Introduction
A striking difference in the quality of oocytes submitted to maturation and fertilization in vitro is considered to be a key factor confounding the repeatability of any assisted reproductive technologies (ARTs) (Chrenek et al., Reference Chrenek, Kubovicova, Olexikova, Makarevich, Toporcerova and Ostro2014; Torres et al., Reference Torres, Batista, Diniz, Silva, Mateus and Lopes-da-Costa2014; Korkmaz et al., Reference Korkmaz, Tekin, Sakinci and Ercan2015; Ulloa et al., Reference Ulloa, Heinzmann, Herrmann, Timmermann, Baulain, Grossfeld, Diederich, Lucas-Hahn and Niemann2015). In this regard, a significant improvement in oocyte survival rates will have a great potential for increasing the efficiency of ARTs (Wang & Sun, Reference Wang and Sun2007; Lasiene et al., Reference Lasiene, Vitkus, Valanciute and Lasys2009). A recent approach to improving the quality of retrieved oocytes involves devising nutritional strategies by changing the proportion of specific metabolites in the oocyte microenvironment. In this regard, dietary intakes of polyunsaturated fatty acids (PUFAs) have been shown to exert a wide range of actions on reproductive and endocrine functions. It is believed that PUFAs not only act as mediators in a series of processes in several reproductive tissues (Mattos et al., Reference Mattos, Staples and Thatcher2000) but also provide the necessary precursors for the synthesis of several classes of reproductive endobiotics such as steroids and prostaglandins (Abayasekara & Wathes, Reference Abayasekara and Wathes1999; Mattos et al., Reference Mattos, Staples and Thatcher2000).
It is well established that the lipid profile of the oocyte is dynamic and is largely dependent on the environment in which it develops (McKeegan & Sturmey, Reference McKeegan and Sturmey2012). Recent works have clearly demonstrated that supplementary fatty acids have a significant role in the maturation of oocytes and development of oocytes into preimplantation embryos (Leroy et al., Reference Leroy, Vanholder, Mateusen, Christophe, Opsomer, de Kruif, Genicot and Van Soom2005; Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009, Reference Marei, Wathes and Fouladi-Nashta2010; Wonnacott et al., Reference Wonnacott, Kwong, Hughes, Salter, Lea, Garnsworthy and Sinclair2009; Veshkini et al., Reference Veshkini, Asadi, Khadem, Mohammadi-Sangcheshmeh, Khazabi, Aminafshar, Deldar, Soleimani and Cinar2015). Results obtained from lipid analysis of follicular fluid have indicated that among PUFAs, ω-6 and ω-3 families are highly enriched in the follicle (Bender et al., Reference Bender, Walsh, Evans, Fair and Brennan2010). There is now clear evidence that dietary supplementation of ω-6 and/or ω-3-fatty acids has an important role in oocyte quality and development (Fouladi-Nashta et al., Reference Fouladi-Nashta, Wonnacott, Gutierrez, Gong, Sinclair, Garnsworthy and Webb2009). The findings of a previous study suggest that dietary supplementation of heifers with high levels of fish oil significantly increase the total amount of n-3 PUFAs and the n-3:n-6 ratio in follicular fluid (Childs et al., Reference Childs, Hennessy, Sreenan, Wathes, Cheng, Stanton, Diskin and Kenny2008). In that study, an improvement in oocyte developmental competence with alteration of PUFAs through the dietary sources of FAs has been observed. In bovine species, depending on the type of fatty acid studied, a positive (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009) or negative (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2010) effect on developmental competence of oocytes has been observed following supplementation of maturation medium with essential fatty acids. Recently, several studies have proven the efficiency of fat and mix of fatty acids in culture medium on oocyte developmental competence (Staples et al., Reference Staples, Burke and Thatcher1998; Funston, Reference Funston2004; Santos et al., Reference Santos, Bilby, Thatcher, Staples and Silvestre2008; Sturmey et al., Reference Sturmey, Reis, Leese and McEvoy2009; Van Hoeck et al., Reference Van Hoeck, Leroy, Arias Alvarez, Rizos, Gutierrez-Adan, Schnorbusch, Bols, Leese and Sturmey2013). However, to our knowledge, not only are there rare data available about the effect of each family or each specific fatty acid on oocyte quality, but also the results are inconsistent in most study cases. Alpha-linolenic acid (ALA,18:3 n-3, an essential fatty acid of the ω-3 family) is commonly found in some vegetable oils as well as sea fish (Barcelo-Coblijn & Murphy, Reference Barcelo-Coblijn and Murphy2009). To the best of our knowledge, only a few studies have ever documented the effect of α-linolenic acid on oocyte developmental competence (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009; Ghaffarilaleh et al., Reference Ghaffarilaleh, Fouladi-Nashta and Paramio2014; Veshkini et al., Reference Veshkini, Asadi, Khadem, Mohammadi-Sangcheshmeh, Khazabi, Aminafshar, Deldar, Soleimani and Cinar2015). However, such an effect has not been observed with goat oocytes, and its role, if any, in oocyte cytoplasmic maturation is less clear until now.
In the present study we evaluated the hypothesis that the developmental competence of goat oocytes is influenced by exogenous ALA. The aims of the present study were: (1) to evaluate the concentration of endogenous ALA in ‘small’ (≤2 mm) and ‘large’ (≥6 mm) follicles using gas chromatography/mass spectrometry analysis; (2) to study the effect of exogenous ALA on oocyte nuclear/cytoplasmic maturation by evaluating the cumulus expansion, nuclear status, and intracellular glutathione (GSH) content after in vitro maturation (IVM); (3) to investigate the effect of exogenous ALA on oocyte developmental potential and quality of derived blastocysts; and (4) to analyze the effect of exogenous ALA on expression profile of genes involved in apoptosis (Bax, Bcl-2, and p53) in blastocysts.
Materials and methods
All chemicals and media used in this study were purchased from the Sigma–Aldrich Corporation (St. Louis, MO, USA) and Gibco (Grand Island, NY, USA), except when noted.
Collection of ovaries
Ovaries were collected from adult goats at a local abattoir during the non-breeding season and transported to the laboratory within 2–3 h in saline solution at a temperature of approximately 35–37°C.
Aspiration of follicular fluid
Upon arrival, ovaries were washed three times in fresh saline. Visible follicles were measured and categorized according to diameters as ‘small’ (≤2 mm) and ‘large’ (≥6 mm). Follicular fluid (FF) was aspirated using 5 ml syringes (18G needles) and pooled within each group. After centrifugation at 2400 g for 5 min at 4°C, the supernatant fluid was aspirated and stored at −20°C until analysis.
Gas chromatography-mass spectrometry (GC-MS)
Gas chromatography-mass spectrometry (GC-MS) analysis was used to obtain the fatty acid profiles of FF. Fatty acid methyl ester (FAME) synthesis was conducted as described previously (O’Fallon et al., Reference O’Fallon, Busboom, Nelson and Gaskins2007). Briefly, FF samples were thawed on ice prior to analysis. After thawing, 1 ml of FF sample was supplemented with 1 ml of C13:0 internal standard (0.5 mg of C13:0/ml of MeOH), 0.7 ml of 10 mol/l KOH in dH2O, and 5.3 ml of MeOH. The tube was incubated in a 55°C water bath for 1.5 h with vigorous hand-shaking every 20 min. After cooling below room temperature, 0.58 ml of 12 mol/l of H2SO4 in dH2O was added. The tube content was mixed by inversion, and with precipitated K2SO4 present incubated again in a 55°C water bath for 1.5 h with hand-shaking every 20 min. After FAME synthesis, the tube was cooled and 3 ml of hexane was added; the tube was vortex-mixed for 5 min and centrifuged for 5 min, and the hexane layer, containing the FAME, was placed into a GC vial.
The fatty acid profile of the FAME was analyzed by capillary GC on a SP-2560, 100 m × 0.25 mm × 0.20 μm capillary column (Supelco) installed on a Hewlett Packard 5890 gas chromatograph equipped with a Hewlett Packard 3396 Series II integrator and 7673 controller, a flame ionization detector, and split injection (Agilent Technologies Inc., Santa Clara, CA). After injection of sample to GC the initial oven temperature was 140°C, held for 5 min, subsequently increased to 240°C at a rate of 4°C/min, and then held for 20 min. Helium was supplied as the carrier gas at a flow rate of 0.5 ml/min, and the column head pressure was 280 kPa. Both the injector and the detector were set at 260°C. The split ratio was 30:1. Fatty acids were identified by comparing their retention times with the fatty acid methyl standards (O’Fallon et al., Reference O’Fallon, Busboom, Nelson and Gaskins2007).
Oocyte collection
Cumulus–oocyte complexes (COCs) were isolated from follicles using the slicing method. Only oocytes completely surrounded by compact and thick cumulus were randomly selected and used for the experiments (Thompson et al., Reference Thompson, Gardner, Pugh, McMillan and Tervit1995).
In vitro maturation (IVM)
COCs were washed three times in TCM-199 medium, supplemented with 20 mM HEPES and 0.4% (w/v) bovine serum albumin (BSA), and three times in a maturation medium consisting of TCM-199 supplemented with 25 mM NaHCO3, 0.6% (w/v) fatty acid-free BSA, FSH (5 μg/ml), LH (5 μg/ml), 17β-estradiol (1 μg/ml), and EGF (20 ng/ml) (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009). A group of 10 COCs was cultured per 50 μl drops of maturation medium in 30 mm Petri dishes for 24 h at 38.5°C in 5% CO2, 5% O2, and 90% N2 of humidified air.
Assessment of cumulus cell expansion
After maturation in vitro, COCs were visualized under a stereomicroscope and classified according to the degree of cumulus expansion in three groups: fully expanded, partially expanded, and not expanded (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009).
Nuclear chromatin evaluation
Following IVM, oocytes were released from the cumulus mass by treating them with 0.1% hyaluronidase (100 IU/ml) and vortexing. Then, denuded oocytes were stained with 2.5 mg/ml Hoechst 33258 in 3:1 (v/v) glycerol/PBS. The meiotic stages of the oocytes were then evaluated under an epifluorescence microscope (Nikon, Tokyo, Japan) and each oocyte was classified as at the germinal vesicle (GV; Fig. 1 A), germinal vesicle breakdown (GVBD; Fig. 1 B), metaphase I (MI; Fig. 1 C), or metaphase II (MII; Fig. 1 D) stage (Turathum et al., Reference Turathum, Saikhun, Sangsuwan and Kitiyanant2010).
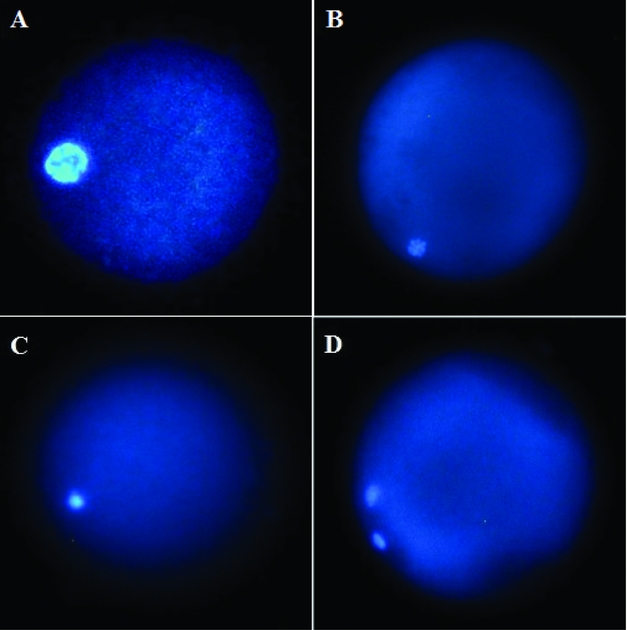
Figure 1 Representative images of goat oocytes stained with Hoechst 33258 and evaluated under fluorescence microscopy. Oocytes in germinal vesicle (GV) (A), germinal vesicle breakdown (GVBD) (B), metaphase I (MI) (C), and MII (D) stages are shown.
Measurement of intracellular GSH content
Intracellular GSH content was measured as described previously (Abazari-Kia et al., Reference Abazari-Kia, Mohammadi-Sangcheshmeh, Dehghani-Mohammadabadi, Jamshidi-Adegani, Veshkini, Zhandi, Cinar and Salehi2014). In brief, immediately after IVM, a group of COCs was denuded and incubated in Tyrodes medium plus 5 mg/ml poly-vinyl alcohol containing 10 μM Cell Tracker blue for 30 min. The oocytes were subsequently washed in mPBS, placed into 10 μl droplets, observed under an epifluorescence microscope (Nikon, Tokyo, Japan) with UV filters, and then all fluorescent images (Fig. 2) were recorded as graphic files. ImageJ software was used for oocyte fluorescence intensities analysis.

Figure 2 Typical images of oocytes (A) under a stereomicroscope. The same oocytes shown for the intracellular glutathione (GSH) content (B) after in vitro maturation (IVM) based on fluorescence intensity (pixel/oocyte).
Oocyte activation
After maturation in vitro, several COCs were freed from surrounding cumulus cells. Denuded oocytes were then washed in TCM-199 supplemented with 10% FBS and activated chemically by applying 1 min exposure to 2.5 μM ionomycin (diluted in TCM-199) followed by 2 mM 6-DMAP (diluted in CR1aa medium) for 3 h (Lan et al., Reference Lan, Han, Wu, Han, Ma, Liu, Chang and Tan2005).
In vitro fertilization
After IVM, a number of COCs were washed two times in HEPES-buffered synthetic oviductal fluid (HSOF) and 10 COCs were placed in 44-μl drops overlaid with equilibrated mineral oil. The fertilization medium was SOF supplemented with 4 IU/ml heparin, PHE (20 μM penicillamine, 10 μM hypotaurine, 1 μM epinephrine), and 2% (v/v) estrous sheep serum. Frozen sperm in straws (Nahadehaye Dami Jahed, Tehran, Iran) were thawed and used for in vitro fertilization. To separate the viable sperm for in vitro fertilization, a swim-up method was applied and high quality sperm was added to COCs at a concentration of 2 × 106 spermatozoa/ml in fertilization drops. Oocytes and sperm were incubated together for approximately 18 h under mineral oil at 39°C, 5% CO2, 5% O2, and 90% N2 in air with maximum humidity (Mohammadi-Sangcheshmeh et al., Reference Mohammadi-Sangcheshmeh, Soleimani, Deldar, Salehi, Soudi, Hashemi, Schellander and Hoelker2011).
In vitro culture
Following either activation or in vitro fertilization, presumable zygotes were after 20 h freed from excess sperm and cumulus cells. After three times washing in CR1aa medium, presumable zygotes were cultured in CR1aa medium supplemented with 10% FBS for 8 days at 38.5°C in a humidified incubator with 5% O2, 5% CO2, and 90% N2. The cleavage rate and blastocyst rate were recorded at days 3 and 8 of culture, respectively. The day of activation/fertilization was considered to be day 0 (Wan et al., Reference Wan, Hao, Zhou, Wu, Yang, Cui, Liu and Zeng2009).
Apoptosis assay with TUNEL
Apoptosis in blastocysts (Fig. 3 A) was detected by the terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) assay. The In Situ Cell Death Detection Kit (TMR red; Roche, Mannheim, Germany) was used for this purpose. Blastocysts were washed three times in PBS/FBS (PBS with 10% fetal bovine serum) and then fixed in 4% paraformaldehyde for 2 h at room temperature. Membranes of the blastocysts were permeabilized by treating them with 0.1% Triton X-100 in 0.1% citrate solution for 1 h at room temperature. Fixed blastocysts were incubated in TUNEL reaction medium for 1 h at 38.5°C in the dark. After the reaction was stopped, the blastocysts were washed and the cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 mg/ml) for 10 min at room temperature. The blastocysts were then mounted onto a slide under a coverslip and the total cell number (Fig. 3 B) and the number of apoptotic cells (Fig. 3 C) were examined with a fluorescence microscope (Hao et al., Reference Hao, Lai, Mao, Im, Bonk and Prather2003).

Figure 3 Example of a blastocyst after terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) assay labelling followed by 4′,6-diamidino-2-phenylindole (DAPI) staining. Blastocyst (A) was stained with DAPI (B) combined with TUNEL labelling (C) for counting total cell number (B) and apoptotic cells (C).
RNA extraction, reverse transcription, and real-time PCR
For reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, total cellular RNA was extracted using TRI-reagent. Synthesis of cDNA was carried out with M-MuLV reverse transcriptase and random hexamer primers, according to the manufacturer's instructions (Fermentas; St. Leon-Rot, Germany). PCR amplification was performed using a standard procedure with Taq DNA polymerase with denaturation at 94°C for 15 s, annealing at 55–60°C for 30 s according to the melting temperature of each primer, and extending at 72°C for 45 s. The number of cycles varied between 30 and 40, depending on the abundance of a particular mRNA. The primers and product lengths are listed in Table 1.
Table 1 Details of primers used for real-time PCR quantitative analysis

F, forward; PCR, polymerase chain reaction; R, reverse.
Real-time PCR reactions of 25 μl were conducted in a Rotor Gene 6000 (Corbett Life Science, Sydney, Australia), by adding 12.5 μl 2× SYBR Premix Ex Taq, 0.4 μM of final concentration for each primer, 2 μl template, and distilled water to reach the volume of 25 μl. Real-time PCR was performed in two steps with the following thermal setting: 3 min at 95°C for initial enzyme activation, followed by 40 amplification cycles (each 5 s at 95°C, and 20 s at 60°C with fluorescence detection) and a final step of melting curve analysis. All samples were analyzed in duplicate, and the average value of the duplicate was used for quantification. Data were normalized to β2m and 2−ΔΔCt methodology was used for relative quantification (Harrington et al., Reference Harrington, Surujballi, Waters and Prescott2007). Expression of YWHAZ was used as an internal housekeeping gene.
Experimental design
Experiment 1. ALA composition of the follicular fluid from small and large follicles
To estimate a normal physiological range of ALA for adding to the medium maturation culture, 1 ml of FF samples were aspirated from small (≤2 mm) and large (≥6 mm) follicles and the fatty acid profile in the FF was analyzed. GC-MS was used to evaluate the composition of fatty acids and compare the types of fatty acids between small- and large-follicle groups. Three biological replicates, each containing 1 ml of FF, were analyzed for this experiment.
Experiment 2. Effects of exogenous ALA in maturation media on cumulus cell expansion and oocyte meiotic competence
To evaluate the effects of ALA on cumulus expansion and meiotic competence after IVM, maturation medium was supplemented with exogenous ALA. ALA [100 mM stock solution in dimethyl sulfoxide (DMSO)] was added to the maturation medium supplemented with fatty acid-free BSA as a carrier. Fatty acid-free BSA was used to eliminate the effect of other fatty acids. The concentration of DMSO was similar in all treatment groups as described previously by Marei et al., (Reference Marei, Wathes and Fouladi-Nashta2009). Based on ALA concentrations estimated in Experiment 1, in small and large follicles, the concentrations of 10, 50, 100, or 200 μM ALA were chosen for use in this experiment. Therefore, COCs (n = 530) were randomly assigned to each 10 (n = 104), 50 (n = 182), 100 (n = 131), and 200 μM (n = 113) ALA group. In addition, several COCs (n = 173) not supplemented with ALA were considered for the control group. After IVM, COCs of each group were evaluated for cumulus expansion and stage of nuclear maturation. This experiment was performed in five replicates.
Experiment 3. Effects of exogenous ALA in maturation media on oocyte GSH level
To evaluate the effects of ALA on oocyte intracellular level of GSH after maturation in vitro, cumulus cells were removed immediately after IVM and intracellular GSH contents were assayed for each group of 50 μM ALA (n = 42) and control (n = 41) oocytes.
Experiment 4. Effects of exogenous ALA in maturation media on oocyte developmental potential and quality of derived blastocysts
To determine whether ALA has positive effects on subsequent embryos, matured COCs were activated and/or fertilized, and cultured in vitro. Activation of 287 and 269 oocytes and fertilization of 113 and 104 oocytes were performed in the control and 50 μM ALA-treated groups, respectively. Rates of cleavage and blastocyst development were recorded and compared between the 50 μM ALA-treated oocytes and the control group. This experiment was executed in five replicates.
To evaluate the effect of ALA on total cell number and cell apoptosis, blastocysts derived from 50 μM ALA-treated (n = 21) and control (n = 18) oocytes were stained with TUNEL and DAPI. The total cell number and the number of apoptotic cells were then evaluated under a fluorescence microscope for each group and compared.
Experiment 5. Effects of exogenous ALA in maturation media on expression of apoptosis-related genes in blastocysts
To evaluate the effects of ALA in maturation media on apoptosis, expression of apoptosis-related genes was analyzed. Blastocysts derived either from 50 μM ALA-treated oocytes or control oocytes were evaluated for the expression of pro-apoptotic (Bax and p53) and anti-apoptotic (Bcl-2) genes. Three biological replicates, each containing five blastocysts were used for RNA extraction, reverse transcription, and real-time PCR.
Statistical analyses
All statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC, USA). Comparisons among dose effect (0, 10, 50, 100, or 200 μM ALA), such as cumulus expansion, maturation, and cleavage and blastocyst rates, were analyzed using the non-parametric chi-squared test. Mean group differences were tested using Student's t-test. Relative gene expression levels of different genes were analyzed by one-way analysis of variance (ANOVA). Results are expressed as mean ± standard error of the mean (SEM) of at least three independent replicates. A probability of P ≤ 0.05 was considered to be significant.
Results
Experiment 1. ALA composition of the follicular fluid from small and large follicles
Regardless of follicle size, about 23 fatty acids were identified and quantified in follicular fluid (Table 2). Notably, among the ω-3 family, ALA had the highest concentration in both small and large follicles and increased as the ovarian follicles enlarged. Comparative analysis between small (≤2 mm) and large (≥6 mm) follicles showed concentration of ALA in follicular fluids ranging from 0.018–0.028 mg/ml or 64.6–100.6 μM, with a mean of ~82.6 μM.
Table 2 Comparison of fatty acid composition of follicular fluid from large and small goat follicles (cumulative results of three replicates). Values are expressed as means (mg/100 ml) ± SEM

P-values are reported for differences between large and small follicles calculated using a general linear model. NS = non-significant; ND = non–detected.
a Value for one sample; not detected in other samples.
Experiment 2. Effects of exogenous ALA in maturation media on cumulus cell expansion and oocyte meiotic competence
Supplementation of maturation medium with 0, 10, 50, or 100 μM ALA had no effect on cumulus cells expansion (Table 3). However, the proportion of oocytes with fully expanded cumulus cells was lower (P > 0.05) for 200 μM ALA concentration than the control and other treatment groups. Accordingly, we observed more (P ≤ 0.05) oocytes with non-expanded cumulus cells in the 200 μM ALA group compared with the control and other treatment groups.
Table 3 Cumulus cell expansion of goat oocytes treated with different concentrations of ALA after 24 h of in vitro maturation (cumulative results of five replicates)

ALA: α-linolenic acid; COCs: cumulus–oocyte complexes.
a,b Different superscripts within the columns indicate a significant difference (P < 0.05).
Treatment with 50 μM ALA resulted in the highest (P ≤ 0.05) maturation rate (MII stage) while 200 μM ALA resulted in the lowest (P ≤ 0.05) maturation rate when compared with the control group (Table 4). However, maturation rates were similar (P > 0.05) among 10 μM ALA, 50 μM ALA, and 100 μM ALA groups.
Table 4 Nuclear status of goat oocytes treated with different concentrations of ALA after 24 h of in vitro maturation (cumulative results of five replicates)

ALA: α-linolenic acid; GV: germinal vesicle; GVBD: germinal vesicle break down; MI: metaphase I; MII: metaphase II.
a,b,c Different superscripts within the columns indicate a significant difference (P < 0.05).
Experiment 3. Effects of exogenous ALA in maturation media on oocyte GSH level
The oocyte intracellular level of GSH was measured after IVM for 50 μM ALA-treated and control groups. No evidence (P > 0.05) of changes in GSH content was detected between the ALA-treated and control oocytes (Fig. 4).

Figure 4 Effect of α-linolenic acid (ALA) on intracellular glutathione (GSH) content of goat oocyte after in vitro maturation. No difference (P > 0.05) was observed among treated and untreated oocytes.
Experiment 4. Effects of exogenous ALA in maturation media on oocyte developmental potential and quality of derived blastocysts
Based on the results of the previous experiments, the concentration of 50 μM ALA was selected for evaluation of oocyte developmental competence after either PA or IVF. Proportional data for cleavage and development to blastocysts after PA or IVF are shown (Table 5). After PA, the cleavage rate was higher (P ≤ 0.05) in the ALA-treated (50 μM ALA) group compared to the control group. Likewise, the parthenogenetic blastocyst rate for 50 μM ALA-treated oocytes was higher (P ≤ 0.05) than for the control oocytes. Although in vitro fertilization cleavage rates were similar (P > 0.05) between 50 μM ALA-treated and control groups, 50 μM ALA-treated oocytes produced more (P ≤ 0.05) blastocysts than the control group.
Table 5 Embryo development of ALA-treated and control oocytes at 8 days after parthenogenetic activation (PA) and in vitro fertilization (IVF) in goats (cumulative results of five replicates)

ALA: α-linolenic acid; IVF: in vitro fertilization; PA: parthenogenetic activation.
a,b Different superscripts within the columns indicate a significant difference (P < 0.05).
In addition, the results of this experiment revealed that blastocysts derived from oocytes supplemented with 50 μM ALA, had not only a greater (P ≤ 0.05) total cell number but also a lower (P ≤ 0.05) number of apoptotic cells when compared with the control blastocysts (Table 6).
Table 6 Mean (± SD) total cell number and apoptotic cell number in blastocysts derived from ALA-treated and control oocytes

ALA: α-linolenic acid
a,b Different superscripts within the columns indicate a significant difference (P < 0.05).
Experiment 5. Effects of exogenous ALA in maturation media on expression of apoptosis-related genes in blastocysts
Expression data for pro-apoptotic (Bax, p53) and anti-apoptotic (Bcl-2) genes in blastocysts are shown (Fig. 5). The relative transcript abundance of Bax and p53 was decreased (P ≤ 0.05) in blastocysts originated from oocytes previously matured in medium with 50 μM ALA when compared with control blastocysts (no ALA used during oocyte maturation). Furthermore, there was an increased (P ≤ 0.05) expression of Bcl-2 transcripts in blastocysts derived from the 50 μM ALA-treated oocyte group when compared to the control.

Figure 5 Relative mRNA expression of Bax, Bcl-2, and p53 transcripts of goat blastocysts derived from α-linolenic acid (ALA)-treated oocytes compared with blastocysts of control oocytes. Bars with asterisks represent differing groups (P ≤ 0.05).
Discussion
In this study, in order to find an optimum range of ALA for designed experiments, we have initially analyzed the follicular fluid of goat antral ovaries from different size categories (i.e. small and large follicles). Irrespective of the follicle size, overall, 23 fatty acids were detected and determined in goat follicular fluid; among them, linoleic acid (LA), oleic acid (OA), stearic acid (SA), palmitic acid (PAL), arachidonic acid (AA) and ALA were the major ones. In agreement with our findings, LA, OA, SA, PAL, and ALA have been reported to be the most abundant fatty acids in bovine preovulatory follicles (Bender et al., Reference Bender, Walsh, Evans, Fair and Brennan2010; Leroy et al., Reference Leroy, Vanholder, Mateusen, Christophe, Opsomer, de Kruif, Genicot and Van Soom2005). Furthermore, the previous authors found that the content of all those fatty acids were higher in follicular fluid of cows compared to heifers. Moreover, each phase of follicular development seems to have its own set of requirements for the metabolites, including fatty acids (Lussier et al., Reference Lussier, Matton, Guilbault, Grasso, Mapletoft and Carruthers1994; Bender et al., Reference Bender, Walsh, Evans, Fair and Brennan2010). We tried to detect the variation of fatty acids, especially ALA, in ovarian follicles and to investigate their relationship to the follicular size. The antral follicles were subdivided into two groups; those 2 mm or less in diameter (as small follicles), and those 6 mm or more in diameter (as large follicles). The fatty acid composition of each group was then analyzed by using chromatography.
There were significant differences in the amount of some fatty acids between the small and large follicles. Among the ω-3 fatty acids family, ALA not only represented the highest concentration in both small and large follicles, but also the amount of ALA increased as the ovarian follicles enlarged. This finding might be explained by the fact that as the follicles grow and mature, there is a greater need for some specific unsaturated fatty acids.
Different concentrations of exogenous ALA were added to the IVM medium to investigate the effect of ALA on cumulus cell expansion, and oocyte nuclear and cytoplasmic maturation. Our results revealed that the physiological concentrations of ALA in follicular fluid of small and large follicles were 0.018 mg/ml (64.6 μM), and 0.028 mg/ml (100.6 μM), respectively. Therefore, to properly mimic the normal physiological situation in vitro, a range of 0, 10, 50, 100, or 200 μM ALA was added to the IVM medium. The addition of ALA had no effect on cumulus cell expansion except at the highest concentration (200 μM), at which a detrimental effect was detected. Supporting our current findings, a previous study (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009) demonstrated that supplementation of maturation medium with ALA at a concentration of lower than 100 μM had no adverse effect on cumulus cell expansion in cattle. The deleterious effect of ALA at a concentration of 200 μM in the present study is consistent with previous reports (Coyral-Castel et al., Reference Coyral-Castel, Rame, Fatet and Dupont2010; Ghaffarilaleh et al., Reference Ghaffarilaleh, Fouladi-Nashta and Paramio2014) in which a concentration higher than 100 μM decreased the viability of goat granulosa cells after 24 h culture. The lower expansion of cumulus cells under high amounts of ALA (200 μM) in maturation medium might be explained by the toxicity effect of fatty acids outside the tolerance threshold of the cells, leading to a reduction in cell viability (Andrade et al., Reference Andrade, de Lima, Curi and Castrucci2005).
To evaluate the effect of exogenous ALA on goat oocyte developmental capacity, meiotic competence as a prerequisite for subsequent embryo development was evaluated after IVM by visually inspecting the nuclear chromatin. Our data showed that supplementation of maturation medium with 50 μM ALA increased the MII rate of goat oocytes, as determined by extrusion of the first polar body. However, addition of 50 μM ALA in maturation medium had no effect on oocyte cytoplasmic maturity, as determined by the fluorescence intensity used to evaluate GSH level. In previous report, Marei et al. (Reference Marei, Wathes and Fouladi-Nashta2009), using bovine oocytes, indicated that supplementation of maturation medium with the concentration of 50 μM ALA increased the IVM rate; however no effect was observed on mitochondrial activity and distribution (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2012). Therefore, the positive effect of ALA on oocyte meiotic competence might be mediated by improvement of cytoplasmic maturation via the mitogen-activated protein kinase pathway and indirectly through PGE2 synthesis (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009) and not through increases in intracellular GSH content and mitochondrial activity. Further evidence to support this concept has been provided previously (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2012) by the indication of differential effects of linoleic and alpha-linolenic fatty acids on spatial and temporal mitochondrial distribution and activity in bovine oocytes. Recently, in a study in prepubertal sheep, no beneficial effect was observed in nuclear maturation by adding up to 100 μM ALA concentration in maturation medium (Ghaffarilaleh et al., Reference Ghaffarilaleh, Fouladi-Nashta and Paramio2014). In contrast to ours and other findings, supplementation of maturation medium with 100 μM ALA improved the nuclear maturation in adult sheep (Veshkini et al., Reference Veshkini, Asadi, Khadem, Mohammadi-Sangcheshmeh, Khazabi, Aminafshar, Deldar, Soleimani and Cinar2015). However, oocyte collection in Ghaffarilaleh's work was from prepubertal sheep and not from adult sheep, a difference that could explain these contradictory observations regarding meiotic competence after IVM.
Based on our previous findings, a 50 μM ALA concentration was selected to investigate oocyte developmental potential and quality of embryos. After PA, higher cleavage and blastocyst rates were obtained from oocytes that were treated with 50 μM ALA than in the control group. After in vitro fertilization, blastocyst rate but not cleavage rate was higher compared to control for oocytes treated with 50 μM ALA. In contrast to our findings, in prepubertal sheep, no differences were observed in blastocyst development among control, 50, 100, or 200 μM ALA-treated oocytes, but an improvement in male pronuclear formation in zygotes was reported (Ghaffarilaleh et al., Reference Ghaffarilaleh, Fouladi-Nashta and Paramio2014). However, similar to the findings of our study, an increased rate of embryonic development has been observed for bovine oocytes treated with 50 μM ALA (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009). In another study in cattle, addition of ALA at 1, 10, or 100 μM concentrations to the culture medium did not improve the cleavage and blastocyst rates (Al Darwich et al., Reference Al Darwich, Perreau, Petit, Papillier, Dupont, Guillaume, Mermillod and Guignot2010). These different findings suggest that ALA might be more important to the oocytes if added to the maturation medium rather than to the culture medium after fertilization. Furthermore, Al Darwich et al. (Reference Al Darwich, Perreau, Petit, Papillier, Dupont, Guillaume, Mermillod and Guignot2010) also found that the viability of the produced bovine embryos was influenced in a positive manner by the presence of ALA in culture medium.
Apart from providing energy for oocytes and/or early embryos, from the previous disclosures, it is apparent that fatty acids are a key component of different biochemical pathways. Accordingly, fatty acids are involved in re-initiation and completion of the first meiotic division and cytoplasmic maturation, and, therefore, are essential to sustain normal fertilization and further embryonic development (Cha & Chian, Reference Cha and Chian1998). An increased synthesis of PGE2 by enhancing the total concentration of ω-6 fatty acids as precursors has been observed following supplementation of maturation medium with ALA (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009). Coincidentally with data from prepubertal sheep, ALA increased both PGE2 and PGF2a levels in the culture media of oocytes (Ghaffarilaleh et al., Reference Ghaffarilaleh, Fouladi-Nashta and Paramio2014). PGE2 is a key paracrine and/or autocrine regulator of cumulus cell functions and has been proposed to play a major role in oocyte nuclear maturation (Elvin et al., Reference Elvin, Yan and Matzuk2000). An elevated concentration of PGE2 positively stimulated the extent of cumulus expansion and nuclear maturation by enhancing the phosphorylation of MAPK1 and MAPK2 in both oocytes and cumulus cells through the elevation of cAMP levels (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009).
It has been well known that carbon-centred radicals, such as the fatty acid radicals, easily react with oxygen to form oxygen-centred lipid peroxy radicals (LOO–). The latter can absorb hydrogen from a neighbouring PUFA to form the corresponding lipid hydroperoxide (Browne & Armstrong, Reference Browne and Armstrong2000). Therefore, it can be postulated that supplementation of PUFAs (such as ALA) in maturation medium results in their accumulation in oocytes cell membranes and increases the oxidative stress, since PUFAs are more susceptible to peroxidation than monounsaturated and saturated fatty acids (Song et al., Reference Song, Fujimoto and Miyazawa2000; Gladine et al., Reference Gladine, Rock, Morand, Bauchart and Durand2007). In contrast with the above expectations, our data revealed that supplementation of maturation medium with ALA not only increased the total cell number in later blastocysts but also reduced the number of apoptotic cells in these embryos. In accordance with this finding, it has been reported that treatment of oocytes with ALA protected the blastocysts from apoptosis (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2009), increased total cell number, and decreased apoptotic cell number in comparison with the control group. In this regard, improvement in the quality of the obtained blastocysts has been associated with the changes in cytoplasmic maturation in vitro (Ghaffarilaleh et al., Reference Ghaffarilaleh, Fouladi-Nashta and Paramio2014). However, the high sensitivity of ALA to oxidative stress in one way, and its ability to protect the embryo from apoptosis in another way, represents a paradox that requires further investigation.
Growing attention has been directed to the mechanisms of Bcl-2 family proteins action, including the regulation of apoptosis and integrating signals from survival-inducing and death-promoting pathways (Ruvolo et al., Reference Ruvolo, Deng and May2001; Burlacu, Reference Burlacu2003). To investigate the molecular mechanism of ALA-mediated improvement in blastocyst quality, we determined the mRNA expression of Bax, Bcl-2, and p53 in blastocyst-stage embryos. Our data showed that compared with the control blastocysts, blastocysts of ALA-treated oocytes had a decrease in Bax mRNA level and an increase in expression of the Bcl-2 mRNA. Bax is considered as a pro-apoptotic Bcl-2 family member whose apoptotic function is antagonized by Bcl-2 expression (Gross et al., Reference Gross, McDonnell and Korsmeyer1999). Our data revealed a down-regulation of pro-apoptotic (Bax), and up-regulation of anti-apoptotic (Bcl-2) genes in blastocysts of ALA-treated oocytes compared to the control blastocysts. It is fascinating to note that the above findings confirmed our earlier results, in which blastocysts of ALA-treated oocytes were found to be of greater quality than the control blastocysts. The tumour suppressor p53 is also stored in the oocyte, and functions as a transcription factor involved in cell-cycle control, DNA repair, apoptosis, and cellular stress responses (Amundson et al., Reference Amundson, Myers and Fornace1998). Our results showed that there was lower expression of p53 in blastocysts of ALA-treated oocytes in comparison with that of control blastocysts. This result supports the possibility that higher expression of p53 in control blastocysts would explain their predisposed apoptotic phenotype when compared with those blastocysts produced in the ALA-treated group. An earlier study in cattle (Marei et al., Reference Marei, Wathes and Fouladi-Nashta2012) revealed a decreased level of ROS following supplementation of the maturation medium with ALA which may also explain the higher apoptosis-suppressing effect of ALA observed in this study.
Evidence from the present study revealed that supplementation of maturation medium with exogenous 50 μM ALA increased maturation rate of oocytes in vitro without any deleterious effect on cumulus cell expansion. ALA-treated medium led to an improvement in blastocyst rate and quality as determined by greater total cell number, lower number of apoptotic cells, and lower expression of apoptosis-related genes. Thus, the findings of this work will lead to further investigations regarding ALA mechanisms during oocyte and embryo in vitro culture, which could in the near future be applied to routine ART programs.
Acknowledgements
This work was supported by a grant from the Stem Cell Technology Research Center, Tehran, Iran. The authors thank the members of their own laboratories for their helpful discussions.













