1. Polymorphisms in repetitive microsatellite motifs within different ribosomal internal transcribed spacers 2 (ITS2) rDNA variants of Atractolytocestus danjiangkouensis from Cyprinus carpio in the People's Republic of China.

Table 2. Mitochondrial cytochrome c oxidase sequences of Atractolytocestus spp. used for the current phylogenetic analysis.

Phylogenetic analysis and genealogy reconstruction
The maximum likelihood (ML) method was used to construct phylogenetic trees using cox1 sequences (table 2) in PhyML3.0 (Guindon et al., Reference Guindon, Dufayard and Lefort2010). Based on the Akaike information criterion, GTR + G was chosen as the optimal nucleotide substitution model for cox1 (Lefort et al., Reference Lefort, Longueville and Gascuel2017). ML trees were obtained with 1000 bootstraps of the data. Khawia sinensis Hsü, 1935 (Accession no. JN004232) and Breviscolex orientalis Kulakovskaya, 1962 (Accession no. JQ034055) were used as outgroups in the cox1 analysis. To visualize the level of intra-population and inter-population genetic variability of the sampled individuals and three valid species of genus Atractolytocestus, parsimony network was prepared for the cox1 datasets using the program TCS (Clement et al., Reference Clement, Snell, Walker, Posada and Crandall2002) implemented in PopART (Leigh & Bryant, Reference Leigh and Bryant2015).
Results
Morphological identification
Based on 13 mounted specimens and seven histological sections, measurements are in micrometres (μm) unless otherwise stated (fig. 1).

Fig. 1. Morphological characteristics of Atractolytocestus danjiangkouensis from Cyprinus carpio in the People's Republic of China. (a) total view; (b) sagittal section at the level anterior to cirrus- sac; (c) sagittal section at the level of cirrus-sac; (d) sagittal section at the level posterior to cirrus-sac; (e) cross-section at the level anterior to cirrus-sac; and (f, g) cross-section at the level of cirrus-sac. Abbreviations: cga, common genital atrium; cs, cirrus-sac; fg, female genital pore; ilm, inner longitudinal musculature; mg, male genital pore; ov, ovary; te, testes; ug, uterine glands; us, uterus; v, vagina; vd, vas deferens; vf, vitelline follicles.
Family Caryophyllaeidae Leuckart, 1878
Genus Atractolytocestus Anthony, Reference Anthony1958
Atractolytocestus danjiangkouensis n. sp.
Host: Cyprinus carpio L. (Cypriniformes: Cyprinidae)
Locality: Danjiangkou Reservoir (Hubei Province) (110°7′–111°53′E, 32°15′N–33°22′N)
Type specimens: Voucher specimens (CN–ihb–PCA401–20) are deposited in the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan (Accession no. IHB20200910-1).
Representative DNA sequences: The newly generated sequences were submitted to the GenBank database under the accession numbers MW534425–39, MW533426–28 and MW506228–30.
Description (fig. 1)
Tapeworm body 7.4–9.6 mm long, with a maximum width of 0.59–0.67 mm. Scolex bulboacuminate or conical (0.62–0.85 mm wide), much wider than neck (0.29–0.43 mm wide). (fig. 1a). Testes medullary, spherical to oval, 96–130 long, 79–104 wide, and the number of testes numerous (approximately 200) (fig. 1b, c, e). Testes arranged in dorso-ventral rows, 10–12 on cross-section (fig. 1e). First testis anterior to the first vitelline follicle, testes anterior to the ovary (fig. 1a). Vitelline follicles cortical, extensive, oval, 53–79 long, 45–66 wide, uninterrupted alongside cirrus sac, uterine coils and ovarian arms (fig. 1a). Vas deferens forms several loops, anterior to cirrus-sac (fig. 1c). Cirrus-sac thick-walled, almost spherical, 263–273 × 255–265 (fig. 1a, c, f, g).
Ovary H–shaped, 560–728 long, 379–485 wide, with long ovarian lateral arms (fig. 1a). Vagina tubular, slightly sinuous, extending forward between ovarian isthmus and uterine loops (fig. 1d). Male genital pore anterior to female pore, opening into the common genital atrium (fig. 1c).
Uterus strongly coiled, forming loops between post-ovarian vitelline follicles and posterior margin of cirrus-sac (fig. 1a, c). Uterine glands present and well developed (fig. 1c, d). Eggs not examined.
Molecular characteristics and phylogenetic analysis
Divergent intragenomic copies were detected in the ITS2 ribosomal spacer of tapeworms from the Danjiangkou Reservoir. A total of 15 recombinant clones (Accession no. MW534425–39) obtained from three tapeworm individuals yielded eight different sequence types caused mainly by single nucleotide polymorphisms (SNPs) and varying numbers of the short repetitive region (GTGC)n(TTGGT)n. This allowed sorting of the ITS2 sequence types into three ITS2 variants (661, 665 and 665 base pairs (bp)) (table 1 and fig. 2). The ITS2 variant three was the most frequent and observed in a majority (nine out of 15) of the recombinant clones. ITS2 variant two was present only in two clones. The pairwise sequence identity among sequence types ranged between 99.1% and 100%. Compared with A. huronensis, A. tenuicollis and A. sagittatus, the ITS2 sequence identity of the newly collected tapeworms was 94.7–95.5%, 91.8–92.7% and 80.0–83.9%, respectively (table 3).

Fig. 2. Schematic presentation of ribosomal internal transcribed spacers 2 variants of the Atractolytocestus danjiangkouensis from Cyprinus carpio in the People's Republic of China.
Table 3. Comparison of sequence similarity of tapeworms of Atractolytocestus danjiangkouensis from Cyprinus carpio in the People's Republic of China with Atractolytocestus huronensis, Atractolytocestus tenuicollis and Atractolytocestus sagittatus in ribosomal internal transcribed spacers 2 (ITS2), cytochrome c oxidase (cox1) and NADH dehydrogenase subunit 3 (nad3), respectively.
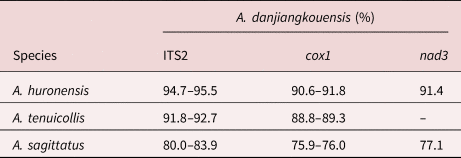
The total length of the amplified cox1 sequences (Accession no. MW533426–28) was 672 bp, encoding for 224 amino acids of the protein. The mitochondrial cox1 sequences revealed the presence of three new haplotypes, which differs from all so far determined Atractolytocestus haplotypes (we designated the new haplotypes as Ha10 to Ha12; haplotypes 1–9 have already been recognized in the three valid species of genus Atractolytocestus; table 3 and fig. 3). The pairwise sequence identity among haplotypes ranged between 99.4% and 99.9% (SNPs). However, all these mutations were silent and did not result in a change in the amino acid sequence. Interspecific pairwise identity of the haplotypes Ha10–12 with A. huronensis, A. tenuicollis and A. sagittatus was 90.6–91.8%, 88.8–89.3% and 75.9–76.0%, respectively (table 3).

Fig. 3. Haplotype network based on mitochondrial cox1 data on Atractolytocestus species. Country codes are as in table 2. EU stands for samples from continental Europe (SK, HU, CR and RO).
The total length of the amplified nad3 sequences (Accession no. MW506228–30) was 348 bp, encoding for 115 amino acids. The identity of nad3 was 91.4% and 77.1% with A. huronensis and A. sagittatus, respectively (table 3).
Phylogenetic tree based on cox1 indicated that tapeworms from the Danjiangkou Reservoir (Hubei, the PRC) were most closely related to A. tenuicollis, followed by A. huronensis, and finally A. sagittatus (fig. 4).
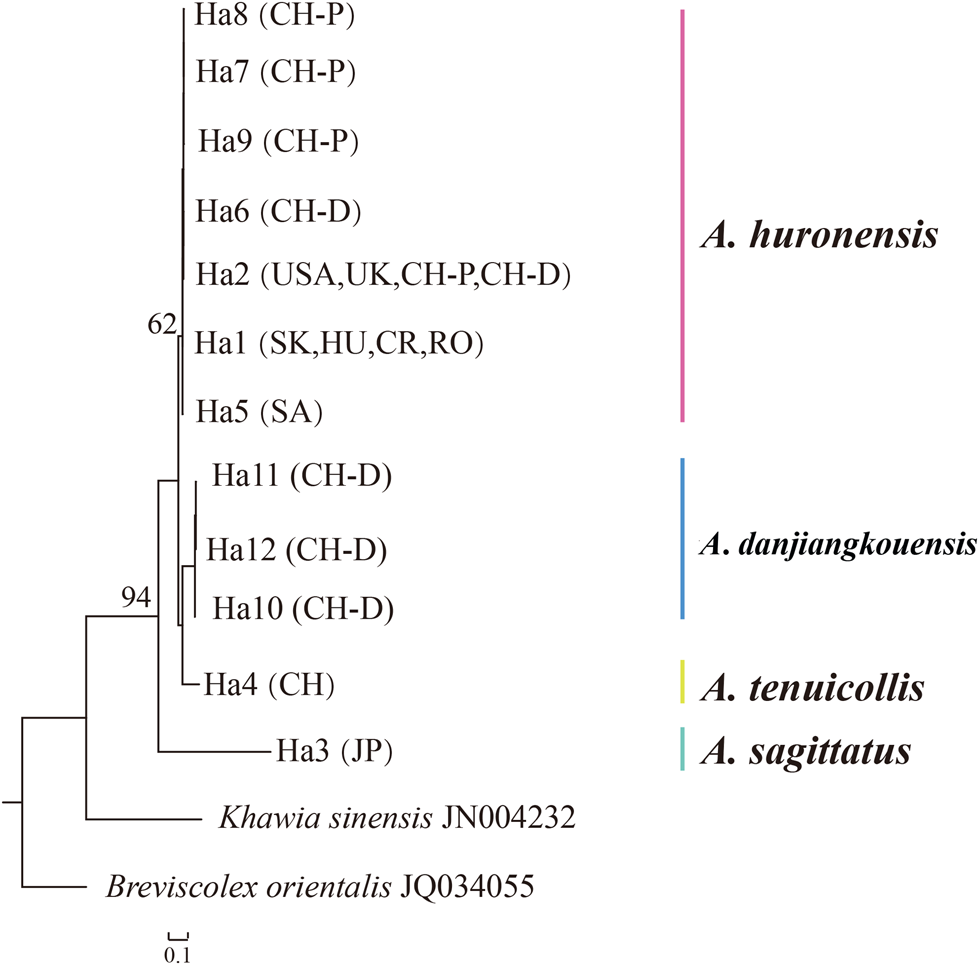
Fig. 4. Phylogenetic relationships of Atractolytocestus danjiangkouensis from Cyprinus carpio in the People's Republic of China inferred using maximum likelihood (ML) method and cox1 sequences. Numbers at the branches represent bootstraps’ values.
Discussion
Morphological differentiation of the three valid species, A. huronensis, A. sagittatus and A. tenuicollis, is mainly based on the number of testes and the relative position of the first testes (fte) and the first vitelline follicles (fvf) (Králová-Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). Based on morphological characters, the newly collected tapeworm found in common carp from the Danjiangkou Reservoir differs from A. huronensis and A. tenuicollis (table 4). In comparison with A. huronensis, the new species had more testes (200 vs. 0–58). The fte were anterior to the fvf, but posterior in A. huronensis. Testes extended posteriorly to the anterior ovary in the new species, but only posterior to the cirrus-sac in A. huronensis. In addition, the ovarian arms of the new species were longer than those in A. huronensis (Oros et al., Reference Oros, Hanzelová and Scholz2004). Compared with A. tenuicollis, the fte were anterior to the fvf in the new species (Králová–Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). The morphological characteristics of the newly collected tapeworms were similar to those of A. sagittatus as characterized by Scholz et al. (Reference Scholz, Shimazu, Olson and Nagasawa2001), which exhibited a larger body size and shorter lateral arms.
Table 4. Morphological comparison of the Atractolytocestus danjiangkouensis from Cyprinus carpio in the People's Republic of China (PRC) with Atractolytocestus huronensis, Atractolytocestus tenuicollis and Atractolytocestus sagittatus.
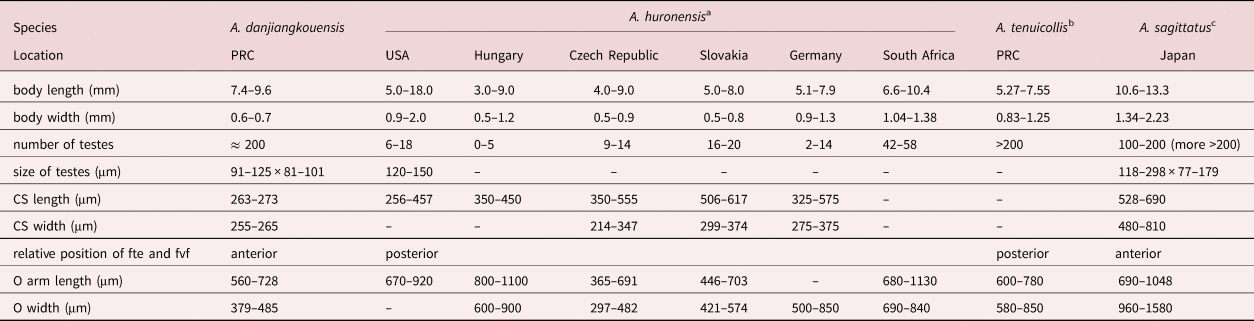
a A. huronensis – data from Anthony (Reference Anthony1958), Oros et al. (Reference Oros, Hanzelová and Scholz2004), Kappe et al. (Reference Kappe, Seifert, El-Nobi and Bräuer2006) and Scholz et al. (Reference Scholz, Tavakol, Halajian and Luus-Powell2015).
b A. tenuicollis – data from Li (Reference Li1964) and Králová–Hromadová et al. (Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013).
c A. sagittatus – data from Scholz et al. (Reference Scholz, Shimazu, Olson and Nagasawa2001). ‘–’: not available; CS: cirrus-sac; fte: first testes; fvf: first vitelline follicles; O: ovary.
However, significant molecular differences between tapeworms from the Danjiangkou Reservoir and A. sagittatus were found. The sequence identity between the nuclear ITS2 and mitochondrial cox1 and nad3 genes was not higher than 84% between the two species (80.0–83.9%, 75.9–76.0% and 77.1%, respectively). This suggests that the Danjiagkou population represents a different Atractolytocestus species, genetically distinct from from A. sagittatus. Although A. sagittatus was reported in Taihu and Niushan lakes, the PRC (Xi et al., Reference Xi, Wang, Wu and Nie2009), some morphological characteristics were different from the original description of A. sagittatus (Kulakovskaya & Akhmerov, Reference Kulakovskaya and Akhmerov1965). The fte were posterior to the fvf (Xi et al., Reference Xi, Wang, Wu and Nie2009), which is opposite from A. sagittatus, but identical to A. tenuicollis. Subsequently, an Atractolytocestus species found in the same locality, Niushan Lake, was identified as A. tenuicollis based on molecular data (Králová-Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). Concerning the geographical distribution, A. sagittatus was firstly described as Markevitschia sagittata in the intestine of C. carpio from the Amur River basin in Russia (Kulakovskaya & Akhmerov, Reference Kulakovskaya and Akhmerov1965), and subsequent studies mostly reported it from Japan (Scholz et al., Reference Scholz, Shimazu, Olson and Nagasawa2001; Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová, Štefka and Scholz2012) Therefore, the identification of A. sagittatus recorded in the PRC by Xi et al. (Reference Xi, Wang, Wu and Nie2009) was most likely incorrect, and the species morphologically most probably corresponded to A. tenuicollis. Regarding the new putative Atractolytocestus species, it morphologically corresponded to A. sagittatus, but it differed in the molecular aspect.
Obvious differences in morphology were found between the putatively new tapeworm species and A. huronensis and A. tenuicolli, but ITS sequence identity was high among these species, with values ranging between 94.7–95.5% and 91.8–92.7%, respectively. Similar sequence identity levels, ranging between 91.7 and 95.2%, were also reported between A. huronensis and A. tenuicolli (Králová-Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). Divergent intra-genomic ITS2 sequences were also observed among different specimens of the new species, but the identity was higher than 99.4%. The same phenomenon had been observed previously in the congeners of A. sagittatus (Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová, Štefka and Scholz2012) and A. tenuicollis (Králová-Hromadová et al., Reference Králová-Hromadová, Štefka, Bazsalovicsová, Bokorová and Oros2013). The molecular differences in ITS2 sequence between the new species and A. huronensis and A. tenuicolli were higher than the within-species variation. The morphological and molecular analyses indicate that this tapeworm was a cryptic species most closely related to A. sagittatus. We named it A. danjiangkouensis n. sp.
In addition, A. huronensis was also recorded in the Danjiangkou Reservoir by Bazsalovicsová et al. (Reference Bazsalovicsová, Králová-Hromadová, Xi and Štefka2018). It would be interesting to study the existence of these two congeners in sympatry, as it may help assess whether the origin of parthenogenetic A. huronensis may involve interspecific hybridization.
Acknowledgement
The authors would like to thank Dr I. Jakovlić for his help in English language editing.
Financial support
This work was funded by the National Key Research and Development Program of China (Grant No. 2019YFD0900703), the National Natural Science Foundation of China (Grant No. 31872604) and the China Agriculture Research System of the Ministry of Finance and the Ministry of Agriculture and Rural Affairs.
Conflicts of interest
None.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed.










