Introduction
Cyanobacteria are prokaryotic photosynthetic organisms that, due to a long evolutionary history, developed morphological, physiological, ecological and biochemical changes that enabled colonization in different types of habitat (Lee, Reference Lee2008). They can also produce secondary metabolites (cyanotoxins), classified according to their action in animals as neurotoxic, hepatotoxic, cytotoxic or allergenic (Hudnell, Reference Hudnell2008). Different strains of Cylindrospermopsis raciborskii (Woloszynska) Seenara & Subba, 1912 can produce cytotoxic (cylindrospermopsin) or neurotoxic (saxitoxin) compounds. In Brazil, there are only reports of saxitoxin-producing strains of this cyanobacterium (Molica et al., Reference Molica, Onodera, García, Rivas and Andrinolo2002; Ferrão-Filho et al. Reference Ferrão-Filho, Soares, Magalhães and Azevedo2009).
The mechanism of action of saxitoxin is the reversible blocking of sodium channels in the neurons, leading to impaired action potentials, paralysis of muscles and respiratory arrest (Sivonen & Jones, Reference Sivonen, Jones, Chorus and Bartram1999). It is recognized as one of the most potent cyanotoxins, with a lethal dose for mice of around 10 μg kg−1 by intraperitoneal injection (Chorus & Bartram, Reference Chorus and Bartram1999). Signs and symptoms in humans can range from tingling, numbness, headaches, weakness and difficulty in breathing. In cases of lethal ingestion, respiratory arrest and death may occur within 3–4 h (Etheridge, Reference Etheridge2010).
Aquatic organisms have direct contact with cyanobacteria and their toxins in the environment, which may affect their growth, development, histology, reproduction and survival (Romo et al., Reference Romo, Soria, Fernandez, Ouahid and Baron-Sola2013; Drobac et al., Reference Drobac, Tokodi, Lujić, Marinović, Subakov-Simić, Dulić, Važić, Nybomd, Meriluoto, Codd and Svirčev2016). The fish Poecilia vivipara Bloch and Schneider, 1801 is one of the most common fish species in small ponds, rivers and coastal lagoon ecosystems from Brazil (Koblitz et al., Reference Koblitz, Andreata, Marca and Andreata2001; Mendonça & Andreata, Reference Mendonça and Andreata2001). They are common hosts for the trematode Pygidiopsis macrostomum Travassos, 1928, and the life cycle of this parasite species was described experimentally by Simões et al. (Reference Simões, Barbosa and Santos2009) using the mollusc Heleobia australis (d´Orbigny, 1835) as the first intermediate host, harbouring the redial and pleurolophocercous cercarial stages. The guppies P. vivipara were reported as the second intermediate hosts, in which metacercariae were found encysted in the gills and mesentery. The mammalian definitive hosts are infected by the ingestion of parasitized fish.
Parasites can be used as monitor species (by measuring the impact of pollutants through the impairment of their vital functions) or as indicator species (via their abundance or absence in certain areas), due to the variety of ways in which they respond to anthropogenic pollution, including domestic and industrial sewage, acidification and eutrophication (Beeby, Reference Beeby1999; Marcogliese, Reference Marcogliese2004; Sures, Reference Sures2004). Lopes et al. (Reference Lopes, Ferrão-Filho, Santos, Cunha and Santos2017) reported that neurotoxic strains of C. raciborskii were able to change the swimming behaviour of wild and reared P. vivipara. Considering the effect of this saxitoxin-producer cyanobacterium on the fish host, it is likely that parasites can also be exposed to saxitoxins. Therefore, the aim of this work was to evaluate the toxicological effect of two concentrations of crude lyophilized extracts of a saxitoxin-producer strain of the cyanobacterium C. raciborskii on the motility of metacercariae of P. macrostomum encysted in the fish P. vivipara, and the accumulation of saxitoxin in fish tissues where parasites are mostly found (guts and gills).
Materials and methods
The saxitoxin-producer strain of the cyanobacterium C. raciborskii (CYRF-01) was isolated from the Funil Reservoir (Rio de Janeiro, Brazil) and cultivated in ASM-1 medium (Gorham et al., Reference Gorham, Mclachlav, Hammer and Kim1964), pH 8.0, under fluorescent light at an intensity of 40–50 μE m−2 s−1, 12:12 h light:dark cycle at 23 ± 1°C (Incubator BOD, Solab SL 224, Solab, Piracicaba, Brazil). The culture was lyophilized (Liotop L101, Liobrás, São Carlos, Brazil) and the crude extract was used in the preparation of 40 mg l−1 and 400 mg l−1 solutions, after previous 48-h toxicity tests where those concentrations were not acutely toxic to fish.
Wild fish were collected from Rodrigo de Freitas lagoon, Rio de Janeiro, Brazil (22°57′02″S, 43°11′09″W) and kept for 20 days in dechlorinated tap water in aerated tanks, at 24 ± 1°C and fed with commercial food. A total of 30 adult P. vivipara were used in the experiments. Measurements and weights of fish are presented as ranges followed by means in parentheses.
During the experiment, the mean values of the physico-chemical parameters of water samples without cyanobacteria were: dissolved oxygen 7.3 ± 0.2 mg l−1, temperature 24 ± 1°C, pH 6.1 ± 0.4 and conductivity 83.7 ± 1.6 μS cm−1. The parameters of water samples with cyanobacteria were: dissolved oxygen 7.4 ± 0.2 mg l−1, temperature 24 ± 1°C, pH 6.6 ± 0.3 and conductivity 165 ± 28.2 μS cm−1.
For the motility test, the 30 P. vivipara were separated into three groups of ten individuals: exposed to clean water (control group), exposed to 40 mg l−1 or exposed to 400 mg l−1 of crude extract of C. raciborskii (CYRF-01), all for 48 h, with aeration and feed. The crude extract solutions were changed after 24 h. At stage 1, immediately after all exposures, five fish from each group were dissected under a stereomicroscope to collect and count metacercariae from gills and guts, after Simões et al. (Reference Simões, Barbosa and Santos2009). At stage 2, after all exposures, the other five fish from each group were kept for an additional 48 h in clean water, to be examined subsequently. The motility of all encysted metacercariae isolated from all fish was observed under a stereomicroscope every 15 min for 1 h. When alive inside the cysts, the metacercariae contract and expand the body, turning upside down or laterally.
A quantitative determination of saxitoxins was performed in the solutions of cyanobacteria, gills and guts of the fish. For the extraction of saxitoxins from the solutions, samples were sonicated for 15 min and centrifuged for 10 min. For the extraction of saxitonins from the tissues, samples were taken from the five fish of stage 1 and five fish of stage 2. Each tissue sample was divided into two similar weights to be used as replicates. Previously weighed tissue samples were homogenized individually (Ultrax-Turrax®T18, IKA®WORKS, Staufen, Germany) with 5 ml of acetic acid 0.1 n. Subsequently the samples were sonicated (Unique Maxiclean 1600, Unique, Indaiatuba, Brazil) for 15 min and centrifuged (Centribio 80-2B, Centribio Co. Ltd, Shangai, China) at 6000 rpm for 10 min. The supernatant was removed and lyophilized; the resulting material was diluted in 1 ml of deionized water and analysed by the enzyme-linked immunosorbent assay (ELISA).
All statistical analyses were performed using the R program (R Development Core Team, 2014). The chi-square test was used to investigate the proportion of metacercariae that were not moving after exposure to cyanobacterial extract in the P. macrostomum motility test. The level of significance assumed for statistical tests was 5%.
Results and discussion
The total length of fish used in the experiments ranged from 3.5 to 5.5 (4.4) cm, and the weight from 0.4 to 1.7 (1.1) g. No deaths or paralysis were observed in fish exposed to 40 mg l−1 or 400 mg l−1 solutions of cyanobacterial crude extract for 48 h. The metacercariae of P. macrostomum were collected from the gills and guts of fish.
In the control group, not exposed to cyanobacteria, 36 metacercariae were recovered from five fishes at stage 1 after 48 h in clean water. From the other five fish in this group, 51 metacercariae were collected at stage 2, after an additional 48 h in clean water. All these metacercariae were all alive and moving (100%) (table 1).
Table 1. Total number of metacercariae of Pygidiopsis macrostomum moving and not moving in stages 1 and 2 of the experiment with C. raciborskii solutions, in comparison with a control group.

In the five fish exposed to a concentration of 40 mg l−1 of cyanobacteria (stage 1), 35 metacercariae were found but only nine (35%) were active and moving (chi-square χ 2 = 10.04, P value = 0.001). From the other five fish exposed to 40 mg l−1 of cyanobacteria and kept for an additional 48 h in clean water (stage 2), 53 metacercariae were recovered and 100% were active (chi-square χ 2 = 0.00, P value = 1) (table 1).
In the group exposed to 400 mg l−1 (stage 1), of 38 metacercariae found, only 10 (36%) were moving (chi-square χ 2 = 10.36, P value = 0.002). From those exposed to 400 mg l−1 and kept for an additional 48 h in clean water (stage 2), 42 metacercariae were collected and 38 (90%) were active (chi-square χ2 = 0.11, P value = 0.77) (table 1).
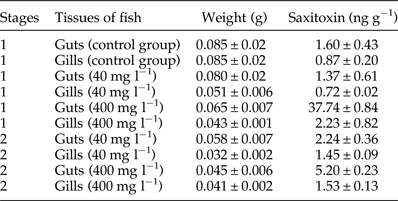
The saxitoxin values quantified from the solutions of the two concentrations of cyanobacterial crude extract used (40 and 400 mg l−1) were 2.6 and 26 μg l−1, respectively. This concentration range of toxins has been reported in Brazilian waters, thus being ecologically relevant (Ferrão-Filho et al., Reference Ferrão-Filho, Soares, Magalhães and Azevedo2009, Reference Ferrão-Filho, Soares, Magalhães and Azevedo2010). The mean values of saxitoxins in fish tissues are shown in table 2, together with the weight of the samples. The saxitoxin (ng g−1) concentration in the guts of all fish was higher than in the gills. At stage 1, it was about 1.8-fold higher in the control group, 1.9-fold in the group exposed to 40 mg l−1 and 16.9-fold in the group exposed to 400 mg l−1 cyanobacterial crude extract. At stage 2, it was about 1.5-fold higher in the group exposed to 40 mg l−1 and 3.4-fold in the group exposed to 400 mg l−1.
Table 2. Quantitative analysis of saxitoxin present in the guts and gills of the fish used in the P. macrostomum motility test. Results are expressed as mean ± SD.
Paralysis was observed in the metacercariae of P. macrostomum when fish hosts were exposed to cyanobacterial extract in both concentrations, 40 mg l–1 and 400 mg l−1. In fact, as conspicuous amounts of saxitoxins were found in fish gills and guts exposed to these extracts, and parasites were collected from the same fish tissues, it is likely that parasites were exposed to saxitoxins. However, measurement of the toxins in metacercariae was not possible due to their small size and the reduced number of individuals collected.
Pygidiopsis macrostomum is a well-studied parasite in our laboratory, and its metacercariae are easily recognizable due to their size, shape and site in the fish host P. vivipara, which facilitates the observation of their motility (Simões et al., Reference Simões, Barbosa and Santos2005, 2009; Borges et al., Reference Borges, Costa, Mantovani, Barros, Santos, Mafra and Santos2017). The data obtained in our experiment for motility of P. macrostomum suggests that saxitoxin causes a temporary paralysis, similar to that observed in the microcrustacean Daphnia sp. exposed to the same saxitoxin-producer strain of C. rasciborskii (Ferrão-Filho et al., Reference Ferrão-Filho, Cunha, Magalhães, Soares and Baptista2007, Reference Ferrão-Filho, Soares, Magalhães and Azevedo2009, Reference Ferrão-Filho, Soares, Magalhães and Azevedo2010; Restani & Fonseca, Reference Restani and Fonseca2014). This fact was corroborated by the fish groups that were returned to clean water for an additional 48 h, in which the metacercariae were active, thus confirming that the paralysis was temporary. This is fully in agreement with the findings of previous studies with invertebrates and thus with the mechanism of action of saxitoxins.
Other metacercariae that parasitize P. vivipara have proved to reduce the locomotor behaviour of this fish host, and the intensity of infection may modulate the host–parasite interaction, thus favouring predation of the fish by the definitive hosts (Santos et al., Reference Santos, Cunha and Santos2011; Santos & Santos, Reference Santos and Santos2013). Likewise, the swimming behaviour of wild and lab P. vivipara changed when exposed to the saxitoxin-producer strain of C. rasciborskii (Lopes et al., Reference Lopes, Ferrão-Filho, Santos, Cunha and Santos2017). This first report of the effect of saxitoxins in metacercariae of P. macrostomum encysted in their fish intermediate host, suggests that the toxins from cyanobacteria could have a similar modulating effect of the host–parasite relationship in the natural environment.
The toxicity of saxitoxins has also been reported in studies with crustaceans in marine and freshwater environments. Such effects include the loss of motor coordination in copepods exposed to dinoflagellates (Ives, Reference Ives, Anderson, White and Baden1985), and a reduction in the beating rate of thoracic appendages and increase in the rejection rate of particles by the post-abdomen of Daphinia carinata when exposed to a filtrate of Aphanizomenom flos-aquae and to purified saxitoxin (Haney et al., Reference Haney, Sasner and Ikawa1995). In addition to the toxic effects reported in animals, cyanotoxins can affect human health through the drinking of water and the consumption of contaminated fish. Cyanobacteria are an important part of the diet of many species of crustaceans and fish, and these toxins can accumulate and be transferred to humans through the food chain (Magalhães et al., Reference Magalhães, Marinho, Domingos, Oliveira, Costa, Azevedo and Azevedo2003; Ferrão-Filho & Kozlowsky-Suzuki, Reference Ferrão-Filho and Kozlowsky-Suzuki2011; Bieczynski et al., Reference Bieczynski, Bianchi and Luquet2013).
Brazilian legislation has established a requirement for cyanobacterial toxins in drinking-water supplies (Portaria 518/04/MS from the Ministry of Health, 2005), setting recommended guideline values of 1 μg l−1 for microcystins, 3 μg l−1 for equivalents of saxitoxins and 15 μg l−1 for cylindrospermopsin. Nevertheless, even when saxitoxin concentrations are low, they may accumulate in zooplankton and fish and become harmful (Teegarden & Cembella, Reference Teegarden and Cembella1996; Nogueira et al., Reference Nogueira, Pereira, Dias, Pflumacher, Wiegand, Franca and Vasconcelos2004).
Comparing the measurement of toxins in the guts and gills of the fish exposed to 40 mg l−1 extract among the stages of the experiment, we observed concentrations of saxitoxin to be 1.6- and 2-fold higher, respectively, in fish kept for 48 h in clean water. However, in the guts and gills of fish exposed to 400 mg l−1 extract, the opposite occurred, with a substantial decrease (7.2- and 1.4-fold, respectively) in fish kept for 48 h in clean water. These high levels of saxitoxin in the guts and gills even in fish kept for 48 h in clean water may be related to the depuration process of the toxin by the fish, given that 2 days were not enough for the total clearance of the toxin. Comparatively, Costa et al. (Reference Costa, Lage, Barata and Ferreira2011) reported that the white seabream fish (Diplodus sargus) can take 2 weeks to completely depurate saxitoxins. Also, biotransformation products of cyanotoxins, conjugated to glutathione via glutathione-S-transferase (Pflugmacher et al., Reference Pflugmacher, Wiegand, Oberemm, Beattie, Krause, Codd and Steinberg1998), can also be detected by ELISA and this may result in overestimation of the toxin concentration in the sample (Metcalf et al., Reference Metcalf, Beattie, Pflugmacher and Codd2000, Reference Metcalf, Beattie, Ressler, Gerbersdorf, Pflugmacher and Codd2002). Thus, even if after 48 h the toxins have been mostly depurated, toxin conjugates could still be detected.
Although at lower levels, saxitoxins were also found in the gills and viscera of fish in the control group. As the wild fish used in this experiment were collected from the Rodrigo de Freitas lagoon, they could have had previous contact with this toxin in their natural environment. However, they were kept in tanks with clean water for at least 20 days before use in experiments, long enough for the clearance of the toxin (15–16 days) according to Costa et al. (Reference Costa, Lage, Barata and Ferreira2011). However, some level of cross-reactivity with by-products of toxin biotransformation can be assumed in this case (Metcalf et al., Reference Metcalf, Beattie, Pflugmacher and Codd2000, Reference Metcalf, Beattie, Ressler, Gerbersdorf, Pflugmacher and Codd2002).
In summary, although no detectable effects were observed in the fish, such as paralysis or swimming behaviour alterations, the saxitoxins produced by C. raciborskii were able to paralyse the metacercariae of P. macrostomum temporarily and bioaccumulated in the tissues of P. vivipara. These changes can lead to biological and ecological consequences for the fish in their natural environment and influence the life cycle of their parasites. This is the first report on the action of neurotoxins in metacercariae of fish.
Acknowledgements
We are grateful to Dr José Augusto Albuquerque dos Santos for chemical analysis of the water, Mario Gatti MSc for lyophilization and to Mr Orlando Marins Filho who provided the fish.
Financial support
The study was supported financially by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq- Universal no. 449658/2014-7), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES-Parasitologia 247/2012) and Fundação Oswaldo Cruz (PAEF no. IOC-008-FIO-04). This study is part of the master's thesis of the first author, as part of the Programa de Pós-Graduação em Biodiversidade e Saúde, Instituto Oswaldo Cruz, Fiocruz.
Conflict of interest
None.
Ethical standards
This study was authorized by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA, license no. 15898-1) and approved by the Animal Ethics Committee of the Oswaldo Cruz Foundation (CEUA- FIOCRUZ no. L-020/2015).




