The cholesterol regulatory system can control the ratio of cholesterol to phospholipids in cell membranes, which is vital for cell viability and function. This regulatory system regulates the uptake and synthesis of cholesterol, maintaining the cholesterol level in the membrane (Radhakrishnan et al., Reference Radhakrishnan, Ikeda, Kwon, Brown and Goldstein2007). Through this process, cells not only obtain extra cholesterol during rapid growth, but also avoid cholesterol toxicity. Studies have shown that inhibition of cholesterol synthesis can be achieved through blocking the proteolytic activation of sterol regulatory element-binding proteins (SREBPs). This process can be manipulated by the insulin-induced genes (INSIGs). The INSIG family has two members, INSIG1 and INSIG2, which are involved in steroid regulation through SREBP, and exert a significant role in lipid synthesis, cholesterol metabolism and glucose homeostasis (Deng et al., Reference Deng, Pang, Ma, Lu, Duan, Zhu and Liang2016).
Milk fat, mainly composed of triglycerides (TAG), is the major energy source in milk. About half of the milk fat is synthesized de novo in ruminants (Harvatine et al., Reference Harvatine, Boisclair and Bauman2009). The recent studies have revealed a key function of SREBP in the milk fat synthesis of cattle and goats (Bionaz and Loor, Reference Bionaz and Loor2008; Xu et al., Reference Xu, Luo, Zhao, Yang, Tian, Shi and Bionaz2016), and INSIG2 is an important protein that regulates the activation of SREBP. Meanwhile, the overexpression and knockdown of goat INSIG2 gene revealed that INSIG2 exerts a key function in milk fat synthesis (Li et al., Reference Li, Wang, Zhang, He, Shi, Luo and Loor2019). Other data also support the evidence that INSIG2 gene was the most representative marker related to saturated/unsaturated fatty acid ratio in milk (Rincon et al., Reference Rincon, Islas-Trejo, Castillo, Bauman, German and Medrano2012). Therefore, we speculate that INSIG2 also exerts a significant role in the milk fat synthesis of buffalo (Bubalus bubalis). Although the study about this gene on goats can potentially provide a reference for other species of Bovidae, they do belong to different genera and their regulatory mechanisms are different (Bernard et al., Reference Bernard, Toral and Chilliard2017). At present, the role of INSIG2 gene in the synthesis of buffalo milk fat is still unclear. In this research, it is hypothesized that buffalo INSIG2 affects the milk fat synthesis of buffalo. In order to confirm this, we explored the function of buffalo INSIG2 in lipid synthesis in buffalo mammary epithelial cells (BuMECs) through lentivirus-mediated gene overexpression and RNA interference experiments.
Materials and methods
All procedures for sample collection were performed in accordance with the Guide for Animal Care and Use of Experimental Animals approved by the Yunnan Provincial Experimental Animal Management Committee under Contract 2007–0069. In addition to the methodologies described here further details are provided in the online Supplementary File.
Animals and tissue preparation
In order to analyze the expression difference of INSIG2 protein in lactating and non-lactating periods, we obtained a small piece of mammary gland tissue by surgical biopsy as previously reported (Farr et al., Reference Farr, Stelwagen, Cate, Molenaar, Mcfadden and Davis1996) from six healthy adult female Binglangjiang buffalo (about four-year-old) with the same management conditions, of which three were in the peak lactation (60 d postpartum) and the other three were at the dry-off period (60 d before parturition). All samples were washed with phosphate-buffered saline and stored in liquid nitrogen.
Fusion expression vector construction
The pLVX-IRES-ZsGreen1 vector (CLONTECH Laboratories, Inc., USA) was used in overexpression of buffalo INSIG2. The CDS of INSIG2 gene (GenBank no. JX534502) was prepared by PCR, which was capped with XhoI and BamHI sites in the 5′ end. The primers used for CDS cloning are shown in online Supplementary Table S1. Then, it was inserted into the vector to get recombinant pLVX-INSIG2-IRES-ZsGreen1 (Lv-INSIG2).
For the knockdown of the INSIG2 gene, three shRNA sequences with the highest ranking were designed using the BLOCK-iT™ RNAi Designer (https://rnaidesigner.thermofisher.com/rnaiexpress/) based on buffalo INSIG2 sequence (GenBank no. JX534502). Those sequences were synthesized at a commercial facility (Sangon Biotech Co. Ltd., China; online Supplementary Table S2) with the EcoRI and AgeI restriction sites at 5′ end. Then, three shRNA were generated by heat treatment annealing and inserted into the pLKO.1-TRC vector (Addgene, USA) via restriction sites to get recombinant pLKO.1-shRNA vectors. In addition, the CDS sequence of buffalo INSIG2 was subcloned into the pEGFP-N1 vector (CLONTECH Laboratories, Inc.) between XhoI and HandIII sites to generate pEGFP-INSIG2-N1 vector (online Supplementary Table S1). All the recombinant vectors obtained in this study have been verified by sequencing.
Cell culture
The 293 T cells were cultured at 37°C in 5% CO2. The medium was composed of Dulbecco's modified Eagle medium (DMEM) (Gibco, USA) compensated with 10% fetal bovine serum (FBS) (Gibco) and 2% penicillin/streptomycin (Gibco). The BuMECs were isolated from buffalo at peak lactation (60 d postpartum) as previously reported by our group (Fan et al., Reference Fan, Qiu, Teng, Zhang and Miao2020). The isolation process of BuMECs is in the supplementary material. To induce lactogenesis, the BuMECs were cultured with 2 μg/ml prolactin (Sigma) in above medium for 24 h before initial experiments.
Lentivirus generation and transduction
The process of preliminary screening of shRNA sequence is in the supplementary material. The process for generation and transduction was performed as previously reported (Fan et al., Reference Fan, Qiu, Teng, Zhang and Miao2020). Briefly, the 239 T cells were respectively transfected with pLVX-INSIG2-IRES-ZsGreen1 and pLVX-IRES-ZsGreen1 vector (Lv-GFP) for packaging lentiviral particles. Furthermore, the 293 T cells were co-transfected with pLKO.1-shRNA (Lv-shINSIG2), psPAX2 and pMD2.G for packaging of knock-down lentivirus particles. The pLKO.1-TRC was used as a negative control (Lv-shNC). The infection titers of concentrated lentivirus particles were assessed in 293 T cells grown in 96-well plates by serial dilutions. The BuMECs at approximately 80% confluence were treated with lentivirus supernatant. After 52 h of transduction, the cells were harvested for mRNA and TAG analysis.
Quantitative PCR and western blot
RNAiso Plus kit (Takara, Dalian, China) was used to isolate and purify total RNA following the manufacturer's instructions. Specific RT-qPCR primers were designed for the INSIG2 gene and 10 genes involved in milk fat synthesis. After screening and evaluating the stability of gene expression, the geometric mean of the Ct values of the beta-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein S23 (RPS23) were used to normalize the targeted mRNA profiles (online Supplementary Table S3). The amplification was executed in accordance with the manufacturer's protocols of SYBR Green (TaKaRa) on a CFX Connect Real-Time System (Bio-Rad Laboratories Inc., USA).
For Western blot, the mammary tissue of buffalo was lysed through a radioimmunoprecipitation assay lysis buffer (Beyotime, Shanghai, China) supplemented with the Cocktail (TransGen Biotech) for the extraction of total protein following the manufacturer's instruction. Equal amounts of total protein were separated through SDS-PAGE. The band from the gel was then transferred to PVDF membrane (Millipore, USA). Then, the membrane was incubated with the polyclonal rabbit anti-INSIG2 (1:750; Absin, China) and monoclonal mouse anti-ACTB (1:7000; TransGen Biotech). After incubation with the goat anti-rabbit IgG (1:7000; Millipore) and goat anti-mouse IgG (1:7000; Millipore), the signals were visualized using the chemiluminescent ECL Western blot detection system (Pierce, USA).
Cellular TAG assays
Total cellular TAG was determined by a commercial kit (Promega, China) according to the manufacturer's protocols. The concentration of TAG was calculated using the linear regression of the standard curve obtained from on the Microplate Reader (Thermo Fisher Scientific, USA) and was normalized to the protein content assessed with BCA method (Thermo Fisher Scientific) based to the manufacturer's recommended instruction.
Statistical analysis
RT-qPCR assays were carried out by the 2−ΔΔCt method. All the treatments were replicated 3 times and the results were displayed with means ± standard error of the mean (sem). The statistical comparison between the means of two groups was determined via Student's t-test with values of P < 0.05 being considered as significant.
Results
Insig2 expression in buffalo mammary gland
As seen in online Supplementary Fig. S1, the expression profiles of INSIG2 revealed a significant difference between dry-off period and peak lactation in mammary gland tissue. The expression level of INSIG2 in peak lactation decreased by 50.9% (P < 0.05) relative to the dry-off period.
Overexpression of INSIG2 inhibited the mRNA expression of genes related to milk fat synthesis
To determine the effect of INSIG2 on milk fat synthesis in BuMECs, we examined the mRNA abundance of key genes related to regulatory factors (INSIG1, SREBP and PPARG), de novo fatty acid synthesis (FASN, ELOVL6 and SCD), TAG biosynthesis (GPAM, DGAT2 and APGAT6) and lipid droplet formation (TIP47). Relative to the control (Lv-GFP), the mRNA expression of INSIG2 gene increased 47.57 fold in the BuMECs treated with Lv-INSIG2 (Fig. 1a) (P < 0.001). In addition, overexpression of INSIG2 markedly up-regulated the expression of INSIG1 (P < 0.01), while down-regulated the expression of SREBP (P < 0.001), PPARG, FASN (both P < 0.01), ELOVL6, SCD, APGAT6 and TIP47 (all P < 0.05) (Fig. 1b–d). However, the Lv-INSIG2 treatment had no effect on GPAM and DGAT2.

Fig. 1. Effect of INSIG2 overexpression on genes involved in milk fat synthesis in the BuMECs. The BuMECs were transfected with the lentivirus expressed INSIG2 (Lv-INSIG1) or the control (Lv-GFP) and then extracted RNA. The values are displayed with means ± sem originated from 3 individual cultures; *P < 0.05, **P < 0.01, ***P < 0.001.
Preliminary shRNA screening
In order to obtain the most effective shRNA, three shINSIG2 sequences and pEGFP-INSIG2-N1 were respectively co-transfected into 293 T cells. The shRNAs targeting INSIG2 would inhibit the expression of green fluorescence protein (GFP) on the pEGFP-INSIG2-N1. In this way, not only the intracellular GFP intensity sensually was used to evaluate the interference efficiency (online Supplementary Fig. S2), but also the mRNA of INSIG2 gene in the cells was further verified via RT-qPCR (online Supplementary Fig. S3). As shown in online Supplementary Fig. S2, the shRNA3 was more efficient than the shRNA1 and shRNA2 to knock down the expression of INSIG2 in the sense. Judging by the RT-qPCR, the shRNA3 (88.00%) was further confirmed to be more efficient than the shRNA1 (84.78%) and shRNA612 (58.69%) in knocking down the expression of INSIG2 in 293 T cells (online Supplementary Fig. S3).
Inhibition of INSIG2 facilitated the expression of genes associated with milk fat synthesis
In order to achieve a comprehensive understanding of the role of INSIG2 in the BuMECs, we explored the effect of INSIG2 knockdown on the genes associated with milk fat synthesis. According to the results of the above screening, the Lv-shRNA3 (Lv-shINSIG2) was chosen to knock down the expression of INSIG2 in BuMECs. The results indicate that the mRNA abundance of INSIG2 gene decreased by 72.8% (P < 0.01) in the BuMECs incubated with Lv-shINSIG2 (Fig. 2a). The knockdown of INSIG2 gene enhanced the mRNA expression of regulator factor SREBP and PPARG (both P < 0.01) (Fig. 2b). Meanwhile, the Lv-shINSIG2 treatment increased the abundance of all genes measured related to de novo fatty acid synthesis and lipid droplet formation, with 3.70 fold increase for FASN, 2.49 fold increase for ELOVL6, 1.75 fold increase for SCD and 3.77 fold increase for TIP47 (Fig. 2c–d). As for the measured genes related to TAG synthesis, only the expression of APGAT6 was increased. Conversely, INSIG1, GPAM and DGAT2 were not affected by Lv-shINSIG2 treatment.
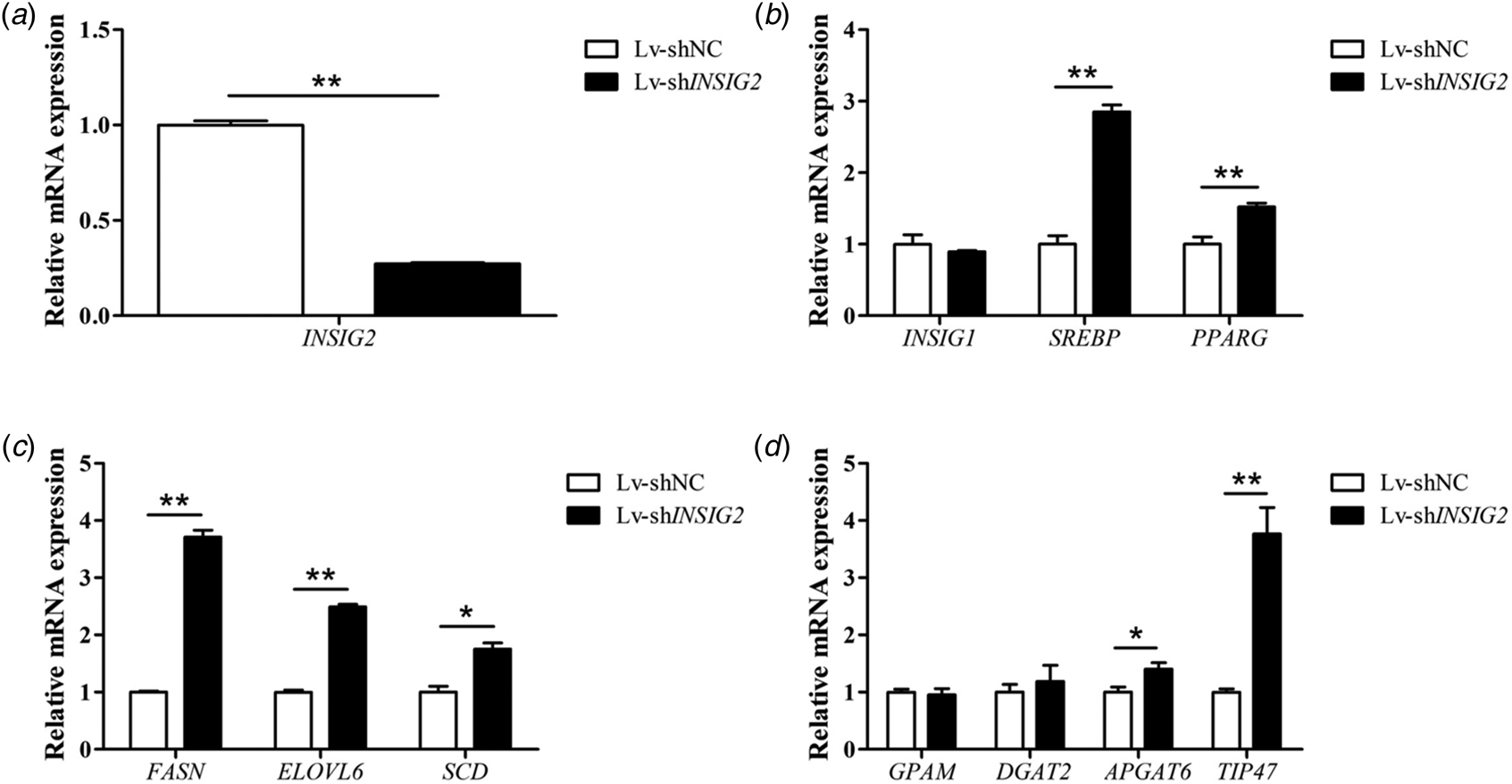
Fig. 2. Influence of INSIG2 knockdown on genes involved in milk fat synthesis. The BuMECs were transfected with the lentivirus contained shRNA targeting INSIG2 (Lv-shINSIG2) or the lentivirus contained negative control (Lv-GFP). The values are displayed with means ± sem originated from 3 individual cultures; *P < 0.05, **P < 0.01.
INSIG2 decreased concentration of cellular TAG in BuMECs
Compared with the control, cellular TAG of the BuMECs treated with Lv-INSIG2 was decreased by 17.4% (P < 0.01) (Fig. 3a). Nevertheless, the quantification of cellular TAG revealed a 1.22 fold increased (P < 0.05) after knockdown of INSIG2 in BuMECs (Fig. 3b).
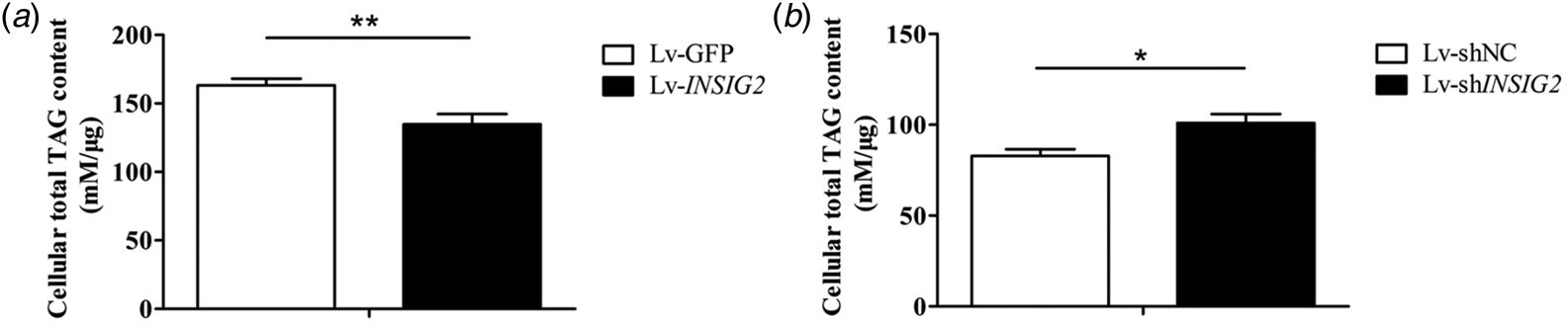
Fig. 3. Insulin-induced gene 2 (INSIG2) suppressed the accumulation of cellular TAG. The BuMECs were transfected with lentivirus expressing INSIG2 (Lv-INSIG2), control (Lv-GFP), lentivirus contained shRNA knocking down INSIG2 (Lv-shINSIG2) and negative control (Lv-shNC), and collected for cellular TAG analysis. The values are displayed with means ± sem from 3 individual cultures; *P < 0.05, **P < 0.01.
Discussion
In view of the importance of SREBP in the network of milk fat synthesis in mammals (Sato, Reference Sato2010), INSIG2 is considered to participate in the regulation of milk fat synthesis. Gene expression profile analysis of 17 human tissues revealed that INSIG2 was ubiquitously expressed (Krapivner et al., Reference Krapivner, Popov, Chernogubova, Hellénius, Fisher, Hamsten and van't Hooft2008). The buffalo INSIG2 was highly expressed in the mammary gland, implying that it is significant in the milk fat metabolism of buffalo (Wu et al., Reference Wu, Liu, Huo, Li, Yuan, Yuan and Miao2014). The expression level of buffalo INSIG2 protein in mammary gland in the period of dry-off was markedly higher than that in peak lactation in this study. The low expression of INSIG2 protein during peak lactation can make more SREBP precursors mature in the Golgi apparatus, and then increase the expression of downstream target genes related to milk fat synthesis, promoting milk fat synthesis. Due to the large amount of milk fat synthesis during the peak of lactation, the results of this study indicate that buffalo INSIG2 is likely to play a vital role in inhibiting milk fat synthesis. However, previous study has shown that compared with the period of dry-off, the mRNA abundance of INSIG2 in peak lactation of dairy goat was markedly increased (Li et al., Reference Li, Wang, Zhang, He, Shi, Luo and Loor2019). This may be due to post transcriptional regulation. In addition, the metabolic mechanisms in the mammary glands of buffalo and goats may also be inconsistent.
When intracellular cholesterol is sufficient, INSIGs bind to SREBP cleavage-activating protein (SCAP) in the form of a INSIGs/SCAP/SREBP complex. Therefore, the SCAP/SREBP complex cannot be transferred to Golgi apparatus. However, when intracellular cholesterol is deficient, the affinity between INSIGs and SCAP decreases, the conformation of SCAP changes, SCAP/SREBP complex dissociates from INSIGs. This enables SCAP/SREBP to move to the Golgi apparatus (Jo et al., Reference Jo, Cha and Moon2017). The N-terminal domain of SREBP is water-soluble and can translocate SREBP into the nucleus (Chen et al., Reference Chen, Hsu, Huang, Goto, Chen and Nakano2017). SREBP binds to sterol regulatory elements on multiple target genes to perform transcriptional regulation and enhance the expression of genes involved in sterol and fatty acid biosynthesis (Rincon et al., Reference Rincon, Islas-Trejo, Castillo, Bauman, German and Medrano2012).
Previous results in mice have revealed that INSIG2 can change the transcription and expression of SREBP through overexpression and knockout of the INSIG2 (Takaishi et al., Reference Takaishi, Duplomb, Wang, Li and Unger2004; McFarlane et al., Reference McFarlane, Liang and Engelking2014). In primary cultures of rat hepatocytes, insulin-induced reduction in INSIG2 protein results in the enhancement of export of SCAP/SREBP complex to the Golgi (Yellaturu et al., Reference Yellaturu, Deng, Park, Raghow and Elam2009). The expression of INSIG2 gene changed significantly after overexpression or interference in BuMECs in this study, which also caused the abundance changes of genes related to milk fat synthesis. The expression of SREBP gene significantly decreased after the overexpression of INSIG2, and increased correspondingly after the knockdown of INSIG2 gene. The results here indicate that the INSIG2 can inhibit SREBP transcription in the BuMECs.
INSIG2 and INSIG1 are members of the same family, and both can inhibit the SREBP processing (Krapivner et al., Reference Krapivner, Popov, Chernogubova, Hellénius, Fisher, Hamsten and van't Hooft2008). Previous findings demonstrated that these two INSIGs have complementary functions in the control of the SREBP activation (Engelking et al., Reference Engelking, Liang, Hammer, Takaishi, Kuriyama, Evers, Li, Horton, Goldstein and Brown2005). The overexpression of INSIG2 decreased the mRNA abundance of INSIG1 in goat mammary epithelial cells (GMECs) (Li et al., Reference Li, Wang, Zhang, He, Shi, Luo and Loor2019). However, the result that the expression of INSIG1 was increased by INSIG2 overexpression in BuMECs was not consistent with those in GMECs. This also reflects the difference in the mechanism of milk fat synthesis between buffalo and goat. Furthermore, the knockdown of INSIG2 gene did not significantly change the expression of INSIG1 gene, which was consistent with the data in goats (Li et al., Reference Li, Wang, Zhang, He, Shi, Luo and Loor2019). INSIG1 gene is the target gene of SREBP, while INSIG2 gene is not regulated by SREBP (Tumanovskaa et al., Reference Tumanovskaa, Swansonb, Serebrovskaa, Portnichenkoa, Goncharova, Kysilova, Moibenkoa and Dosenko2019). The knockdown of buffalo INSIG2 increased the expression of SREBP, and SREBP may further increase the expression of INSIG1, which may be the reason why INSIG1 did not exhibit the expected decrease in expression.
PPARG exerts a key function in fatty acid (FA) metabolism by regulating the expression of its target genes (Shi et al., Reference Shi, Luo, Yao, Zhu, Xu, Shi and Loor2013). Moreover, the overexpression of SREBP markedly up-regulated the mRNA abundance of PPARG, suggesting that this nuclear receptor is the target gene of SREBP (Xu et al., Reference Xu, Luo, Zhao, Yang, Tian, Shi and Bionaz2016). The present data suggest that the mRNA abundance of PPARG was altered by INSIG2, and the change trend of PPARG expression was consistent with that of SREBP expression, indicating that INSIG2 can affect the expression of PPARG by controlling SREBP. In addition, it is speculated that INSIG2 is implicated in process of milk fat synthesis through regulating SREBP and PPARG.
The proteins encoded by FASN, ELOVL6 and SCD exert an important function in de novo fatty acid synthesis in the mammary gland, and they are regulated by SREBP in participating in fatty acid synthesis (Xu et al., Reference Xu, Luo, Zhao, Yang, Tian, Shi and Bionaz2016). In mice, INSIGs were confirmed to have a significant enhancement effect on target genes of SREBP by knockout INSIGs (McFarlane et al., Reference McFarlane, Liang and Engelking2014). In this study, the expression level of SREBP gene changes with the change of INSIG2 gene expression. In addition, after overexpression and knockdown of INSIG2 gene, the mRNA abundance of FASN, ELOVL6 and SCD genes involved in de novo fatty acid synthesis was significantly altered. Given that these three genes are the target genes of SREBP, we speculate that buffalo INSIG2 may participate in the de novo fatty acid synthesis by controlling the expression of SREBP.
The GPAM, DGAT2 and AGPAT6 genes are involved in TAG synthesis (Shi et al., Reference Shi, Wu, Zhu, Zhang, Yao, Luo and Loor2017). GPAM is responsible for the first step of catalytic reaction, AGPAT6 catalyzes the esterification of TAG sn-2 position, and DGAT2 adds a fatty acid acyl-CoA to diacylglycerol to produce TAG (Abeni et al., Reference Abeni, Degano, Calza, Giangiacomo and Pirlo2005). Milk fat is in the form of lipid droplets and is almost completely composed of TAG (Bionaz and Loor, Reference Bionaz and Loor2008). The TIP47 participates in lipid droplets formation in cells (Ren et al., Reference Ren, Wang, Fan, Jia, Zhang, Deng, Deng and Wang2017). Overexpression of buffalo INSIG2 reduced the content of TAG in this study, which was consistent with the finding that overexpression of rat INSIG2 can prevent increase of TAG accumulation (Takaishi et al., Reference Takaishi, Duplomb, Wang, Li and Unger2004). It is suggested that buffalo INSIG2 has a negative effect on milk fat synthesis. This hypothesis was further enhanced by INSIG2 knockdown leading to an increase in TAG content. However, the expression of GPAM and DGAT2 was not affected by the INSIG2 alteration, suggesting that they may also be affected by other regulatory factors.
In conclusion, the protein abundance of INSIG2 in buffalo mammary gland was higher during dry-off period than that at peak lactation. The overexpression or knockdown of INSIG2 lead to changes in the expression of different genes involved in milk fat synthesis and a change of TAG content in BuMECs. These results reveal that INSIG2 gene can negatively affect milk fat synthesis by inhibiting the expression of SREBP gene.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029921000881
Acknowledgments
This research was jointly funded by the National Natural Science Foundation of China (no. 31760659 and no. 31460582) and the Natural Science Foundation Key Project of Yunnan Province, China (no. 2014FA032 and no. 2007C0003Z).





