INTRODUCTION
Numerous specimens of a terebellid with a single pair of plume-shaped branchiae resembling those of Pista were collected during benthic sampling in the Adriatic. Mikac (Reference Mikac2015) in her checklist of the polychaetes from this region lists three species of Pista of which one, Pista unibranchia Day, Reference Day1963, is characterized by a single pair of plume-shaped branchiae and short-handled thoracic uncini. He described the species from South Africa and states it is endemic, which questions the listing by Mikac (Reference Mikac2015). However, these characteristics of Day's species suggest it should be transferred to the genus Pistella erected by Hartmann-Schröder (Reference Hartmann-Schröder1996) to accommodate species previously considered as belonging to Pista by SafronovaFootnote 1 (Reference Safronova1988) in her partial revision of the type species of the genus, Pista cristata (Müller, Reference Müller1776). Safronova (Reference Safronova1988) transferred Scionella lornensis Pearson, Reference Pearson1969 to Pista and subsequently Hartmann-Schröder (Reference Hartmann-Schröder1996) then moved it into her new genus Pistella and designated it as the type species. This species was described from Loch Linnhe, Argyll, Scotland and is characterized by a single pair of plume-shaped branchiae and short-handled uncini present throughout (Hartmann-Schröder, Reference Hartmann-Schröder1996).
Recently, Jirkov (Reference Jirkov2001) synonymized Pistella lornensis with Pista cristata, based on a reinterpretation of the original description of the latter species. He suggests that the original description of P. cristata states that only a single pair of branchiae is present and that short-handled thoracic uncini are present throughout. Whereas Malmgren (Reference Malmgren1866) when erecting the genus Pista and designating the type species P. cristata states that the genus is characterized by two pairs of branchiae and anterior thoracic long-handled uncini restricted to anterior uncinigerous segments. This definition of Pista has been widely used and over 70 species have been described (Hutchings et al., Reference Hutchings, Nogueira, Carrerette, Schmidt-Rhaesa, Hrsg. v. Beutel, Glaubrecht, Kristensen, Prendini, Purschke, Richter, Westheide and Leschen2017); however, the number of uncinigerous segments with long-handled uncini is variable. An acceptance of Jirkov's proposal, would require that all species currently assigned to Pista would need to be transferred to a new, still unestablished genus, due to the presence of long-handled uncini on the first few thoracic segments, which are completely absent in P. cristata sensu Jirkov (Reference Jirkov2001). It appears that two species co-occur in the locality where Müller (Reference Müller1776) collected his worms. One form had a single pair of branchiae and short-handled uncini throughout, and the other with two pairs of branchiae and long-handled uncini on anterior segments. At this stage, we prefer to follow Malmgren's interpretation of P. cristata and consider both Pista and Pistella as valid genera. Hutchings et al. (Reference Hutchings, Nogueira, Carrerette, Schmidt-Rhaesa, Hrsg. v. Beutel, Glaubrecht, Kristensen, Prendini, Purschke, Richter, Westheide and Leschen2017) provide a generic diagnosis of both genera and these are that: Pistella is characterized by a single pair of plumous branchiae on segment 2, presence of lateral lobes and all neurochaetae as short-handled avicular uncini, initially in single rows but in partial to completely intercalated double rows on segments 11–20. In contrast Pista is characterized by arborescent, pectinate or plumous branchiae present from segment 2, typically 2 pairs on segments 2 and 3, rarely a single pair or 3 pairs; neurochaetae as long-handled avicular uncini, at least on anterior neuropodia, initially in single rows but in partial to completely intercalated double rows on segments 11–20.
However, material from the type locality (Christiansandensdis, Norway, 58°N 8°E) needs to be re-examined and a neotype designated for P. cristata as well as clarifying the status of the other species present.
Jirkov (Reference Jirkov2001) reported that the number of pairs of branchiae is not a valid generic character and also that the long-handled thoracic uncini are really ligaments and that they develop as the animal grows and so is also not a valid generic character. He illustrates this with figures of the thoracic uncini of Pista bansei Safronova, Reference Safronova1988 from various populations, and although the exact locality details are not given, they are from the Atlantic and the Pacific, so perhaps they are not all the same species. We suggest that a detailed study of the development of the thoracic uncini of worms from a range of locations is necessary to confirm or disprove Jirkov's comments regarding the origins of the long-handled uncini and whether or not they are ligaments (which may be called filaments in some descriptions). A detailed morphological study on the development of the uncini and branchiae is currently being undertaken combined with molecular studies (Londoño Mesa, in prep.). Pearson (Reference Pearson1969) illustrates some filaments arising from both the heel and the dorsal button (following the terminology of Nogueira et al., Reference Nogueira, Hutchings and Fukuda2010) although his material does include some large specimens (>35 mm in length) suggesting that long-handled uncini are never present in this species. An alternative explanation to the findings of Jirkov (Reference Jirkov2001) is that two cryptic species co-occur in his sampled populations, one with long-handled thoracic uncini and one without them, but this needs to be tested using both morphological and molecular studies. However, all this is beyond the scope of this paper and so in the meantime we describe the species found in the Northern Adriatic as a new species of Pista and one of Pistella. Both these species co-occur at some stations (see Table 1).
Table 1. Locations, sampling details, sediment characteristics of sampling stations shown in Figure 1 and occurrence of two new species.

MATERIALS AND METHODS
All specimens were collected by B. Mikac while undertaking several different research projects at the Center for Marine Research of the Ruđer Bošković Institute in Rovinj (Croatia) from 2003 to 2010. Soft bottom samples were taken with a Van Veen grab at 11 stations in the Northern Adriatic Sea (Figure 1, Table 1). Sediment was sieved through 1 mm mesh and fixed in 4% formaldehyde water solution. After removing the polychaetes from their tubes, they were preserved in 70% ethanol and examined using stereo- and light- microscopes. One to two specimens were examined under the scanning electronic microscope after being dehydrated in ethanol, critical point dried and covered with 20 nm of gold, at the Australian Museum (Zeiss Evo LS15 scanning electron microscopy, using Robinson Backscattered and ET secondary electron detectors) and at Macquarie University (JEOL JSM 6480LA) and imaged with a secondary detector.

Fig. 1. Map of sampling area and stations 1–11, Scale bars: (A) 200 km; (B) 20 km.
The following abbreviations are used: AM Australian Museum, Sydney; NHMR Natural History Museum in Rijeka, Croatia.
RESULTS
Taxonomic account
SYSTEMATICS
Family terebellidae Johnston, Reference Johnston1845
Genus Pista Malmgren, Reference Malmgren1866
Type species: Amphitrite cristata Müller, Reference Müller1776, by original designation.
DIAGNOSIS
Transverse prostomium attached to dorsal surface of upper lip; basal part as thick crest, eye spots sometimes present; distal part shelf-like. Buccal tentacles all uniformly cylindrical. Peristomium restricted to lips; relatively short upper lip, hood-like; swollen, cushion-like and mid-ventral lower lip. Segment 1 reduced dorsally, with pair of lobes of variable size and position; segments 2–4 also with pairs of lobes of variable size and position, sometimes extending for a few more segments. Anterior segments highly glandular ventrally, with discrete, smooth to slightly corrugated, rectangular to trapezoidal mid-ventral pads. Paired arborescent, pectinate or plumous branchiae present from segment 2, typically 2 pairs, on segments 2 and 3, rarely a single pair or 3 pairs. Conical to rectangular notopodia beginning on segment 4, typically extending for 17 segments, until segment 20; notochaetae all distally winged, frequently broadly winged. Neuropodia beginning on segment 5, as low ridges in conjunction with notopodia and short pinnules posteriorly; neurochaetae as long-handled avicular uncini, at least on anterior neuropodia, frequently until segment 10 or termination of notopodia, then short-handled; uncini in partial to completely intercalated double rows on segments 11–20. Nephridial papillae present on segment 3, genital papillae on variable number of segments, usually on segments 6–7, posterior and dorsal to notopodia. Pygidium smooth to slightly crenulated.
The above generic diagnosis is from Hutchings et al. (Reference Hutchings, Nogueira, Carrerette, Schmidt-Rhaesa, Hrsg. v. Beutel, Glaubrecht, Kristensen, Prendini, Purschke, Richter, Westheide and Leschen2017).
Pista adriatica sp. nov.
Figures 2–4
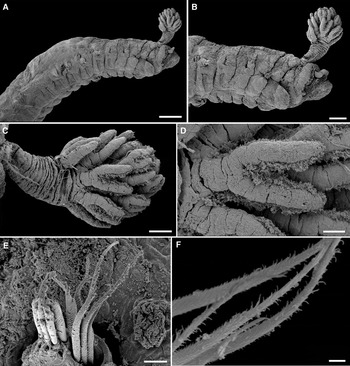
Fig. 2. Pista adriatica sp. nov.: (A) antero-lateral view; (B) anterior view; (C) branchia; (D) branchial filaments; (E) anterior thoracic notochaetae; (F) close up of thoracic notochaetae. Scale bars: (A) 200 µm; (B) 100 µm; (C) 50 µm; (D) 20 µm; (E) 10 µm; (F) 2 µm. All from AM W. 49065.
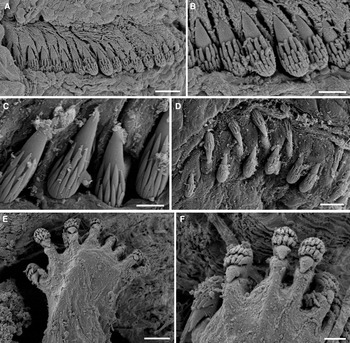
Fig. 3. Pista adriatica sp. nov.: (A) anterior thoracic neurochaetae; (B) close up of anterior thoracic neurochaetae; (C) neurochaetae of 3rd chaetiger; (D) 8th chaetiger; (E) posterior abdominal tori; (F) posterior abdominal neurochaetae. Scale bars: (A, D and E) 10 µm; (B and F) 5 µm; (C) 4 µm. All from AM W.49065.

Fig. 4. Pista adriatica sp. nov.: (A) anterior end showing unequal sized branchiae; (B) anterior dorsal view; (C) anterior view of ventral pads; (D) line drawing of 1st thoracic neurochaeta. Scale bars: (A) 400 μm; (B, C) 250 μm; (D) 10 μm. All from AM W.49057. b, showing branchial filaments coming off in a spiral fashion; bs, branchial stalk; ll, lower lip; ul, upper lip; vp, ventral pads.
MATERIAL EXAMINED
Holotype: AM W.49056, Station 1, 23.7.2003.
Paratypes:AM W.49057, Station 4, 20.9.2010, complete, 8 mm in length, 1 mm in width, about 82 segments, posterior segments very compacted; AM W.49058, Station 2, 28.5.2003, 2 specs: (1) incomplete, 6 mm in length, 1 mm in width, complete thorax plus 2 abdominal chaetigers, (2) complete, 8 mm in length, 0.5 mm in width, difficult to accurately count numbers of abdominal segments, covered in mucous; NHMR PMR 18356. Station 8, 29.9.2010, complete, 10 mm in length, 1 mm in width, about 67 chaetigers; AM W.49062.001, Station 1, 5.12.2008, mounted for SEM; AM W.49065.001, Station 6, 30.09.2010, mounted for SEM.
A total of seven specimens were examined.
DESCRIPTION
The following description is based on the holotype, a complete specimen, 18 mm in length, 1 mm in width anteriorly, with 87 chaetigers. Preserved worm pale cream with no pigmentation. Transverse prostomium attached to dorsal surface of upper lip, basal part thickened, crest with numerous scars where buccal tentacles were attached, those remaining uniform in width and length. No eye spots present. One pair of unequal sized plumose branchiae on segment 2, with filaments arranged in a spiral fashion around central stalk, about 8 whorls, each filament with about 2–3 branches (Figures 2A–D & 4A). Branchial stalk rounded, long, markedly annulated. Shorter branchia approximately length of stalk of larger branchia, with about 5 whorls.
Peristomium restricted to lips, upper lip large, rectangular, glandular, cushion shaped, ventral surface grooved down towards mouth. Lower lip mid ventral, compact, U-shaped, glandular. Segment 1 reduced dorsally, forms narrow glandular ventral bi-lobed structure connected mid dorsally. Segment 2 with narrow lateral lobe, which continues across ventrum as narrow glandular ridge. Segment 3 has rectangular lateral lobes with rounded margins, connected across ventrum by narrow strip and continuing across dorsum as a narrow ridge (Figure 2A, B). Segment 4 lacking lateral lobes but anterior segmental margins glandular. Dorsal margins of segments 2 and 3 glandular forming thickened ridges.
Anterior notopodia more dorsally displaced than subsequent ones, which progressively increase in size along the body becoming more erect, glandular. Notochaetae broad-winged capillaries, slightly curved with smooth tips, surface of capillaries ornamented (Figure 2E, F) of two lengths. First six pairs of thoracic neuropodia with long-handled uncini, handles progressively becoming shorter and by 6th neuropodia lost and uncini avicular. Anterior long-handled uncini with back straight and prow slightly curved (following terminology of Nogueira et al., Reference Nogueira, Hutchings and Fukuda2010) (Figure 4D) and posterior thoracic uncini also with back straight and prow slightly curved, dorsal button absent in both. Uncini initially arranged in single rows (Figure 3A–C), but arranged in partially to completely intercalated double rows on segments 11–20 (Figure 3D) and then reverting to single rows on abdomen. Thoracic notopodia and neuropodia initially widely spaced forming separate structures (Figure 2B) but by mid to posterior thorax become a single structure. Anterior thoracic uncini with dental formula MF: 3–4 : 4–5 : 4–5 (Figure 3B, C). Abdominal neuropodia becoming more erect posteriorly with about 8–9 uncini each elongate extending from torus, dental formula MF: 5–6 : 7–8 (Figure 3E, F).
Nephridial papillae conspicuous ventro-lateral, spherical, white, glandular papillae inserted posterior to chaetigers 3 and 4 (Figure 2A), more conspicuous on paratypes than holotype, nephridia visible through body all, more marked in paratypes. Ventrum with discrete elongate rectangular pads from segments 4–18, raised, glandular, each pad dissected into 2 with mid pale line of pigment spots, well demarcated segmentally (Figure 4C), then becoming restricted to mid-ventral stripe.
Anal plate with 2 short anal cirri.
VARIATION
Some paratypes exhibit pale brown pigmentation on posterior neuropodia and in some the nephridial papillae are more conspicuous than those present on the holotype. Material not well preserved as samples were fixed before the animals were removed from their tubes and none are gravid. However, none of the specimens show any sign of branchial scars, only a single pair of branchiae. The paratype NHMR has no pygidial cirri and presents a simple rounded pygidium, suggesting the anal plate of the holotype may be an artefact or represent a damaged specimen as posterior end of holotype not very well preserved.
REMARKS
Pista adriatica sp. nov. is described as a new species characterized by a single pair of branchiae with the branchial filaments coming off the main axis in a continuous spiral, and by the shape and orientation of the lateral lobes and the presence of dorsal glandular ridges on segments 2 and 3. Only four other species of Pista with a single pair of branchiae have been described: P. dibranchis Gibbs, Reference Gibbs1971 from the Solomon Islands, P. godfroyi (Gravier, Reference Gravier1911) from the Antarctic, P. mirabilis McIntosh, Reference McIntosh1885 from deep water off Argentina and P. spinifera (Ehlers, Reference Ehlers and Chun1908) from the Antarctica. Two other species originally described as Pista, P. anthela Hutchings & Glasby, Reference Hutchings, Glasby, Wells, Walker, Kirkman and Lethbridge1990 and P. unibranchia Day, Reference Day1963 both belong to Pistella as they do not have any long-handled uncini. Pista adriatica sp. nov. can be distinguished from both P. spinifera and P. mirabilis as they both lack plumose branchiae, having either a thick bifurcated stem, which is then divided many times, or with a thick long stalk terminating in three main branches each with numerous branches. The other two species P. dibranchis and P. godfroyi both lack any long-handled uncini and should therefore be transferred to Pistella. For these reasons, we describe Pista adriatica as a new species.
ETYMOLOGY
The name of the species refers to its finding from the Adriatic Sea.
HABITAT
Specimens were collected from 5 to 37 m, from soft substrates (gravelly sand, gravelly muddy sand, silty gravelly sand and silty sand).
Genus Pistella Hartmann-Schröder, Reference Hartmann-Schröder1996
DIAGNOSIS
Transverse prostomium attached to dorsal surface of upper lip; basal part as thick crest, eye spots absent. Buccal tentacles all uniformly cylindrical. Peristomium restricted to lips; relatively short upper lip, hood-like; swollen, cushion-like and mid-ventral lower lip. Segment 1 reduced dorsally, usually with pair of ventro-lateral lobes connected to each other across ventrum by low lobe, marginal to mouth; segment 2 with pair of ventro-lateral lobes connected to each other by raised crest across ventrum; segments 3, or 3 and 4, with pair(s) of short lateral lobes; absent on following segments. Anterior segments highly glandular ventrally. Single pair of plumous branchiae, on segment 2, with long main stalks. Notopodia from segment 4, extending for 17 segments, notochaetae all distally winged. Neuropodia from segment 5, as low ridges in conjunction with notopodia and short pinnules posteriorly; neurochaetae as short-handled avicular uncini throughout, in partial to completely intercalated double rows on segments 11–20. Nephridial papillae present on segment 3, genital papillae usually on segments 6–7, posterior and dorsal to notopodia. Pygidium crenulated or with small rounded papillae.
The above generic diagnosis is from Hutchings et al. (Reference Hutchings, Nogueira, Carrerette, Schmidt-Rhaesa, Hrsg. v. Beutel, Glaubrecht, Kristensen, Prendini, Purschke, Richter, Westheide and Leschen2017).
Pistella rovignensis sp. nov.
Figures 5–7

Fig. 5. Pistella rovignensis sp. nov.: (A) anterior lateroventral view; (B) anterior ventral view; (C) anterior lateral view; (D) branchia; (E) branchial filaments. Scale bars: (A) 500 µm; (B–D), 250 µm; (E) 50 µm. All from AM W.49064.

Fig. 6. Pistella rovignensis sp. nov.: (A) thoracic notochaetae of 2nd segment; (B) close up of thoracic notochaetae of 2nd segment; (C) 1st neurochaetae; (D) 13th chaetiger; (E) close up of 13th chaetiger; (F) abdominal tori; (G) abdominal uncini. Scale bars: (A) 50 µm; (B and F) 20 µm; (C) 5 µm; (D) 40 µm; (E and G) 10 µm. All from AM W.49064.

Fig. 7. Pistella rovignensis sp. nov.: (A) anterior end showing unequal sized branchiae; (B) anterior dorsal view; (C) anterior ventral view; (D) 1st thoracic uncinus; (E) head on view of the uncini of 5th thoracic segment. Scale bars: (A, C) 500 μm; (B) 250 μm; (D) 10 μm; (E) 30 μm. All from AM W.49050. b, unequal sized branchiae; dr, dorsal ridge; vp, ventral pads; 2, 3, 4, lateral lobes.
MATERIAL EXAMINED
Holotype: AM W.49050, Station 3, 22.10.2008.
Paratypes: AM W.49064.001, 1 spec., Station 6, 30.9.2010, mounted for SEM; AM W.49063.001, 1 spec., Station 6, 30.9.2010, mounted for SEM; AM W.49052, Station 9, 29.9.2010, 2 specs, 12 mm in length, 1 mm in width, complete with about 87 chaetigers, 4 mm in length, 1 mm in width, posteriorly incomplete with 31 chaetigers; NHMR PMR 18355, Station 5, 20.9.2010, 2 specs, both incomplete, (1) 8 mm in length, 1.5 mm in width, 32 chaetigers, 16 mm in length, 1 mm in width, about 36 chaetigers, (2) posterior thorax damaged; NHMR PMR 18354, 1 spec., Station 10, 19.1.2011, incomplete, 11 mm in length, 2 mm in width, 26 chaetigers.
Non-type material: AM W.49066, 8 specs, Station 11, 24.11.2009; AM W.49053, 3 specs, Station 7, 29.9.2010; AM W.49061, 1 spec., Station 5, 20.9.2010; AM W.49054, 6 specs, Station 6, 30.9.2010; AM W.49055, 1 spec., Station 2, 27.2.2003.
A total of 27 specimens were examined.
DESCRIPTION
The following description is based on the holotype, which is posteriorly incomplete, 17.5 mm in length, 1 mm in width, with 31 chaetigers. Preserved specimen pale cream with speckled pale brown pigmentation anteriorly. Numerous colourless buccal tentacles all of similar width but of 2 lengths, short ones about length of longest branchial stalk, more numerous long ones just longer than the longest branchia (Figures 5A, B & 7A). Eye spots absent but some scattered pigment on margin of lower lip.
One pair of unequal sized plumose branchiae, filaments arranged in distinct tiers around a main shaft, filaments themselves branched 2 or 3 times, each branchia with stout, wide stalk with faint basal pigmentation on segment 2 (Figures 5D, E & 7A, B).
Peristomium consisting of short rectangular upper lip, forming a swollen cushion with rounded margins, mid ventral lower lip slightly V shaped, glandular. Segment 1 reduced dorsally continues across ventrum, covered by base of buccal tentacles. Segment 2 with well developed lateral almost rectangular lobes with anterior margins rounded that merge with the ventral pad to form a continuous ventral glandular collar (Figures 5A–C & 7B, C). Segment 3 small but conspicuous elongated asymmetrical triangular lateral lobes slightly displaced dorsally, not connected across ventrum with greater concentration of pale brown pigmented spots and continuing across the dorsum as a raised glandular anterior margin forming a dorsal crest (Figure 7B). Segment 4 with thickened glandular anterior margins (Figure 5C). Anterior dorsum very glandular (Figure 7B).
First pair of notopodia slightly displaced dorsally compared with subsequent ones. Notopodia short rectangular podia not extended from body wall initially, becoming more erect and larger posteriorly. Notochaetae broadly winged capillaries with marked inflection tapering to fine tips arranged in two tiers, short and long (Figure 6A, B). All thoracic neuropodia sessile, with all thoracic uncini short-handled avicular, initially arranged in single rows (Figure 6C), from segments 11–20 arranged in completely intercalated double rows (Figure 6D, E). Uncini with well developed main fang, small dorsal button and basal heel (following Nogueira et al., Reference Nogueira, Hutchings and Fukuda2010) (Figure 7D). Thoracic uncini with the following dental formula MF: 6–8: 4–5:∞. Initially thoracic notopodia and neuropodia well separated (Figure 5C) but by mid to posterior thorax, contiguous on a raised glandular ridge (Figure 5A). Abdominal neuropodia becoming more erect posteriorly as raised tori with about 12 avicular uncini with the following dental formula MF: 5–8: 8–9: 6–9, with individual uncini projecting from torus (Figure 6F, G).
Nephridial papillae present on segments 6 to 7. Entire ventrum very glandular with well developed and well defined (Figure 7C) rectangular glandular pads extending to chaetiger 10–11, appear to be divided into anterior and posterior sections but without any corresponding pigmentation in preserved material; subsequently pads not discrete just entire ventrum glandular, leading to a thin ventral glandular streak which continues on all remaining segments.
Holotype posteriorly incomplete.
VARIATION
The type and non-type material vary in the intensity of pigmentation on anterior segments and while one pair of unequal sized branchiae are always present, they vary in relative size to each other. There is no evidence of any branchial scars meaning that only one pair of branchiae was ever present.
All the material examined exhibits two lengths of buccal tentacles, regardless of the size of the individual, which indicates that the shorter tentacles are not just regenerating ones. The non-type material varies in terms of the quality of fixation and with it the ability to really resolve the nature of the lateral lobes, as the worms were fixed within their tubes in the sediment and only later sorted and removed from their tubes.
REMARKS
Pistella rovignensis sp. nov. can be distinguished from P. lornensis (Pearson, Reference Pearson1969) by the arrangement of the branchial filaments, which are arranged in distinct tiers, whereas the latter species has them arranged in a spiral. The shape and orientation of the lateral lobes also differs but both species have anterior dorsal crests, those of P. rovignensis sp. nov. occur only on segment 3 (Figure 7B) whereas P. lornensis has them on segments 2–4 (J.M.N. Noguiera, personal communication). Pistella lornensis has large lateral lobes on segment 2 which project anteriorly, and those of segment 3 are very small, whereas in the new species those on segment 3 are discrete elongated triangular and project dorsally (see Figures 5A & 7A, B). The number of pairs of ventral pads is greater in P. lornensis where they extend from segment 6 to 20, whereas in the new species they begin from segment 2 and continue to 14 or 15. No information is given in the P. lornensis description regarding the dentition of the uncini. While PH has examined the holotype, she did not have access to photographic equipment and was unable to prepare SEMS, so this material needs to be re-examined and the description updated.
Pistella rovignensis sp. nov. can be distinguished from other described Pistella species (P. anthela (Hutchings & Glasby, Reference Hutchings, Glasby, Wells, Walker, Kirkman and Lethbridge1990) and P. franciscana Nogueira, Hutchings & Carrerette, Reference Nogueira, Hutchings and Carrerette2015) by the number and arrangement of the lateral lobes. Pistella anthela has lateral lobes on segments 1, 2 and 3, which continue across the dorsum as crests, whereas in P. franciscana these lobes are on segments 1, 2 and 3 but do not continue across the dorsum, in contrast in P. rovignensis sp. nov. they are only present on segment 3 and they continue across the dorsum as crests. This has been disputed by Londoño Mesa (personal communication) who suggests they are just dorsally swollen anterior margins of the segments, which we disagree with and instead follow Nogueira et al. (Reference Nogueira, Hutchings and Fukuda2010) and refer to them as dorsal crests as they appear to be discrete structures.
Pistella franciscana occurs in shallow waters around Lizard Island on the Great Barrier Reef and P. anthela also occurs in shallow tropical waters in Dampier Archipelago in Western Australia, so these tropical coral reef habitats are very different to the environment in the Northern Adriatic where P. rovignensis sp. nov. was collected.
In addition, as mentioned earlier, some other species currently assigned to the genus Pista should be transferred to the genus Pistella, these include Pista unibranchiata Day, Reference Day1963, P. dibranchis Gibbs, Reference Gibbs1971 and P. godfroyi Gravier, Reference Gravier1911, as all lack any long-handled uncini and should therefore be transferred to Pistella according to the illustrations and text provided in their descriptions. Such a transfer should occur when the entire genus is revised.
ETYMOLOGY
The name of the species refers to the location where it was found: the city of Rovinj in the Northern Adriatic Sea (Croatia).
HABITAT
Specimens were collected from 6 to 37 m, from soft substrates (gravelly sand, gravelly muddy sand, silty sand and gravelly mud).
DISCUSSION
This study highlights the need to clarify the validity of Pista as currently accepted (Hutchings et al., Reference Hutchings, Nogueira, Carrerette, Schmidt-Rhaesa, Hrsg. v. Beutel, Glaubrecht, Kristensen, Prendini, Purschke, Richter, Westheide and Leschen2017) and this must include an examination of material from the type locality of Pista cristata and the designation of a neotype. That study should ideally consider the taxonomic value of the numbers of pairs of branchiae, and a study on the development of the long-handled thoracic uncini and how these characters change if at all as the animal grows and allow the proposals by Jirkov (Reference Jirkov2001) to be evaluated. Only then can the validity of the genus Pistella be determined.
Further adding to the confusion of the validity of these two genera, is that the type species have been widely reported geographically. Pista cristata has been reported from around the world in part due to its characteristic plume-shaped branchiae, which seems unlikely (P. Hutchings, personal communication). In the Adriatic Sea, four species of Pista have been recorded, P. cretacea, P. cristata, P. maculata and P. unibranchia, the first three being distributed also in the Northern Adriatic (Mikac, Reference Mikac2015). Pista cretacea and P. cristata were previously found (Fauvel, Reference Fauvel1934; Vatova, Reference Vatova1935, Reference Vatova1943; Bellan, Reference Bellan1969; Katzmann, Reference Katzmann1971, Reference Katzmann1972; Zavodnik, Reference Zavodnik1971; Marcuzzi, Reference Marcuzzi1972; Amoureux, Reference Amoureux1975; Mikac, Reference Mikac2015) close to or in the same area (Mikac, Reference Mikac2015) from which the two newly described species were found. All these species of Pista have the plume-shaped branchiae similar to those of P. cristata.
Similarly Pistella lornensis has subsequently been reported from many localities apart from its original type locality of the West coast of Scotland (Pearson, Reference Pearson1969), namely Yellow Sea (Saphronova, Reference Saphronova1991), Saguenay Fjord in Canada (north-west Atlantic Ocean) (Bossé et al., Reference Bossé, Sainte-Marie and Fournier1996), Mediterranean (Arvanitidis, Reference Arvanitidis2000), north-west of Portugal (Mucciolo, Reference Mucciolo2015) and western Africa (an ongoing survey of the University Museum of Bergen, Norway: http://miwa.b.uib.no/). The species was reported for the first time in the Mediterranean in the north Aegean Sea, Greece, by Arvanitidis (Reference Arvanitidis2000) and subsequently Musco et al. (Reference Musco, Mikac, Mirto, Vega Fernández, Agnetta, Pipitone, Di Stefano and Badalamenti2013) reported it from the southern coast of Sicily. Pearson (Reference Pearson1969) provides some ecological data of the two sites where he collected his species from Lochs Linnhe and Eil, in depths of 25–94 m, salinities varying from 28–34‰, temperatures of 6 and 12°C and dissolved oxygen concentration varying between 6 and 10 ppm over 4 years of hydrographic surveys. These lochs are well protected from the open ocean although connected. We suggest that all of the above records for P. lornensis need checking to confirm this very wide distribution range and the type species need to be redescribed. We further suggest that the Mediterranean records may represent a mixture of Pista adriatica sp. nov. and Pistella rovignensis sp. nov.
ACKNOWLEDGEMENTS
Many thanks to Sue Lindsey (currently at Macquarie University, formerly of the Australian Museum) for taking the SEM photographs. We should also like to thank the two reviewers who provided very constructive comments.










