INTRODUCTION
Nitrogen (N) and phosphorus (P) are thought to be the principal growth-limiting nutrient elements in tropical rain forests (Tanner et al. Reference TANNER, VITOUSEK and CUEVAS1998) but their relative importance is not entirely clear and seems to vary with site conditions. While P likely is limiting the productivity of many tropical lowland forests, N shortage may be more decisive in tropical montane forests on younger soils and under lowered temperatures (Paoli et al. Reference PAOLI, CURRAN and ZAK2005, Tanner Reference TANNER1981, Vitousek & Sanford Reference VITOUSEK and SANFORD1986). Studies along altitudinal gradients in tropical mountains found marked decreases in ammonification rate with altitude and even steeper decreases in nitrification rate, because the activity of autotrophic nitrifiers is particularly sensitive to the cool and often acidic soil conditions at higher altitudes (Jones et al. Reference JONES, KIELLAND, SINCLAIR, DAHLGREN, NEWSHAM, FARRAR and MURPHY2009, Marrs et al. Reference MARRS, PROCTOR, HEANEY and MOUNTFORD1988, Wolf et al. Reference WOLF, VELDKAMP, HOMEIER and MARTINSON2011). Thus, not only the relative importance of N and P shortage may vary with altitude, but the supply rates of NH4+ and NO3− and the relative availability of the two N forms as well. Moreover, the depth of organic layers on top of the soil was found to greatly increase with altitude on tropical mountains (Moser et al. Reference MOSER, LEUSCHNER, HERTEL, GRAEFE, SOETHE and IOST2011, Wolf et al. Reference WOLF, VELDKAMP, HOMEIER and MARTINSON2011) suggesting that organic N forms should be more readily available for plant use at high altitudes. As a consequence, N supply should vary greatly across altitudinal and exposure gradients in tropical montane forests. Given that many tropical mountain forests are rich in tree species, one might assume that the heterogeneity in N supply patterns is associated with plurality in N acquisition strategies in the trees. Studies in temperate and boreal forests suggest that trees with apparent preference of ammonium are more abundant in cold environments and that the importance of organic N forms for tree nutrition increases with decreasing decomposition and N mineralization rate (Finzi & Berthrong Reference FINZI and BERTHRONG2005, Kielland Reference KIELLAND1994, Reference KIELLAND1997; Näsholm et al. Reference NÄSHOLM, EKBLAD, NORDIN, GIESLER, HÖGBERG and HÖGBERG1998). If these patterns are also valid on tropical mountains, we predict a higher abundance of nitrate-preferring trees at lower elevation and a dominance of trees with preference for organic N and/or ammonium at higher elevation. However, in contrast to temperate and boreal forests, the large majority of tropical tree species seem to form arbuscular mycorrhizas (AM) (Kottke et al. Reference KOTTKE, BECK, OBERWINKLER, HOMEIER and NEILL2004) and not ectomycorrhizal associations (ECM).
So far, information on the preference of different N forms by tropical trees is lacking. N acquisition strategies in tropical forest plants have been studied for hemi-epiphytic Clusiaceae (Arndt et al. Reference ARNDT, WANEK, HOCH, RICHTER and POPP2002, Wanek et al. Reference WANEK, ARNDT, HUBER and POPP2002), understorey palms (Andersen & Turner Reference ANDERSEN and TURNER2013), and rain-forest bryophytes (Wanek & Pörtl Reference WANEK and PÖRTL2008), but not for trees. The first study showed that hemi-epiphyte Clusia minor can use ammonium, nitrate and also glycine under greenhouse conditions, but three other Clusia species seem to prefer NH4+ or glycine over NO3− under field conditions (Wanek et al. Reference WANEK, ARNDT, HUBER and POPP2002). Andersen & Turner (Reference ANDERSEN and TURNER2013) found seedlings of understorey palms to be able to use organic nitrogen with no preferences for chemical forms of N but an overall acquisition pattern of glycine ≥ NO3− ≥ NH4+. It is not known whether N form preferences differ among co-occurring dicotyledonous tree species in species-rich tropical forests and whether preferences change with alteration in inorganic and organic N availability along mountain slopes.
In this study, we examined the uptake of ammonium, nitrate and glycine by seedlings of six native tree species from three Ecuadorian montane forests that were grown under natural conditions in pots inside the forest at 1000, 2000 and 3000 m asl (two species per altitude). NH4+, NO3− and glycine were added at low doses (5 kg N ha−1) with 15N-labelled solutions (or dual-labelled glycine solution) and the plants were harvested after 5 d. For measuring N uptake under conditions as close to nature as possible, we used intact plants instead of excised roots and grew the plants under the characteristic low-light conditions on the forest floor where they received natural rainfall. The main objectives of the study were (1) to search for a significant altitudinal effect on the N form preference in tropical tree species, and (2) to examine the role of organic nitrogen (glycine) for the nutrition of trees in tropical mountain forests. More specifically, we tested the hypotheses that (1) the saplings of tropical trees are capable of using organic N even though they form arbuscular mycorrhiza, and (2) with increasing altitude, tree saplings increasingly prefer ammonium and glycine over nitrate due to a lowered nitrification rate and increased humus accumulation.
METHODS
Study sites
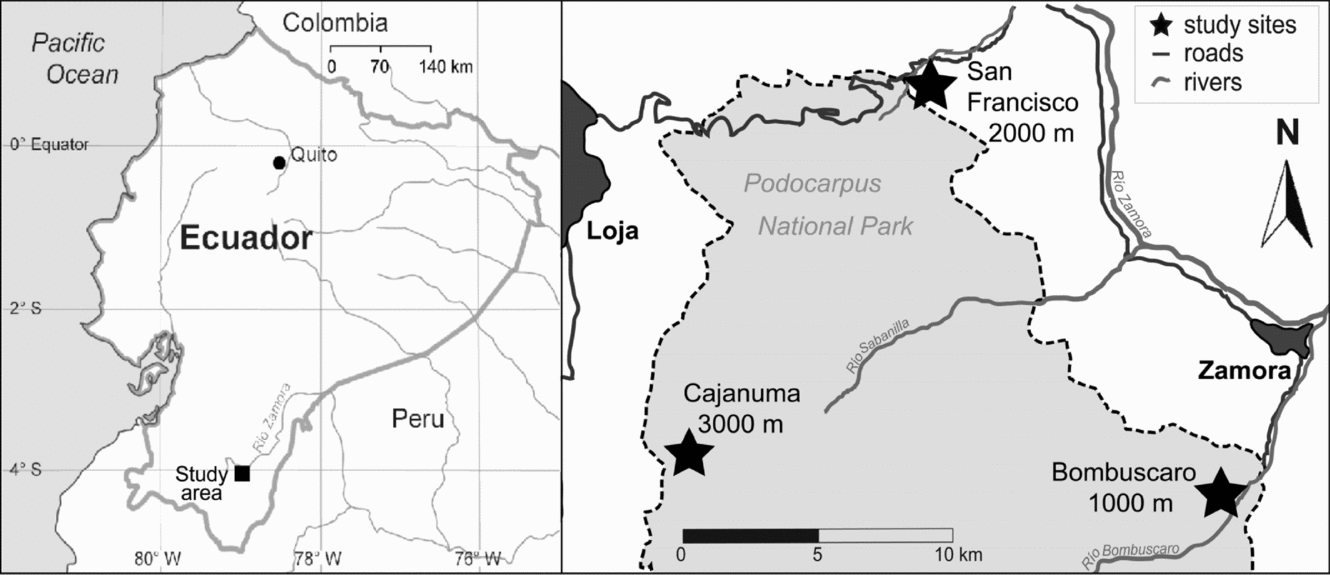
This study took place in tropical montane forests on the eastern slope of the South Ecuadorian Andes along a 2000-m altitudinal transect. Three study sites were selected at c. 1000, 2000 and 3000 m asl in Podocarpus National Park and in the Reserva San Francisco in the Provinces of Loja and Zamora-Chinchipe (Figure 1). The study area has a tropical humid climate with a very wet season in April–July and experiences a less humid period from September to December (Bendix et al. Reference BENDIX, HOMEIER, CUEVA ORTIZ, EMCK, BRECKLE, RICHTER and BECK2006). Regularly occurring longer dry periods do not exist. Table 1 gives further details of the climatic conditions at the study sites.

Figure 1. Location of the study area in southern Ecuador with the three stands at 1000, 2000 and 3000 m asl.
Table 1. Characteristics of the studied forest stands in the altitudinal transect in southern Ecuador (climate data from Moser et al. (Reference MOSER, HERTEL and LEUSCHNER2007); soil data from Wolf et al. (Reference WOLF, VELDKAMP, HOMEIER and MARTINSON2011) and A. Baldos, unpubl. data). C/N ratio, pH, net nitrification and net N mineralization rate (in situ buried-bag method) refer to the topsoil (0–10 cm), organic N concentrations to 0–5 cm.

According to Homeier et al. (Reference HOMEIER, WERNER, GRADSTEIN, BRECKLE, RICHTER, Beck, Bendix, Kottke, Makeschin and Mosandl2008), the forests at the study sites can be classified as follows: At 1000 m (4°7′S, 78°58′W), in the transition zone between tropical lowland and lower montane forest, evergreen forest with tree heights of up to 40 m is present. Common tree families of this forest type are Fabaceae, Melastomataceae, Moraceae, Myristicaceae, Rubiaceae and Sapotaceae. The evergreen lower montane forest at 2000 m (3°58′S, 79°04′W) achieves a canopy height of 18–22 m. Characteristic tree families are Euphorbiaceae, Lauraceae, Melastomataceae and Rubiaceae. At 3000 m (4°7′S, 79°11′W), evergreen upper montane forests and elfin-forests are found that extend up to the tree line; canopy height rarely exceeds 8–10 m. Dominant tree families are Aquifoliaceae, Clusiaceae, Cunoniaceae, Lauraceae and Melastomataceae.
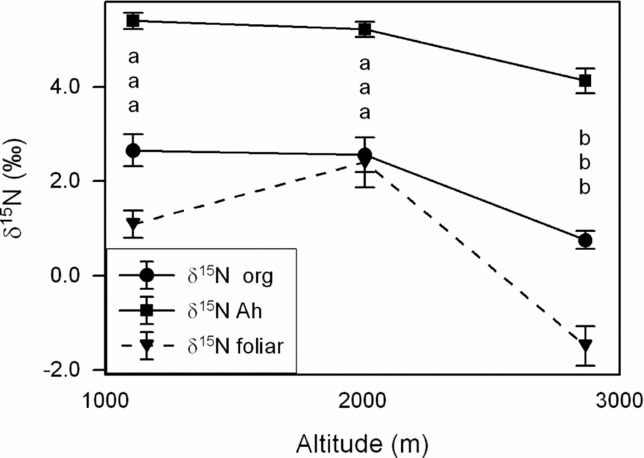
All soils are acidic with a progressive pH decrease toward higher elevation. With increasing altitude, soil nutrient availability also decreases along the transect. Both N net mineralization and nitrification rate decreased with altitude, as does the mineral N concentration of the topsoil (Table 1, after Wolf et al. Reference WOLF, VELDKAMP, HOMEIER and MARTINSON2011). The δ15N signature of the mineral topsoil material and the organic layer decreases in general from 1000 to 3000 m by about 1.5‰ (difference significant between 2000 and 3000 m). The mean δ15N value of tree sun leaves increased from +1.1‰ at 1000 m to c. +2.4‰ at 2000 m and rapidly dropped to −1.5‰ at 3000 m; foliar N concentration followed this pattern with a mid-slope peak at 2000 m (Wittich et al. Reference WITTICH, HORNA, HOMEIER and LEUSCHNER2012) (Figure 2). Kottke et al. (Reference KOTTKE, BECK, OBERWINKLER, HOMEIER and NEILL2004) investigated the mycorrhizal status of the tree species in the montane forests of southern Ecuador and found more than 95% of the species to be colonized by AM fungi without the formation of ECM. In our stand at 2000 m, five of c. 300 abundant tree species including the genus Graffenrieda were found to form ECM (I. Haug, pers. comm.).

Figure 2. δ15N signatures (mean ± SE; 18 plots per altitude) in canopy leaves (mean of various tree species per altitude) and in the soil (organic layer and 0–30 cm of mineral soil) at 1000, 2000 and 3000 m asl in the study transect after data from Wolf et al. (Reference WOLF, VELDKAMP, HOMEIER and MARTINSON2011) and unpublished data of K. Wolf.
Plant material
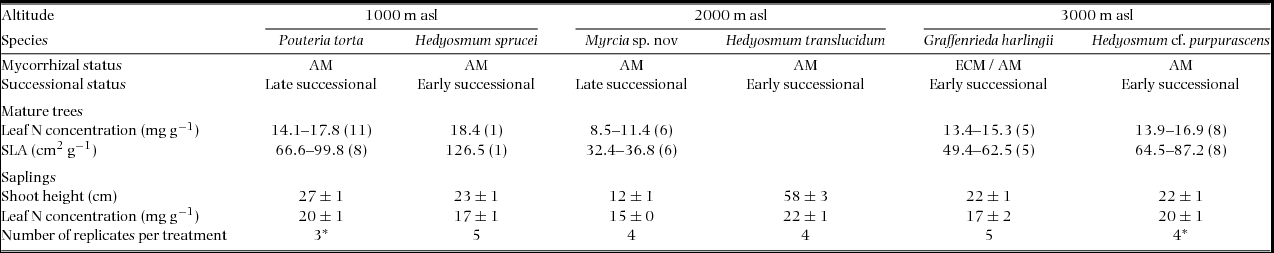
The forests in the study area are extremely diverse with ≥800 tree species present (J. Homeier, unpubl. data) and no tree species was found to be abundant at all three study sites (1000, 2000 and 3000 m). We selected six tree species (two each per site) that were considered to be representative for the sites because they occurred more frequently in the stands than elsewhere: Pouteria torta (Mart.) Radlk. (Sapotaceae) and Hedyosmum sprucei Solms (Chloranthaceae) at 1000 m asl, Myrcia sp. nov. (undescribed species, Myrtaceae) and Hedyosmum translucidum Cuatrec. (Chloranthaceae) at 2000 m, and Graffenrieda harlingii Wurdack (Melastomataceae) and Hedyosmum purpurascens Todzia (Chloranthaceae) at 3000 m. Hedyosmum is one of the very few genera found from 1000 to 3000 m asl in the study area. Characteristics of the six species are summarized in Table 2.
Table 2. Characteristic parameters of the tree species investigated in this study. Given are ranges for mature trees growing in the study region (unpublished data) and means (± SE) for the seedlings studied for the tracer study. AM: arbuscular mycorrhiza; ECM: ectomycorrhiza (after I. Haug, pers. comm.). Number of replicates per treatment and per date (*three harvest dates for P. torta and H. purpurascens). The number of replicate measurements in parentheses.

Some 4–6 mo before the start of the experiment, saplings of all species were collected from the three stands and planted into plastic pots. According to sapling monitoring studies in the field (Homeier et al., unpubl. data), the plants were approximately 3–5 y in age at the time of the experiment. Average shoot height was 27 cm. The pots of 25 cm diameter and 25 cm height were filled with local forest soil from the sites where the saplings had been collected. We used mineral topsoil (upper 25 cm) from patches of undisturbed primary forest. The pots, each one sapling growing in it, were placed on wooden tables at the three study sites in the interior of the local stands under closed forest canopy. Photosynthetically active radiation at the height of the pots was on average 4.5% of incident flux density (range = 2.04–7.14%; measured with a LI-1000 Quantum Sensor, Licor Biosciences, Lincoln, NE, USA). By placing two layers of fine-meshed polypropylene net on the soil surface of the pots, we prevented waterlogging after strong rainfall events.
15N and 13C tracer application
For determining the optimal time of harvest in the 15N labelling experiment, we conducted a preliminary study with Pouteria torta saplings at 1000 m and with Hedyosmum purpurascens saplings at 3000 m. We harvested the leaves of selected plants at seven different time steps (2 h–18 d) after adding 15N-ammonium, 15N-nitrate or 15N-glycine solution and calculated the temporal development of 15N accumulation into leaf biomass. This preliminary experiment with all three N forms indicated a measurable increase in the first 6 d and a very slow further increase (or even a decrease) in the 15N values in the leaves when more than 6 d (up to 18 d) had passed after application. The harvest times were chosen according to these results and they are also based on the time lag of response found by Graefe et al. (Reference GRAEFE, LEUSCHNER, CONERS and HERTEL2011) who conducted an experiment on the stimulation of tree fine-root growth by locally adding N, P or K at the study sites.
For every tree species, four treatments with three- to five-fold replication (Table 2, depending on plant availability) were established: (1) control (only water added), (2) addition of labelled nitrate (NH415NO3, 98 atom-%), (3) addition of labelled ammonium (15NH4NO3; 98 atom-%), and (4) addition of 15N13C dual-labelled glycine (H215N13CH213CO2H; 98 atom-%). Thus, the experiment consisted of c. 32 pots each (2 species × 4 treatments × 4 (3–5) replicates) at the three altitudes. Since exclusive uptake of ammonium or nitrate leads to acidification or alkalinization of the rhizosphere, we applied ammonium-nitrate with specific labelling of only one of the components (NH4+ or NO3−) in order to exclude soil pH effects on uptake kinetics. The 15N tracer was added on 13 April 2010 to all pots (except for the control) at 1000 m asl and on 14 April 2010 to the pots at 2000 and 3000 m asl as 50 ml solution in a dose of 5 kg N ha−1 (0.3 g N per pot) calculated on the basis of the pot surface area.
In order to avoid losses of the added 15N ammonium through nitrification, we added the nitrification inhibitor dicyandiamide (DCD, AlzChem Trostberg GmbH, Trostberg, Germany) to all 15N-ammonium pots (20 mg 14 atom%-DCD-N); DCD is widely used in agriculture and decomposes in soil into non-toxic components (Di & Cameron Reference DI and CAMERON2004, Zacherl & Amberger Reference ZACHERL and AMBERGER1990). The concentration used was shown to inhibit nitrification for 6–10 d in tropical soils (Verma et al. Reference VERMA, TYAGI and SINGH2007).
Harvest and analysis
All investigated plants were harvested either 5 d after nutrient application (H. sprucei (1000 m), Myrcia, H. translucidum (2000 m), Graffenrieda (3000 m)), or 2, 5 and 8 d after application (Pouteria (1000 m), H. purpurascens (3000 m)) to document the temporal course of 15N acquisition in plant biomass. In the latter two species, three times the number of experimental plants was cultivated.
Plants were cut into leaves, shoot and roots. The roots were washed immediately to remove all soil. The plant material was dried at 70°C for 48 h and transferred to Germany. Roots were separated into coarse roots and fine roots (diameter of dried fine roots < 1.5 mm) and all plant material was weighed.
The 15N and 13C concentrations and the total concentrations of N and C in the plant biomass were determined with an elemental analyser (NA 1108, Fisons-Instruments, Rodano, Milano, Italy) coupled with an isotope mass ratio spectrometer (Delta plus, Finnigan MAT, Bremen, Germany) in the Laboratory for Stable Isotope Research at Göttingen University (KOSI). The 15N concentration in the dry mass of the organs of all treatments including the control was calculated as atom% 15N of total N. Percentage recovery of 15N in a given organ is the total amount of 15N (minus the background level, i.e. untreated control plants) detected in the organ's biomass related to the 15N amount added to the pot at the experiment's start.
In the case of dual-labelled glycine, we calculated the 15N concentration of the sample after 15N13C-glycine addition with two different approaches. The first enrichment value was derived directly from the 15N values measured with the mass ratio spectrometer (termed glycine-15N approach hereafter). This calculation should include all 15N that is accumulated in that plant organ (the balance of influx into minus efflux out of the organ) from the labelled glycine either through uptake of intact glycine or glycine deaminated prior to plant uptake. The second approach (glycine-13C) corrects this figure by considering the accumulation of 13C based on the following equation:
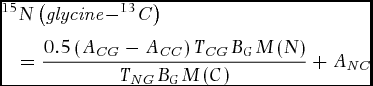 \begin{eqnarray*}
&&{}^{{\rm 15}}N\left( {\it glycine{\rm - }{}^{{\rm 13}}C} \right)\\
&&\quad = \frac{{{\rm 0}{\rm .5}\left( {A_{\it CG} - A_{\it CC} } \right)T_{\it CG} B_G M\left( N \right)}}{{T_{\it NG} B_G M\left( C \right)}} + A_{\it NC}
\end{eqnarray*}
\begin{eqnarray*}
&&{}^{{\rm 15}}N\left( {\it glycine{\rm - }{}^{{\rm 13}}C} \right)\\
&&\quad = \frac{{{\rm 0}{\rm .5}\left( {A_{\it CG} - A_{\it CC} } \right)T_{\it CG} B_G M\left( N \right)}}{{T_{\it NG} B_G M\left( C \right)}} + A_{\it NC}
\end{eqnarray*}
with ACG being the 13C concentration of the glycine-treated plants (atom-%), ACC the mean 13C concentration of the control plants (atom-%), TCG the total C concentration of the glycine-treated plants (g g−1), BG the biomass of the glycine-treated plants (g), TNG the total N concentration of the glycine-treated plants (g g−1), M(N) the molar mass of 15N (g mol−1), M(C) the molar mass of 13C (g mol−1) and ANC the 15N concentration of the control (atom-%).
This calculation assumes that 13C enrichment is a reliable indicator of the synchronous uptake of the C skeleton and the amino group of the glycine molecule. The glycine-15N approach may overestimate the amount of glycine taken up by the plant due to deamination in the soil prior to plant uptake, while the glycine-13C approach may underestimate the amount due to 13C loss in the form of 13CO2 respired after plant uptake (Näsholm & Persson Reference NÄSHOLM and PERSSON2001). By plotting the 13Cexcess values against the corresponding 15Nexcess values, we tested for glycine uptake in intact form which would show a 2:1 line.
Data analysis
Data analysis focused on treatment differences within a species, i.e. acquisition of NH4+, NO3− or glycine relative to the untreated control plants which served for obtaining the 15N background levels. The control was included as one of the treatments (15N concentration) or NH4+, NO3− and glycine acquisition values were calculated after taking into account the values of the controls (recovery of 15N in the biomass). A fourth treatment was introduced in the analysis through the 13C-based uptake calculation for glycine. We refrained from analysing for species differences because the six species differed largely in growth rate. Analysis of variance (Scheffé's test) was used for conducting comparisons among the four treatments of a species. If the data were not normally distributed according to a Shapiro–Wilk test, the Mann–Whitney two-sample test (Wilcoxon U-test) was used instead of Scheffé's test. All calculations were conducted with SAS software (version 9.1; SAS Institute, Cary, NC, USA). A significance level of 5% was used throughout the analysis.
The relationship between 15N-excess and 13C-excess values of a species was analysed by simple linear regressions conducted with the software Xact, version 8.03 (SciLab, St Yrieix, France).
RESULTS
Effects of altitude on the uptake of different N forms
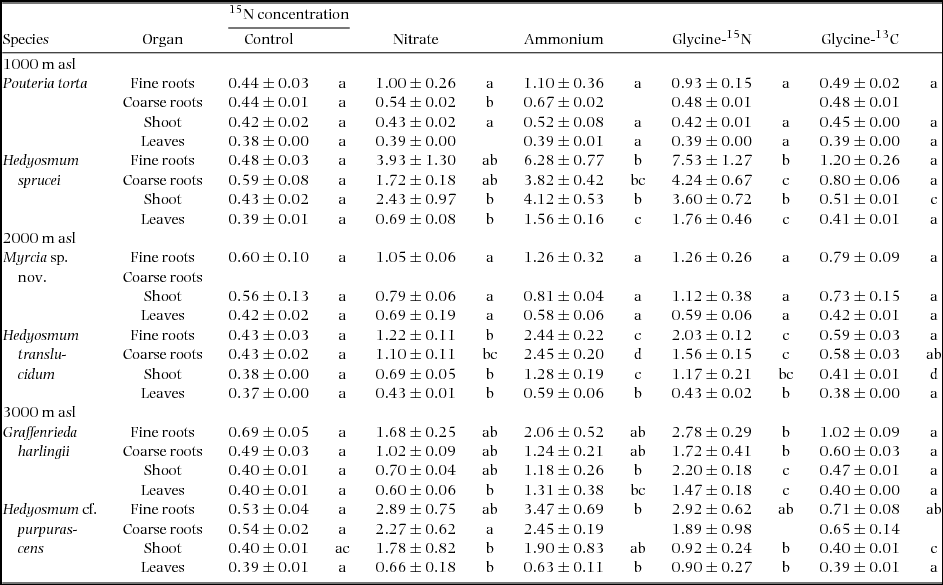
Five days after tracer application, we found a characteristic pattern of tracer enrichment in the plants with generally highest 15N concentrations in fine roots and a decrease in the sequence coarse roots – shoot – leaves in all six species and all three N forms (Table 3). High atom-% 15N values were found in the fine roots of H. sprucei (7.53 atom-%) while leaves typically did not exceed 1 atom-% (except in H. sprucei and G. harlingii). In the two treatments with inorganic N addition (ammonium-nitrate), in general more 15N label was accumulated when NH4+ was labelled as compared with NO3− labelling which indicates higher ammonium uptake when both N forms were equally available (Table 3). However, the difference between the two treatments was only significant in certain species and biomass fractions (H. sprucei: leaves; H. translucidum; fine roots, coarse roots and shoot).
Table 3. 15N concentration (in atom-%) in the saplings of six tree species grown in pots outdoor inside three tropical montane forests in southern Ecuador at 1000, 2000 and 3000 m asl that were harvested 5 d after the application of labelled nitrate, ammonium or glycine (means ± SE, N = 3–5). The 15N enrichment in the glycine treatment is presented either as uncorrected value (glycine-15N) or corrected to the amount of 13C accumulated which may indicate uptake of intact glycine molecules (glycine-13C). Some saplings (such as all Myrcia saplings) had no coarse roots; consequently, some values and statistics are missing. Different letters indicate significant differences between treatments.

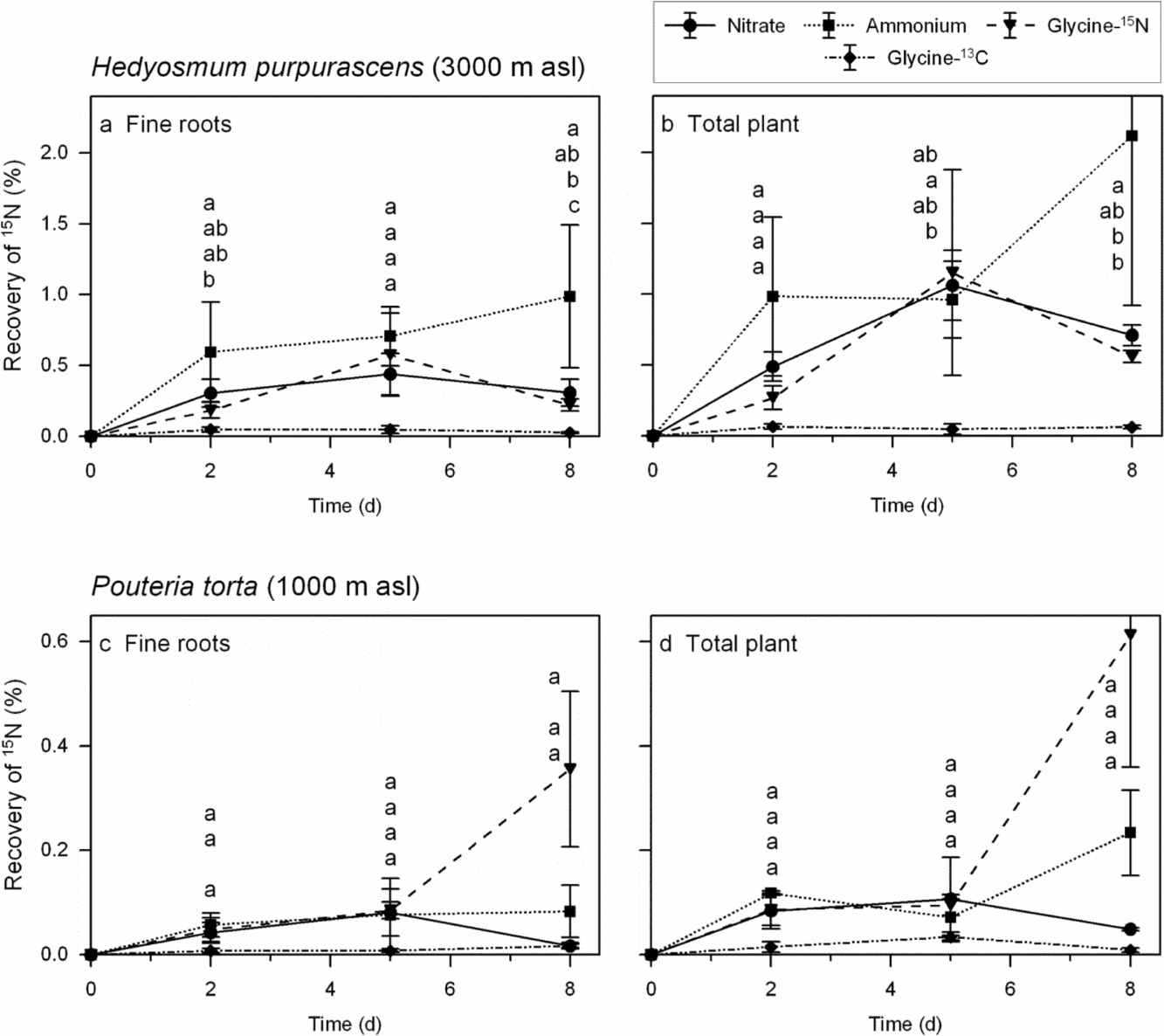
Figure 3 gives the temporal development of 15N accumulation in two species, P. torta at 1000 m asl and H. purpurascens at 3000 m, 2, 5 and 8 d after application. In H. purpurascens at the high-elevation site (3000 m), 15N recovery increased from day 0 to day 2 and further to day 5 (slight increase in fine roots, marked increase in total biomass) in all treatments, and decreased from day 5 onwards (except for the ammonium treatment which showed further increase). In P. torta, the accumulation patterns were in general similar but 15N recovery in the biomass was much lower in this species. In contrast to H. purpurascens, the 15N content in the glycine treatment of the P. torta saplings increased strongly between day 5 and day 8 when calculated as glycine-15N.

Figure 3. Temporal development of the 15N content in the fine-root biomass or total plant biomass of saplings of Hedyosmum purpurascens (3000 m asl, a, b) and Pouteria torta (1000 m asl, c, d) 2, 5 and 8 d after application of labelled fertilizer to the soil. The saplings were cultivated in pots and grown in the natural forest under a closed forest canopy. The 15N enrichment in the glycine treatment is presented either as uncorrected value (glycine-15N) or corrected to the amount of 13C accumulated which may indicate uptake of intact glycine molecules (glycine-13C). Some saplings (such as all Myrcia saplings) had no coarse roots; consequently, some values and statistics are missing. Different letters indicate significant differences between treatments. N = 3–4.
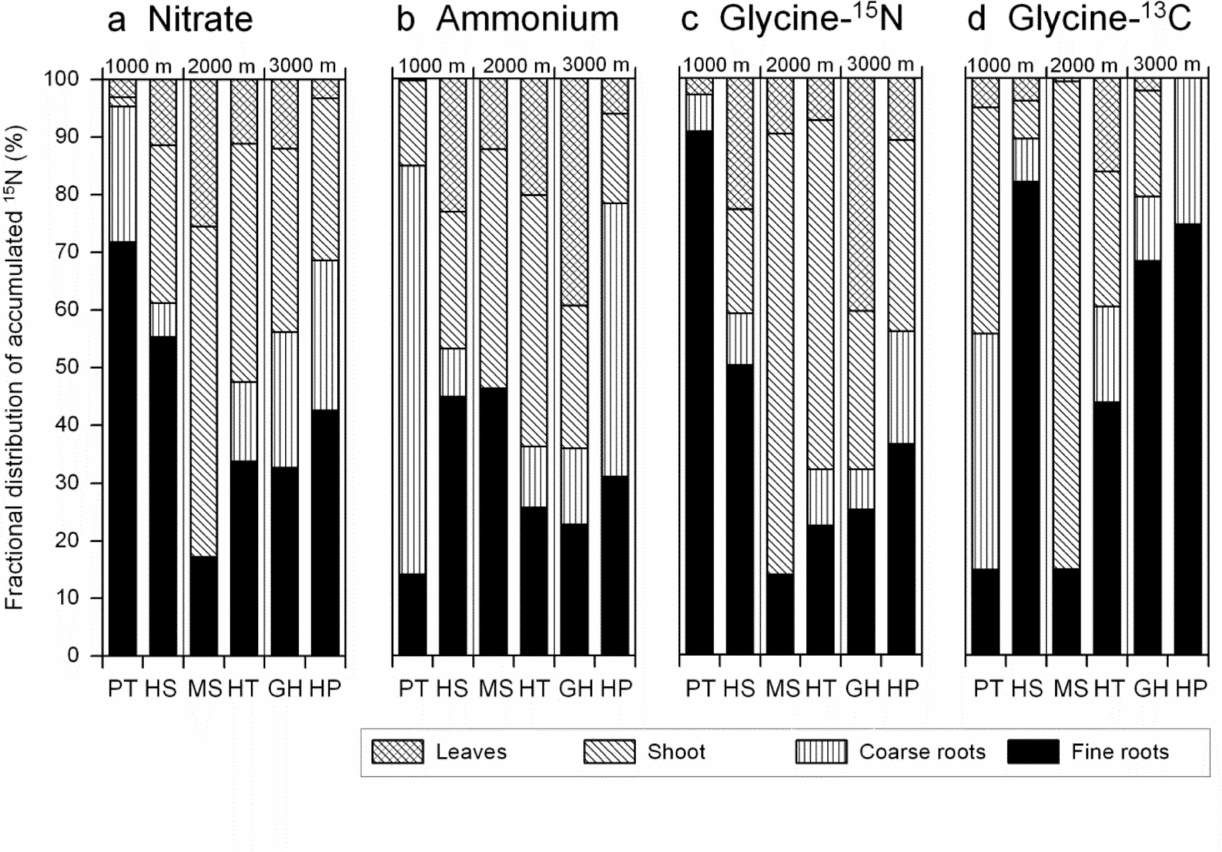
The distribution to different organs of the 15N accumulated in the plants after 5 d revealed a considerable variation in N allocation patterns among the six species and also for the different treatments but no clear altitudinal trend (Figure 4). The species with very low 15N accumulation, P. torta, accumulated a relatively large proportion of the N tracer in the fine or coarse roots (nitrate and glycine-15N vs. ammonium treatment) with 85–95% of the 15N remaining in the below-ground organs. These differences are partly related to species differences in carbon allocation patterns; P. torta and Graffenrieda saplings had a particularly large root biomass (35% of total, Table 4).

Figure 4. Distribution to leaves, shoot, coarse and fine roots of 15N taken up by the plant from labelled nitrate (a), ammonium (b) or glycine solution (in % of total 15N uptake). The 15N enrichment in the glycine treatment is presented either as uncorrected value (glycine-15N, c) or corrected to the amount of 13C accumulated which may indicate uptake of intact glycine molecules (glycine-13C, d). Some individuals (such as all Myrcia seedlings) had no coarse roots; consequently, this category is missing here.
Table 4. Biomass of the saplings at the date of harvest (in g per plant and in % of total biomass). Some individuals (such as all Myrcia seedlings) had no coarse roots; consequently, values are missing.

Glycine incorporation
After adding dual-labelled 15N13C-glycine, much more 15N was accumulated in the biomass than 13C which resulted in the calculation of much higher apparent glycine uptake rates when considering the 15N enrichment (glycine-15N calculation) than when calculating with the accumulation of 13C (glycine-13C approach); the 15N enrichment was often two-fold higher than the corresponding 13C enrichment. The difference between the glycine-15N and glycine-13C values was significant in H. sprucei (all organs), H. translucidum (all organs), G. harlingii (all organs) and H. purpurascens (shoots). An extreme case was the 15N concentration in the root biomass of H. sprucei which exceeded the 13C-concentration more than five-fold (Table 3). In contrast, P. torta reached slightly higher glycine uptake rates according to the 13C approach in the shoots than when calculated through 15N (glycine-15N approach), but all values were very low in this species. All values of apparent glycine uptake according to the 13C approach (glycine-13C) were lower than the 15N content after 15N-nitrate and 15N-ammonium addition. The glycine-13C values were only in a few cases significantly higher than those of the respective control treatment (in H. sprucei, H. translucidum and in H. purpurascens in the shoot). Thus, all three Hedyosmum species exhibited a significantly higher 13C label in one plant organ after addition of dual-labelled glycine. Plotting the 13Cexcess values against the corresponding 15Nexcess values showed much lower slopes (typically <0.4) than expected for the case of complete glycine incorporation as intact molecule (slope = 2.0) (Appendix 1).
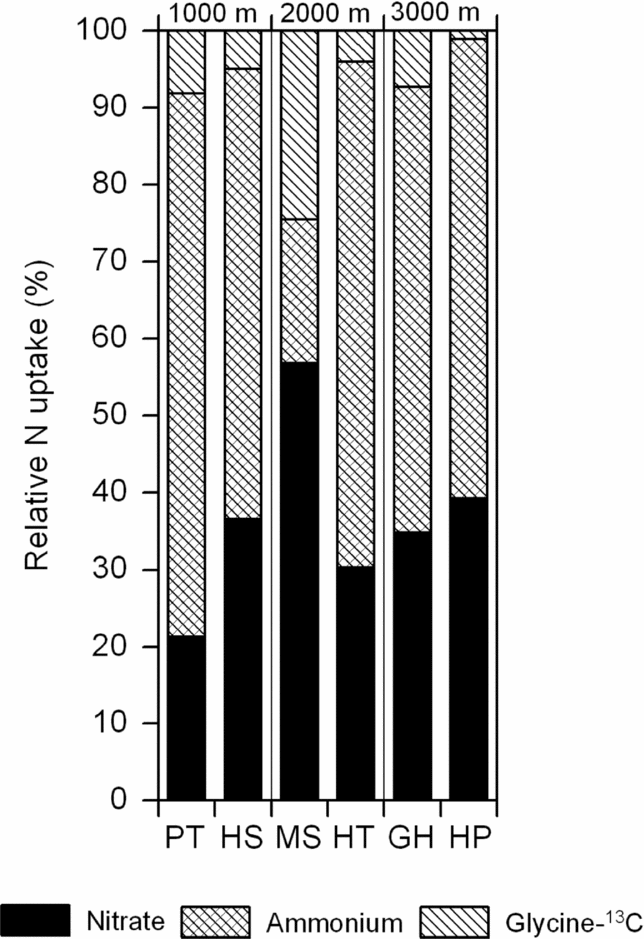
A simple addition of the whole-plant uptake rates from the respective ammonium, nitrate and glycine (glycine-13C approach) experiments may be used for estimating the relative importance of the three N forms for the nitrogen nutrition of the six species, given that all N forms were available at similar abundances (Figure 5). Accordingly, 20–70% would have been taken up as NH4+, 20–50% as NO3− and 5–20% as glycine in the six species.

Figure 5. Relative importance of nitrate, ammonium and glycine (calculated with the glycine-13C calculation approach) in assumed total N uptake of the six species if all N forms were equally available. This calculation is a simple addition of the 15N incorporation data for ammonium, nitrate and glycine and does not consider interactions among the uptake of the three N forms.
Tracer recovery in the biomass
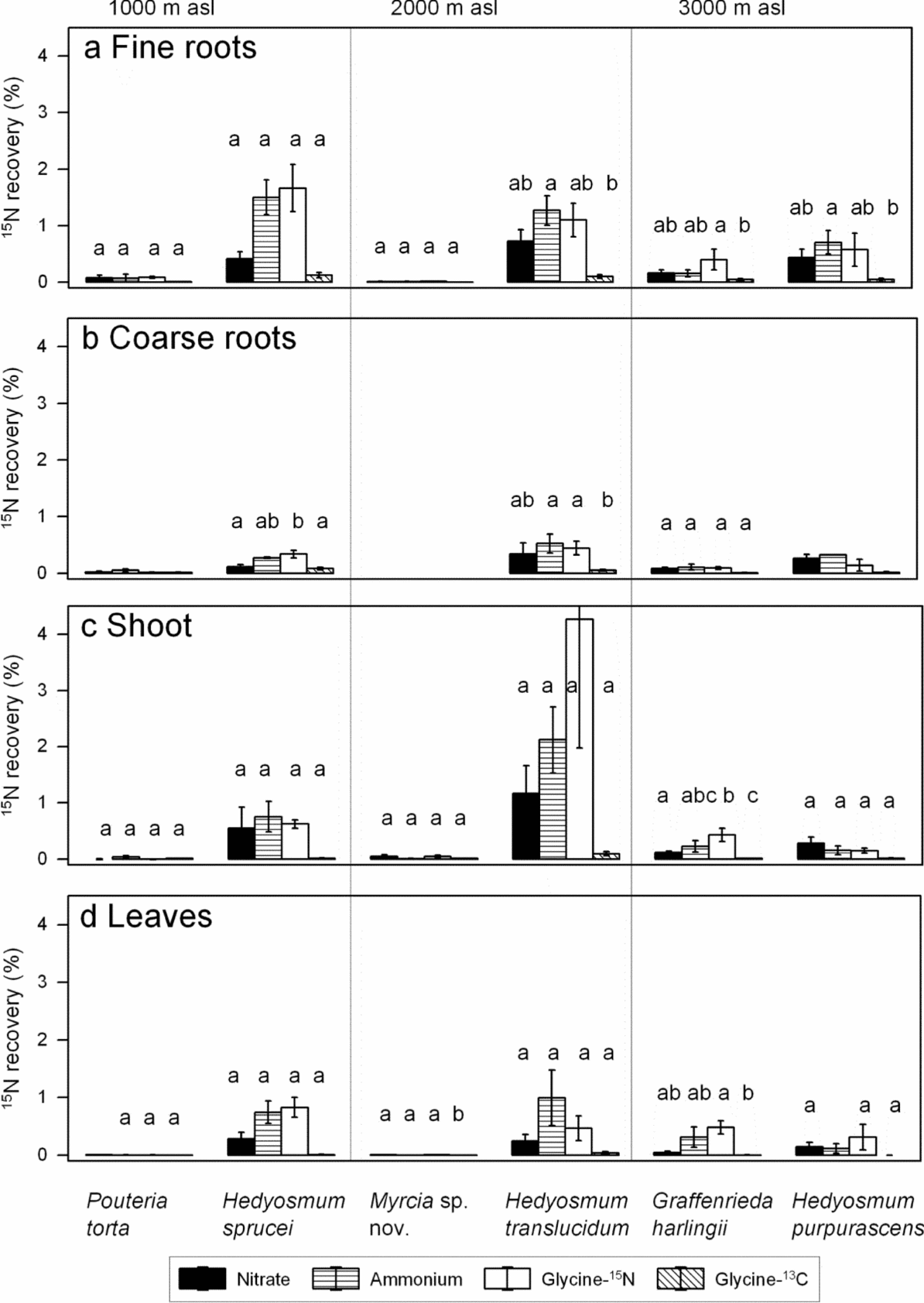
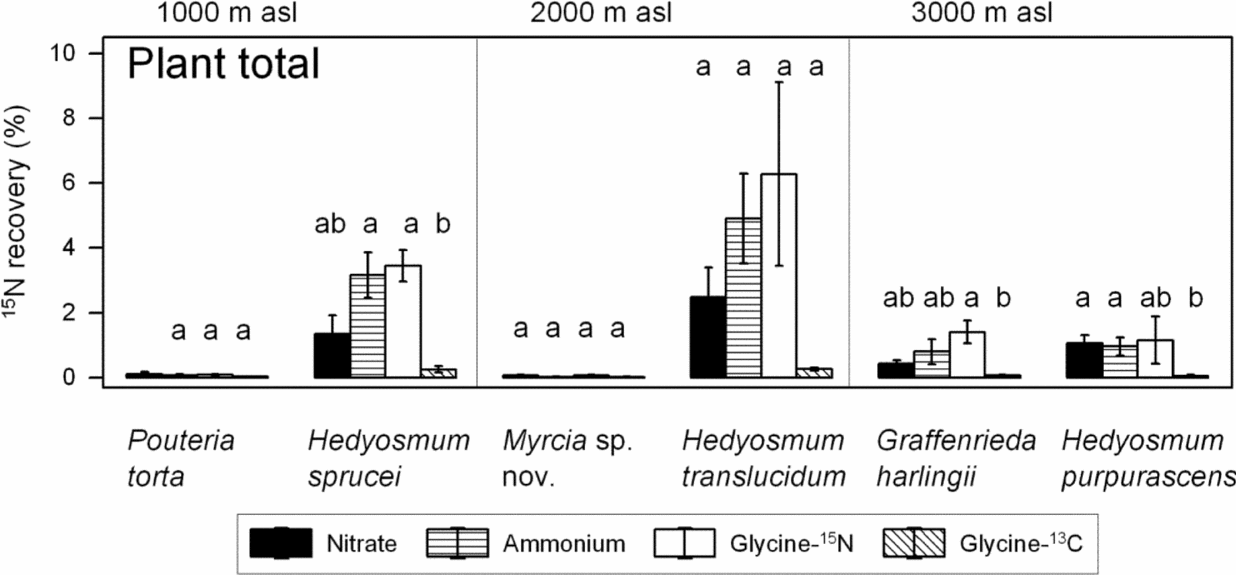
Between 0.02% and 6.28% of the added amount of 15N was recovered in the biomass of the saplings 5 d after application (Figures 6, 7). The total amount of 15N recovered showed no significant preference for either ammonium or nitrate in any of the species. However, NH4+ tended to reach a higher accumulation in the total biomass than nitrate in H. sprucei and H. translucidum and in the fine-root biomass of all three Hedyosmum species. Similar to the 15N atom% values, the mean recovery of 15N in total biomass was always lower in the glycine-13C than the glycine-15N approach (significant in H. sprucei and G. harlingii).

Figure 6. Recovery of 15N in the biomass of saplings in % of the 15N added for the six species in the fine roots (a), coarse roots (b), shoot (c) and leaves (d) 5 d after labelling with 15N-nitrate, 15N-ammonium, or 15N13C-glycine (means ± SE). The 15N enrichment in the glycine treatment is presented either as uncorrected value (glycine-15N) or corrected to the amount of 13C accumulated which may indicate uptake of intact glycine molecules (glycine-13C). Some saplings (all Myrcia plants) had no coarse roots; consequently, some values and statistics are missing. Different letters indicate significant differences between treatments within a species.

Figure 7. Recovery of 15N in the biomass of saplings in % of the 15N added for the six species in the total plant biomass 5 d after labelling with 15N-nitrate, 15N-ammonium or 15N13C-glycine (mean ± SE). The 15N enrichment in the glycine treatment is presented either as uncorrected value (glycine-15N) or corrected to the amount of 13C accumulated which may indicate uptake of intact glycine molecules (glycine-13C). Different letters indicate significant differences between treatments within a species.
DISCUSSION
The altitudinal transect in southern Ecuador is characterized by a large decrease in net N mineralization rate and an even steeper decrease in nitrification rate from 1000 to 3000 m (Wolf et al. Reference WOLF, VELDKAMP, HOMEIER and MARTINSON2011). With mineralization and subsequent nitrification, the upper montane forest receives less than 5% of the NH4+ and less than 1% of the NO3− of the pre-montane forest. At 1000 m, about 80% of the NH4+ released through mineralization is oxidized to NO3−, while it is only c. 10% at 3000 m resulting in increasing dominance of ammonium over nitrate on the cation or anion exchangers in the soil with increasing elevation (c. 80% of the exchangeable mineral N pool at 1000 m and c. 98% at 3000 m consists of NH4+). Data on the concentration of dissolved organic N (DON) show a marked increase from 1000 to 2000 m with growing humus layer thickness. At 2000 m, Goller et al. (Reference GOLLER, WILCKE, FLEISCHBEIN, VALAREZO and ZECH2006) found 50–70% of the soil solution N to be DON and 27–43% NH4+; only 3–5% referred to NO3−. As DON is released from soil organic matter mainly by microbial degradation (Guggenberger et al. Reference GUGGENBERGER, ZECH and SCHULTEN1994, Michalzik et al. Reference MICHALZIK, KALBITZ, PARK, SOLINGER and MATZNER2001, Uselman et al. Reference USELMAN, QUALLS and LILIENFEIN2012), the DON fraction should increase in importance with increasing organic matter content of the soil. Thus, we expected that the relative abundance of organic N compounds and of ammonium both should increase with altitude at the expense of nitrate.
The great dominance of DON and NH4+ over NO3− in the soils at 2000 and at 3000 m is only partly reflected in the N form preference of the investigated tree species. Only one of the four species from 2000 and 3000 m (H. translucidum) took up ammonium more rapidly than nitrate when both N forms were equally available. Another species (G. harlingii) showed a tendency for NH4+ preference but the 15N accumulation from added ammonium was not significantly higher than that from nitrate in any of the organs examined. At 1000 m with a higher abundance of nitrate in the soil, one species (H. sprucei) seemed to prefer NH4+ over NO3−, but the other species showed no difference in the uptake of nitrate and ammonium. Thus, our data from six relatively abundant montane forest tree species indicate that there seem to be species-specific differences in the N form preference but they were not related to the abundance of ammonium and nitrate in the soil and thus apparently independent of altitude. While no species seemed to prefer NO3− over NH4+, we found apparent ammonium preference in a minority of tree species, in particular the species with highest sapling growth rates (unpubl. data). It must be kept in mind that experiments adding different N forms at equal concentrations (as done here) may not reflect actual N form preferences in the stands because the three forms occurred at very different abundances which could influence root uptake kinetics. Nevertheless, it appears that balanced uptake of NH4+ and NO3− seems to be preferred by the majority of species when both N forms are equally available. A similar conclusion was drawn from 15N-uptake experiments in a five-species temperate broad-leaved forest by Jacob & Leuschner (Reference JACOB and LEUSCHNER2014). The existing two N-uptake studies for tropical rain-forest plants reported a higher ammonium than nitrate uptake in three hemiepiphytic Clusia species (Wanek et al. Reference WANEK, ARNDT, HUBER and POPP2002) and no preferences for ammonium or nitrate for understorey palms (Andersen & Turner Reference ANDERSEN and TURNER2013).
Indirect evidence for differences in the use of ammonium or nitrate in tropical forests may be derived from the δ15N signature of foliage and soil. Brearley (Reference BREARLEY2012) concluded that the trees of a montane forest on acidic soil in Jamaica must prefer NH4+ over NO3− due to the isotopic similarity between the leaf and bulk soil signatures. In our transect, the altitudinal decrease in bulk soil δ15N from 1000 to 3000 m matches well with the measured decrease in mineralization and nitrification rates along the slope and the very low nitrate availability at high elevations. However, low foliar δ15N values in the trees at 3000 m in our study should not be mistaken as indication of NH4+ preference; in fact, the uptake experiments showed that nitrate and ammonium were incorporated at roughly similar rates by the two species from this altitude. It should be noted that the soil chemical conditions measured at 1000, 2000 and 3000 m in the stands (Table 1) are not necessarily exactly those established in the pots, even though we used local soil. A possible stimulation of N and P mineralization in the pots and nitrate leaching could have changed soil chemistry.
Our data from a dual-labelling experiment provide some evidence that intact glycine molecules are used as an additional N source by certain montane forest species and that this capability is not restricted to ECM species. According to the 13C incorporation data of the glycine-13C calculation approach, glycine skeletons showed a significant accumulation relative to the control in at least one plant organ in three species (H. sprucei, H. translucidum and H. cf. purpurascens), with all three species having exclusively AM symbionts.
The amount of 15N incorporated in the biomass after feeding with labelled glycine was in most cases similar to the 15N accumulation after adding an equal amount of NH4-N. Adding dual-labelled glycine typically resulted in two- to four-times larger 15N accumulation according to the ‘glycine-15N calculation’ than estimated from the 13C incorporation data. This is reflected by very shallow slopes (typically <0.4) of the regression line 13Cexcess vs. 15Nexcess values in the biomass of the plants (Appendix 1). The low gradients suggest that much, if not all, of the glycine has been deaminated in the soil in the 5 d before harvest and that the glycine-15N was subsequently taken up as NH4+ or NO3−. Our ‘glycine-15N calculation’ should therefore largely overestimate glycine uptake. However, the 13C-based calculation from dual-labelling studies has also been criticized for possible shortcomings such as assumed uptake of labelled inorganic C through the roots (Rasmussen & Kuzyakov Reference RASMUSSEN and KUZYAKOV2009). Therefore, our findings cannot be judged as a proof of the use of intact organic N sources in tropical trees and our results suggest that 15N tracer studies on the uptake of organic N in the tropics using single-labelled glycine, as in the study on understorey palms in a lower montane forest in Panama by Andersen & Turner (Reference ANDERSEN and TURNER2013), might overestimate the actual uptake of intact organic N molecules, if it really occurs at all. While the pathway of glycine-N incorporation by tropical montane-forest tree species is still unclear, our experiment has demonstrated that the large organic N pool in these forests is significantly contributing to plant N supply, be it directly or indirectly after conversion to NH4+.
The decreasing 13C content in the biomass of P. torta after day 5 of the experiment (Figure 3) may relate to respirative C losses. Our data are not comprehensive enough to prove an altitudinal increase in the use of glycine as it was found in the tree species of a temperate mountain by Averill & Finzi (Reference AVERILL and FINZI2011).
One of the factors that could lead to contrasting N uptake rates and differences in N-form preference among the co-occurring tree species of a species-rich tropical forest is phenology. Two species of our sample (P. torta at 1000 m and the unnamed Myrcia species at 2000 m) showed only poor sapling growth in the experiment and the plants accumulated only very small amounts of 15N from the added tracer which made it impossible to detect preferences for certain N forms. Pouteria torta shows leaf flushing in January and February and reduces growth thereafter with presumably reduced N demand. This species and also the Myrcia species are typical late-successional trees with normally slower growth than more light-demanding species. The small fine- and coarse-root systems of the two species may be related to the generally slow growth rates which are a likely explanation of the low N uptake of these species.
Future studies on N uptake patterns in species-rich tropical forests should examine possible relationships between light demand, growth rate, type of mycorrhiza and N uptake capacity and N-form preference among the co-occurring species. Relationships between these traits may only become visible when a much larger number of species is investigated. Further, the study of organic N use should be extended to include other larger and charged amino acid species as well.
Conclusions
Our knowledge about the nitrogen uptake capacity and N form preference of tropical montane forest trees is rudimentary. This study with six tree species provides some of the first information on uptake rates into fine roots and the whole plant under field conditions, on possible preferences for ammonium or nitrate, and on the role of organic N (glycine) for the N nutrition of trees. Despite the large decrease in N supply rate from 1000 to 3000 m asl, we found no indication of an altitudinal shift in N-form preference. Future studies in a larger number of tree species should search for more profound evidence (e.g. through triple-labelling of amino acids) that organic N indeed is playing a significant role in the N nutrition of these forests on humus-rich cool soils, and how uptake rates are dependent on tree functional traits and mycorrhiza type. In addition, this could lead to a better understanding of the importance of phylogeny versus elevation in the N nutrition of tropical trees.
ACKNOWLEDGEMENTS
We thank Katrin Wolf for data on the δ15N signature of tree leaves and Angelica Baldos (both at Department of Tropical Soil Science, University of Göttingen) for data on salt-extractable concentrations of organic N. We are also grateful to AlzChem Trostberg GmbH, Trostberg, Germany for supplying us with dicyandiamide. The authors are indebted to the German Academic Exchange Service (DAAD) for funding part of the field work and the DFG (FOR 816, subproject A2.2) for financial support. Logistic support by the Foundation Nature and Culture International (NCI, San Diego – Loja) is gratefully acknowledged. We thank the Ministerio del Ambiente de Ecuador for the research permit.
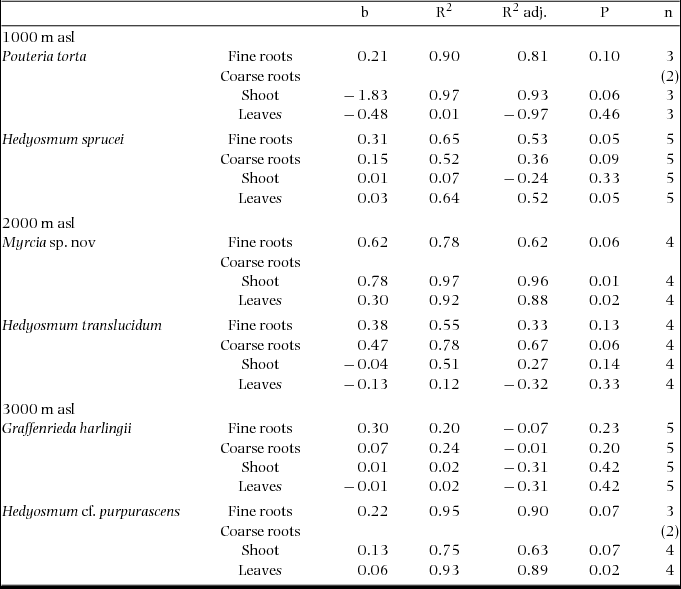
Appendix 1. Slope b, R2, adjusted R2 and P value of the regression of 13Cexcess values (μmol g−1) on the corresponding 15Nexcess values (μmol g−1) in different organs of the six tree species 5 d after the application of dual-labelled glycine. A slope of 2.0 would indicate 100% uptake of glycine-derived N in form of intact molecules. Note that the slope is always much smaller than 2.