INTRODUCTION
The ability of parasites to modify the external features or behaviour of their hosts, in order to enhance the parasite's chances of completing its life-cycle has been documented and reviewed by various different authors (Holmes and Bethel, 1972; Dobson, 1988; Hadeler and Freedman, 1989; Moore, 2002; Poulin, 2002). In parasites with a life-cycle that is based on the predator-prey relationship between final and intermediate hosts these modifications tend to increase the susceptibility to predation of infected individuals, by making them less active, less motile and/or more conspicuous (Holmes and Bethel, 1972; Dobson, 1988; Hadeler and Freedman, 1989; Moore, 2002). Small fish that are infected with the parasitic eye-fluke Diplostomum spathaceum have reduced visual acuity and spend a greater proportion of their time feeding in better-illuminated surface water than those that are not infected, and are thus more susceptible to predation by fish-eating birds (which are the fluke's definitive host) (Crowden and Broom, 1980). Brown rats (Rattus norvegicus) that are infected with Toxoplasma gondii are more susceptible to predation by domestic cats because they are more active, less neophobic, do not avoid areas scented with the odour of their predators and may even show a preference for such areas (Macdonald, Mathews and Berdoy, 1999 and references therein). In some isopods infected with spiny-headed worms Acanthocephalus, the parasite impairs the ability of the intermediate host to use its chromatophores as an effective camouflage mechanism and makes them more susceptible to predation by aquatic vertebrates that act as final hosts (Dobson, 1988 and references therein). For Echinococcus granulosus in moose, Joly and Messier (2004) found empirical support for an increase in vulnerability to predation.
In the case of E. multilocularis, there is at present no direct evidence for the occurrence of parasite-increased susceptibility to predation in the intermediate host. Regarding E. multilocularis, at present the evidence that the parasite does actually increase the susceptibility to predation of its intermediate hosts is limited to observations of infected animals in the laboratory. The rapid and progressive proliferation of the metacestode stage can result in a considerably extended abdomen (Romig, personal communication). This suggests that infection will eventually lead to impaired mobility and an increased vulnerability to predation but whether this is significant in nature is unknown. Observations on captive M. arvalis revealed that even in the late stage of infection, no obvious deterioration in the nutritional status was observed and feeding continues normally (Romig, personal communication). This would imply that for M. arvalis, that mainly feed above ground, infected individuals would continue to be active on the surface and with impaired mobility would be more exposed to predation. Muskrats which are heavily parasitized by E. multilocularis or other hepatic cestode larvae can behave abnormally (e.g. slower movements, less aggressive), show impaired reflexes for flight while on land and may even die there (Romig et al. 1999). Thus, although direct evidence is still poor, parasite-increased susceptibility to predation in the intermediate host is certainly plausible in the case of E. multilocularis. As seen in R. norvegicus infected with Toxoplasma gondii (Macdonald et al. 1999), the effects on the intermediate host can be subtle.
Infection with the larval form of the small endoparasitic tapeworm, E. multilocularis is considered the most pathogenic autochthonous parasitic zoonosis in central Europe and is usually lethal if left untreated (Eckert et al. 2001; Romig, 2002; Kern et al. 2003). The life-cycle of E. multilocularis is based on the predator-prey relationship between its final and intermediate hosts and is predominantly sylvatic, involving wild carnivores (mainly foxes) as final hosts and small mammals (mainly voles) as intermediate hosts. Adult worms live in the intestines of the final host and shed eggs with the host's faeces; intermediate hosts are infected by ingesting eggs which then develop to larvae that form cysts in internal organs. Humans are an aberrant host and become infected by ingesting or inhaling eggs. The parasite is widely distributed in the northern hemisphere and while it is unclear whether E. multilocularis has extended its range, a trend towards increased parasite density is apparent for central and parts of western Europe (Romig, 2002). Increasing fox populations in most European countries over the last decades (Artois, 1997; Romig et al. 1999; Vervaeke et al. 2003) and their invasion of urban and suburban settings (Hofer et al. 2000; Deplazes and Eckert, 2001; Giraudoux et al. 2001; Gloor et al. 2001) have raised awareness of scientists and authorities of the public health risk.
Options for control of the sylvatic cycle of E. multilocularis are limited due to difficulties in gaining access to final and intermediate hosts. The management option that is presently under evaluation is the antihelminthic treatment of final hosts by means of baits containing praziquantel (Schelling et al. 1997; Tackmann et al. 2001; Hegglin, Ward and Deplazes, 2003). A mathematical model suggested that, in areas with low prevalence rates in foxes, praziquantel-baiting could eradicate E. multilocularis (Roberts and Aubert, 1995). However, two field trials in Germany succeeded in lowering the prevalence considerably during the treatment period but the parasite was not eliminated and there was a rapid post-control recovery. A spatially explicit model (Hansen et al. 2002, 2003) suggested two biological mechanisms that might explain the swift recovery to pre-control prevalence rates; spatial heterogeneity in the distribution of the parasite and a lower than expected proportion of foxes that acquire immunity. Here we use a simpler (and more general) model to show that increased susceptibility to predation is a third plausible biological mechanism that can undermine baiting campaigns.
MATERIALS AND METHODS
Our model is a compartment model for a homogeneously mixing population of intermediate and final hosts, with a free-living stage of the parasite. Final hosts (the predator) are infected by consuming infectious intermediate hosts (prey) which are infected by ingesting the free-living stage of the parasite (eggs). Susceptible prey that ingest eggs and become infectious are assumed to do so instantaneously. Similarly, predators that consume infectious prey and subsequently become infected are assumed to begin shedding eggs immediately. This implies there are only two classes of individuals, susceptible and infectious. A delay between the moments an individual is infected and the state of being infectious may be included by having an additional infected but not yet infectious class. We investigated such an extended, more realistic model, but since it did not change our conclusions, hence we focus on the simpler model here.
The model includes neither spatial nor seasonal variation in its parameters. Thus we assume the abundance of the final and intermediate host populations are constant. For the intermediate host population this implies that as preys are removed by predators they are quickly replaced by newly recruited individuals. In fact for all sources of mortality in the predator and prey population, individuals are quickly replaced. In making these assumptions we follow the earlier example of Roberts and Aubert (1995).
The process by which susceptible predators become infected may be represented by (see Begon et al. 2002)

where S is the number of susceptible individuals, I is the number of infected individuals, α is the rate of contacts that are of an appropriate type for transmission to occur, p is the probability that the contact will be with infectious material or an infectious individual and v is the probability that transmission actually does occur given such contact. The relevant rate of contacts between susceptible predators and infectious prey is the rate at which intermediate hosts are consumed. If there is no difference in susceptibility to predation between infected and healthy prey then the probability that such a contact is with an infectious animal is equal to the proportion of the prey population that is infectious. However, in the case that the parasite affects the intermediate host such that predation by the final host is more likely, then the likelihood that taken prey are infectious is greater than the frequency of infectious prey. Increased susceptibility to predation is included by raising the chance that individual prey taken by a predator will be infectious. We set the probability that prey taken by predators are infectious equal to the proportion of infectious prey raised to a power 1/c, where c[ges ]1 (Fig. 1). Equation (1) becomes

The rate of contacts between healthy prey and the eggs of the parasite, deposited by infected predators, is assumed to depend linearly on the density of eggs.

Fig. 1. Increased susceptibility to predation was included in the model by increasing the chance that prey taken by predators are infectious above the frequency of infection in the prey population. In the absence of any relevant effect of the parasite, the chance that captured prey is infectious is exactly equal to the frequency of infection in the prey population (c=1). With increasing values of c the effect of increased susceptibility is increasingly pronounced. With c=1·5 and prevalence of infection in the prey population is 0·05, the chances that captured prey are infectious is 0·14. This probability increases to 0·5 when c=5 reflecting the idea that any infectious prey are rapidly taken by predators.
This set of assumptions produces a model that can be reduced to 3 differential equations;

Here the equations represent the proportion of the predator population that is infectious (I), the abundance of eggs in the environment (E), and the proportion of prey that is infectious (Q). Susceptible predators become infected at the rate given by equation (2) (where S is replaced by 1−I and p is replaced by Q) and infected predators recover at a per capita rate R and die at a per capita rate τ. Eggs enter the environment at a rate determined by a shedding rate (k) and the number of infected individuals (the product NI). Eggs also lose their viability at some rate μ. Finally the proportion of prey that is infectious increases as susceptible prey ingest eggs (with transmission coefficient γ), decreases as infectious prey are consumed (at a rate

where N/K represents the abundance of predators relative to the abundance of prey) and decreases as infectious prey die of the infection (at a per capita rate m).
As a model for the sylvatic life-cycle of E. multilocularis our model is not dissimilar to that of Roberts and Aubert (1995) though there are several noteworthy differences: we explicitly model the density of eggs in the environment, we do not include an infected but not yet infectious class of individuals for either the final or intermediate hosts, and there is a parameter allowing increased susceptibility of infectious prey to predation (c). Parameter values were obtained from the literature on the sylvatic life-cycle of E. multilocularis in Europe.
Parameter values
(1) Predation rate (α=3 rodents per fox per day)
Extremes of 60 rodents eaten per week per fox have been reported (Hansen et al. 2002), but we use a realistic mean predation rate of approximately 3 rodents per fox per day (Schmitt et al. 1997).
(2) Probability of transmission to foxes (ν=0·1)
Experimental infections have shown that foxes are highly susceptible to E. multilocularis: after experimental application of a sufficient quantity of infectious tissue (considered adequate to produce numerous cestodes in susceptible animals) all animals acquired the infection (Eckert et al. 2001; Kapel et al. 2006). This suggests a value of 1 but transmission in nature is likely to be less efficient. For example the role of immune responses of foxes against E. multilocularis remains unclear and the infectiousness of intermediate hosts will vary. We therefore take 0·1.
(3) Turnover rate (τ=0·003 per day)
There are few published estimates of turnover in fox populations in Europe. Lloyd et al. (1976) noted a total annual population turnover that is about two-thirds (64%) of the entire post-breeding fox-population. This implies a daily rate of 0·003.
(4) Life-expectancy of adult worms (R=1/150=0·0067)
The survival time of E. multilocularis in final hosts is estimated to be about 5 months (Deplazes and Eckert, 2001).
(5) Shedding rate (k=10000 eggs per fox per day)
The amount of eggs shed by an individual fox depends on its worm burden and the shedding rate of the adult worms. In Europe the distribution of the adult stage of E. multilocularis in foxes is overdispersed (Hofer et al. 2000; Raoul et al. 2001) with most foxes carrying less than 1000 cestodes (Eckert et al. 2001). Surveys indicate that the mean number of eggs per proglottid of the mature parasite isolated from dogs or foxes is approximately 300, and the number of proglottids produced per worm per day is in the range 0·08 to 0·14 (Eckert et al. 2001 and references therein). This implies that a fox infected with 1000 mature E. multilocularis worms could theoretically excrete 80 to 140 proglottids per day corresponding to approximately 24000 to 42000 eggs. As an order of magnitude, we use a rate of 10000 eggs per fox per day.
(6) Egg decay rate (μ=0·013 per day)
The eggs of E. multilocularis are highly resistant with egg survival depending on microclimate and on the properties of the soil. Under natural climatic conditions in south western Germany, E. multilocularis eggs were frequently observed to remain viable for more than 100 days (Veit et al. 1995) but if conditions are unfavourable (high temperatures, low humidity) then the eggs can lose their viability within hours after being shed. The maximal survival time was 240 days during autumn and winter and 78 days in summer. Hansen et al. (2003) assumed that eggs lose their infectivity already after 1 week. We present results for a range of decay rates that include values as high as that used by Hansen et al. (2003) and as low as the extremes observed by Veit et al. (1995).
(7) Mortality of infected rodents (m=0·01 per day)
Most rodent species have a short life expectation, becoming mature in 1 or 2 months and having a life-span that rarely exceeds 1 year. Relevant field data about the mortality rates of rodents infected with E. multilocularis are lacking. We assume here that an infected rodent will survive approximately 100 days but alternative values are briefly explored.
(8) Rodent density (K=100 rodents/ha)
The population densities of Microtus arvalis and Arvicola terrestris are thought to vary over up to 4 orders of magnitude with both seasonal and multiannual fluctuations (Petavy and Deblock, 1983; Giraudoux, personal communication). Delattre et al. (1988) have suggested that cohabitation of different rodent species that fluctuate asynchronously facilitates a more or less constant presence of infectious intermediate hosts. We use 100 rodents/ha as an order of magnitude estimate.
(9) Fox density (N=0·012 foxes/ha)
The density of fox populations fluctuates from year to year and population dynamics are thought to be heavily influenced by dispersal (Lloyd et al. 1976; Eckert et al. 2001). For European fox populations, Lloyd et al. (1976) noted a range of densities in spring, when they are highest, between 0·5 and 1·8 adults per km2 (mean=0·012/ha).
(10) Transmission rate of rodents (γ=1×10−7).
This parameter is the product of the contact rate between infectious eggs and rodents, and the likelihood that a rodent is infected given contact has occurred. Neither is easy to measure in the field. Here the parameter was set to an order of magnitude which produced (model) prevalences for the final and intermediate host populations that were comparable with those observed in wildlife populations. The meaning of the parameter is better understood by noting that γE is the rate at which voles become infected. With an average egg density of 5000 eggs per hectare (see Figs 2–4) then a rate of 0·0005 may be interpreted as 1 rodent per 20 hectares becomes infected per day (assuming a naïve rodent population and a density of 100 per hectare).

Fig. 2. Model results with the parameter values for Echinococcus multilocularis showing the dependence on final host density with and without increased susceptibility to predation in the intermediate host. Prevalence in rodent and fox populations are long-term equilibrium values, as are those shown for egg density. All suggest that the parasite is able to persist at low final host densities if it can increase the chances of infected rodents being taken by foxes. At low fox densities the equilibrium values are 0 for (a) but not for (b) to (d) though they eventually become unrealistically small.

Fig. 3. Model results with the parameter values for Echinococcus multilocularis. Sensitivity to the turnover rate of infected foxes differs markedly for different values of c (i.e. with and without increased susceptibility to predation in the intermediate host). The turnover rate is determined by the natural death rate of the final host and the life-expectancy of adult worms. Prevalence in rodent and fox populations are long-term equilibrium values, as are those shown for egg density.

Fig. 4. Model results (parameter values for Echinococcus multilocularis) showing the sensitivity of the parasite to the decay rate of the free-living stage of the parasite, with and without increased susceptibility to predation in the intermediate host. Prevalence in rodent and fox populations are long-term equilibrium values, as are those shown for egg density.
RESULTS
We denote the equilibrium solutions of (3) by (I*, E*, Q*). These are the values of prevalence in the predator population, density of eggs and prevalence in the prey population that the system may settle on after a period of time. Apart from the zero equilibrium, one that represents the disappearance of the parasite, there is a second possible equilibrium given by
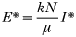

and Q* satisfying

We do not formally derive the stability properties of this solution but all of the simulations shown (and numerous others) suggest that when it exists, it is stable and the system converges to this equilibrium.
Since the right-hand side of equation (6) must increase with Q* and the left-hand side must decrease, then positive solutions for Q* are only possible if
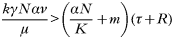
when c=1, and

when c>1. The difference between (7) and (8) is that the latter condition does not depend on the density of foxes. This implies that when increased susceptibility is a feature of the parasite, the infection will persist even at very low predator densities. The response of the parasite to changes in predator abundance, the rate at which the parasite is cleared from infected predators (either naturally or due to baiting with a helminthicide) and egg survival is shown in Figs 2, 3 and 4 with different values of the parameter c. They all illustrate a qualitative shift in the ability of the parasite to persist when it is under pressure from either a lack of final hosts, a high turnover in the final host, or adverse survival conditions for the free-living stage of the parasite. Without increased susceptibility (c=1) there are values for predator density, turnover and egg survival rates that predict extinction of the parasite. With increased susceptibility (c>1) the prevalence rates of the parasite in both intermediate and final hosts become relatively insensitive to changes in egg survival or predator density or predator turnover. This qualitative shift is also seen in an extended model that includes an infected but not yet infectious class of rodents (results not shown).
The model does not include seasonal variation in any of the parameters. For the sylvatic cycle of E. multilocularis in central and western Europe there are seasonal differences in rodent abundance, fox abundance and egg survival. With respect to the management issues we tackle in this paper, the importance of seasonality is that there may be a time of the year for which there are particularly adverse conditions for transmission of the parasite; for example, particularly low survival of E. multilocularis eggs combined with low fox abundance. The results of our modelling suggest that increased susceptibility to predation in the intermediate host will help the parasite maintain itself during periods of adverse conditions, i.e. Fig. 4 shows that with increased susceptibility to predation the parasite is robust to changes in egg survival. Because this is true under permanently adverse conditions in our model, it should also hold when such conditions are only seasonal. Thus, including seasonality in model parameters should not change the conclusions about the effects of increased susceptibility to predation.
DISCUSSION
With a simple mathematical model for the dynamics of a parasite such as E. multilocularis, that relies on its final host consuming infected intermediate hosts, we have established that increased susceptibility to predation is expected to enhance parasite persistence.
This finding may have significant effects on control programmes, such as antihelminthic treatment of foxes. In central Europe there have been 3 field trials of antihelminthic baiting to control E. multilocularis infections in final hosts (Schelling et al. 1997; Tackmann et al. 2001; Hegglin et al. 2003). In northern Germany, aerial application of praziquantel-containing baits resulted in a distinct reduction in the prevalence of E. multilocularis but only during the periods of actual control (27 months) and prevalence increased rapidly afterwards to return to pre-control levels (Tackmann et al. 2001). Hansen et al. (2002) developed a spatially explicit simulation model to predict how the host-parasite system would react to control. They showed that spatial heterogeneity in egg survival can greatly reduce the effectiveness of a baiting campaign. Hansen et al. (2003) added a lower proportion of foxes that acquired immunity due to a lower infection pressure under control, as a second explanation for the unexpected rapid return to pre-control levels. If increased susceptibility is a feature of E. multilocularis then this is an additional explanation for the disappointing rapid post-control recovery of the parasite.
There are currently no practical methods available for direct large-scale treatment of E. multilocularis eggs in the field that would render them sterile. However, such a method seems attractive since humans may become infected by consumption or inhalation of viable eggs (Eckert et al. 2001; Kern et al. 2003). Our results indicate that although the effect on egg density is always strongly non-linear, increased susceptibility again reduces the impact of that control option.
Another management option is to reduce the abundance of foxes. In some endemic areas an increase in fox density was accompanied by an increase in E. multilocularis prevalence (Romig et al. 1999; Romig, 2002), while a survey in Germany (Tackmann et al. 1998) found no major fluctuations in prevalence despite a considerable increase in fox density. Raoul et al. (2003) found a complex dependence between fox abundance and E. multilocularis presence in fox faeces, with the lowest level of infection 1 year after the decline in fox density, suggesting that a reduction in population density of foxes in a high endemic area could reduce the contamination of the environment with eggs of E. multilocularis. Our results show that with and without increased susceptibility, removal of foxes has a direct and almost linear effect on the density of eggs. Fox removal may also increase the turnover of the predator population which would place additional pressure on the parasite. However, the direct effect of reducing the density of foxes is sensitive to whether or not increased susceptibility occurs. Without increased susceptibility there is a density of the final host below which the parasite cannot persist. Such a density threshold is not unexpected since such thresholds are a common outcome of host-pathogen models for wildlife disease (Hudson et al. 2002). However, when increased susceptibility is included then there is no threshold, implying the parasite should persist even at extremely low predator densities. In such circumstances the benefits of a fox removal campaign for the management of E. multilocularis will be reduced and only ever temporary.
Our results suggest that increased susceptibility to predation will enable persistence of a parasite under control programmes that would otherwise lead to its (local) extinction. This effect seems to require only a slight increase in susceptibility to predation. Empirical support for an increase in vulnerability to predation in the field is difficult to obtain though Joly and Messier (2004) were able to do so for E. granulosus in moose. We suggest similar attempts are made for E. multilocularis. The implications for management of a zoonosis such as E. multilocularis are simple; if increased susceptibility is a feature of the parasite then management will be undermined and eradication of the parasite from a particular region extremely difficult.
This research was possible thanks to a grant from F. W. O. Vlaanderen to Muriel Vervaeke. The authors wish to thank Thomas Romig (Universität Hohenheim, Germany) for his useful advice concerning increased susceptibility to predation for E. multilocularis and also Kurt Jordaens for his constructive comments.






