INTRODUCTION
Because terrestrial small mammals are formidable predators and dispersers of seeds they may have important consequences for plant populations, secondary succession, and species composition and coexistence (Crawley Reference CRAWLEY1983, Janzen Reference JANZEN1970, Reference JANZEN1971; Harper Reference HARPER1977, Maron & Crone Reference MARON and CRONE2006, Vander Wall Reference VANDER WALL2010). Unlike for seeds, however, far fewer studies have investigated post-establishment seedling mortality from small mammals (Alvarez-Clare & Kitajima Reference ALVAREZ-CLARE and KITAJIMA2009, Osunkoya et al. Reference OSUNKOYA, ASH, GRAHAM and HOPKINS1993, Paine & Beck Reference PAINE and BECK2007, Sork Reference SORK1987, Theimer et al. Reference THEIMER, GEHRING, GREEN and CONNELL2011), especially in forests of equatorial Africa (Clark et al. Reference CLARK, POULSEN and LEVEY2012, Hall Reference HALL2008, Norghauer & Newbery Reference NORGHAUER and NEWBERY2011, Struhsaker Reference STRUHSAKER1997) where more mammalian biomass is ground-dwelling than in the Neotropics (Malcolm Reference MALCOLM, Lowman and Rinker2004).
Of the small mammals, the rodents (Rodentia), have continuously growing incisor teeth that must be worn down by gnawing. Long ago, Watt (Reference WATT1919) thought this was why 18% of British oak seedlings and saplings had their stems severed or ‘cut off’ by mice, although beech stems were less susceptible (Watt Reference WATT1923). That gnawing of seedlings by voles and mice differs starkly among temperate tree species is now appreciated (Ida & Nakagoshi Reference IDA and NAKAGOSHI1996, Ostfeld & Canham Reference OSTFELD and CANHAM1993, Pigott Reference PIGOTT1985, Sato Reference SATO2000). Yet this ‘stem-cutting’, essentially a lethal form of browsing, remains unstudied in primary tropical forest except for some large mammals (e.g. pigs, Ickes et al. Reference ICKES, PACIOREK and THOMAS2005).
Stem-cutting begets two pertinent and intriguing considerations. First, that co-occurring tree species are not equally vulnerable to it suggests that it could be non-random and selective. If so, interspecific differences in stem quality from physical and chemical traits in their wood or bark may deter, or attract, such attacks (Gill Reference GILL1992). These traits might include stem density and fibre content (Alvarez-Clares & Kitajima Reference ALVAREZ-CLARE and KITAJIMA2009, Bozinovic et al. Reference BOZINOVIC, NOVOA and SABAT1997), nutrients and minerals (Hansson Reference HANSSON1991, Hjältén & Palo Reference HJÄLTÉN and PALO1992), secondary plant metabolites (Bryant et al. Reference BRYANT, PROVENZA, PASTOR, REICHARDT, CLAUSEN and DUTOIT1991, Freeland & Janzen Reference FREELAND and JANZEN1974, Lee et al. Reference LEE, LEE and AHN1999), or even aromatic organic volatiles (Bedoya-Perez et al. Reference BEDOYA-PEREZ, ISLER, BANKS and MCARTHUR2014) cued upon by individuals especially active at night, namely rodents (Howard et al. Reference HOWARD, MARSH and COLE1968, Vander Wall Reference VANDER WALL1998).
The second consideration is the habitat use of small mammals (Gill Reference GILL1992), which can remove seeds faster in canopy ‘gaps’ created by treefalls than in the more closed-canopy areas of tropical rain forest (Norghauer & Newbery Reference NORGHAUER and NEWBERY2011, Norghauer et al. Reference NORGHAUER, MALCOLM, ZIMMERMAN and FELFILI2006, Schupp Reference SCHUPP1988). Indeed, liana tangles and dense vegetation of gaps may protect some rodent species from their predators to increase their fitness and abundance (Beck et al. Reference BECK, GAINES, HINES and NICHOLS2004, Lambert et al. Reference LAMBERT, MALCOLM and ZIMMERMAN2006, Malcolm Reference MALCOLM, Lowman and Nadkarni1995, Malcolm & Ray Reference MALCOLM and RAY2000), while the greater light there accelerates the recruitment of tree species across the shade-tolerance spectrum (Denslow Reference DENSLOW1987, Rüger et al. Reference RÜGER, HUTH, HUBBELL and CONDIT2009) which is critical for understanding their regeneration dynamics and life-history strategies (Connell Reference CONNELL1989, Hartshorn Reference HARTSHORN, Tomlinson and Zimmermann1978).
This paper compares stem-cutting predation of two dominant, masting tree species that differ markedly in their population structures and shade-tolerance as seedlings. The incidence of stem-cutting in 40 gap-understorey areas and the species stem tissue traits were investigated to test three hypotheses: (1) Seedlings of each species are not equally prone to stem-cutting. (2) Such attacks are more common in gaps than nearby forest understorey. (3) The two species differ in key wood and bark traits, which might influence their vulnerability to stem-cutting by small mammals.
METHODS
Study site
The field research was done in lowland primary rain forest at Korup National Park, Cameroon, in and around the 82.5-ha permanent ‘P-plot’ (5°1’N, 8°5’E; 125 m asl) that is situated within a large grove of the canopy-emergent tree, Microberlinia bisulcata A. Chev. (Newbery et al. Reference NEWBERY, SONGWE, CHUYONG, Newbery, Prins and Brown1998, Reference NEWBERY, VAN DER BURGT, WORBES and CHUYONG2013). Here the soils are quite sandy and resource-poor, and especially deficient in P and K nutrients (Newbery et al. Reference NEWBERY, ALEXANDER and ROTHER1997). Annual rainfall is heavy, at c. 5100 mm, coming almost entirely during a 9-mo wet season (March–November; Newbery et al. Reference NEWBERY, CHUYONG and ZIMMERMANN2006). Korup reportedly has 47 species of rodent; the giant pouched rat (Cricetomys emini Wroughton) is confirmed as present in the park and region (Norghauer pers. obs.; Fa et al. Reference FA, SEYMOUR, DUPAIN, AMIN, ALBRECHTSEN and MACDONALD2006).
Natural history
The population of M. bisulcata at the site is mixed with that of another dominant canopy-emergent species, Tetraberlinia bifoliolata (Harms) Haumann (Newbery et al. Reference NEWBERY, VAN DER BURGT, WORBES and CHUYONG2013). However, the adult trees of T. bifoliolata are smaller than those of M. bisulcata (≈125 cm vs. ≈220 cm maximum stem diameters), because they grow slower but also die faster than M. bisulcata, with seeds of both species ballistically dispersed during masting events (Norghauer & Newbery Reference NORGHAUER and NEWBERY2015). Early in development, however, the seedlings and saplings of M. bisulcata are much less tolerant of shade, yet can grow faster but also die faster than those of T. bifoliolata (Green & Newbery Reference GREEN and NEWBERY2001, Norghauer & Newbery Reference NORGHAUER and NEWBERY2013, Norghauer et al. Reference NORGHAUER and NEWBERY2014). For both species, seed losses to small mammals are more likely in gap areas than in more closed-canopy areas of the forest (Norghauer & Newbery Reference NORGHAUER and NEWBERY2011). And without the extra light provided by gap areas, new seedlings of either species scarcely increase in height following establishment (Green & Newbery Reference GREEN and NEWBERY2001, Norghauer & Newbery Reference NORGHAUER and NEWBERY2013). That animals occasionally cut down M. bisulcata seedlings is known (Green & Newbery Reference GREEN and NEWBERY2002). Signs of such attacks are conspicuous (Figure 1): stems are cut near the ground floor, at ≈5–10 cm height, usually at an angle (Figure 1a, c) with teeth marks evident on thicker stems (Figure 1b–d). For unknown reasons part of the stem is often removed and occasionally found shredded a few metres away (Figure 1b) (Norghauer pers. obs.).

Figure 1. Natural history photos of Microberlinia bisulcata seedlings established in gap areas that were damaged (encircled) by small mammals in rain forest at Korup, Cameroon. Shown are three stems cut (a); a stem that was cut and shredded thereafter (b); a stem cut but also partly chewed about the cutting point (c); a close-up of the characteristic angled cut made to a stem, with teeth impressions slightly visible, from small-mammal predators (d) (likely rodents (Muridae)).
Field data on seedling predation
Incidence of stem-cutting was systematically recorded as part of a large insect herbivore exclusion experiment. This experiment applied fine-mesh cages and control tops to individual, new seedlings that had naturally established in 40 paired gap and understorey areas after the 2007 masting event (applied between 13 December 2007 to 13 January 2008; see Norghauer & Newbery Reference NORGHAUER and NEWBERY2013, Reference NORGHAUER and NEWBERY2014). The initial sample of M. bisulcata consisted of 97 seedlings per control and caged treatment; the sample size was smaller, at 46 seedlings per treatment, for the more dispersal-limited and less-fecund T. bifoliolata (Norghauer & Newbery Reference NORGHAUER and NEWBERY2015). All these seedlings were re-censused three times over c. 22 mo for their growth and mortality (9–14 November 2008, 11–15 March 2009 and 4–10 October 2009: corresponding to the first full wet, second dry and second wet season, respectively). The key response variable here is whether or not a control seedling had its stem cut during this monitoring period (none was cut when caged). This subset of data also included ‘replacements’: same-aged, nearby wild seedlings added to the sample in the first or second re-census to replace control ones that had died (if possible) (see Norghauer & Newbery Reference NORGHAUER and NEWBERY2014).
Collection and preparation of stem tissues from a separate sample of seedlings
It is illegal to destructively harvest wild juvenile stems within Korup. However, in a parallel seed addition experiment, both study species had been dispersed artificially into gaps outside the main M. bisulcata grove, and these now large seedlings had to be eventually killed and removed. Hence, in the process, their bark and wood tissues were collected (full details in Appendix 1).
Stem tissue density
This trait is defined as the mass of a woody stem with its bark dried at 70°C for 72 h, divided by its ‘fresh volume’ (Pérez-Harguindeguy et al. Reference PÉREZ-HARGUINDEGUY, DIAZ, GARNIER, LAVOREL, POORTER, JAUREGUIBERRY, BRET-HARTE, CORNWELL, CRAINE, GURVICH, URCELAY, VENEKLAAS, REICH, POORTER, WRIGHT, RAY, ENRICO, PAUSAS, DE VOS, BUCHMANN, FUNES, QUETIER, HODGSON, THOMPSON, MORGAN, TER STEEGE, VAN DER HEIJDEN, SACK, BLONDER, POSCHLOD, VAIERETTI, CONTI, STAVER, AQUINO and CORNELISSEN2013). Each 15-cm-long sample was measured twice, perpendicularly, for its diameter using digital callipers at five points: 2.5, 5.0, 7.5, 10.0 and 12.5 cm. At that point closest to a perfect circle, a 1-cm-wide disc centred on that measured point was removed. Its volume was calculated as V = л r2h; where r is the radius and h is the height. These volumes, though comparable between species, were not absolutely ‘fresh’; some drying was needed to prevent fungal activity in the ≈4 wk between sample collection and arrival in Bern, Switzerland.
Tissue separation
From the now 14-cm-long samples, their bark was removed from stem wood using a kitchen knife cleaned with ethanol between sample separations. Bark and wood tissues – the latter cut into small pieces using clean shears – were fine-milled into a powder using a planetary micro-mill (‘Pulverissette 7’ by Fritsch GmBH, Germany). These samples (n = 56, from 14 locations) were dried once more for 18 h at 45°C and then stored in glass vials in a refrigerator (4°C) until used.
Nutrients
From each wood and bark sample, ≈300 mg (300–359 mg) and ≈20 mg (16.6–36.1 mg) respectively, were digested in a 2.5-ml mixture of selenium, sulphuric acid and salicyclic acid. These digestions were analysed for nitrogen (N) using the modified Bertholet reaction, and for phosphorus (P) with the molybdenum-blue one. Concentrations were determined colorimetrically on a Skalar San++ continuous flow auto-analyser (Skalar Analytical B.V., Breda, the Netherlands). The tissue concentrations of calcium (Ca), potassium (K) and magnesium (Mg) were determined using the same digests on an Optima 7000 inductively coupled plasma, optical emission spectrometer (ICP-OES; Perkin Elmer, Waltham, USA).
Total phenolics
Concentrations of total phenolic compounds were determined using an automated procedure. The tissue material is first mixed with a solution of carboxymethyl cellulose and EDTA (ethylenediamine tetra acetic acid); to this is added an alkaline ferric solution that in turn reacts with polyphenols to yield a red dye measured at 600 nm: this method has been found to be as robust as the more laborious Folin-Ciocalteu method (de Mattos & Zagal Reference DE MATTOS and ZAGAL2010). Phenolics were extracted from 16 mg of tissue samples in 60% methanol and stored at −20°C. Phenolic concentrations were compared using gallic acid, GA, as the standard (3,4,5-trihydroxybenzoic acid, from Sigma-Aldrich, USA).
Non-volatiles
From each powdered sample, 10 mg was dissolved in 2 ml of a solution of 70% MeOH (HPLC grade), 29.5% H2O (Milli-Q) and 0.5% formic acid (‘puris’), in a 2-ml Eppendorf tube. Then, to each tube, 5–;10 tiny glass beads were added and centrifuged for 4 min at 14000 rpm; 1 ml of solution was removed and re-centrifuged as before; finally, 700 μl was pipetted into HPLC vials and stored at −20°C until use. Extracts were analysed in an untargeted manner for low-molecular-weight primary and secondary metabolites. To separate and detect these compounds, ultra-high pressure liquid chromatography coupled to high-resolution mass spectrometry was used (UHPLC-HRMS; full details in Appendix 2).
Volatiles
Wood and bark powdered samples were stored in Eppendorfs, at −80°C, until analysed for volatile organic compounds using gas chromatography coupled to mass spectrometry (GC-MS). To do this, 1.5 mg of powder was accurately weighed and placed in microvial glass inserts (Gerstel GmBH), then placed into individual glass liners that were closed with metallic adaptors. To allow relative quantification of detected compounds, 1 μl of internal standard (solution of ethanol with 1% of cis-3-hexenyl acetate), was added manually in the upper part of each microvial, avoiding direct contact with the sample. All samples were further handled by a multipurpose GC-MS sampling system (MPS2, Gerstel GmBH, Mellinghofen, Germany) that positioned them randomly within the tray. The system was equipped with both a thermal desorption unit and a cooled injection system (TDU & CIS, Gerstel GmBH) (full details in Appendix 2). Plastic scratched from inner Eppendorf was analysed as a corresponding control and determined which of the compounds were not of plant origin. In addition, blank analyses were conducted every five samples in order to clean the system.
Data analyses
Stem-cutting
The probability of an individual seedling having its stem cut was determined in a generalized linear mixed model (GLMM) that used the logit link and a binomial error distribution for the binary response (cut or not-cut). The GLMM considered the incidence of stem-cutting for the entire starting population of seedlings that died over the c. 2-y study period; it included canopy cover (gap vs. understorey) at the highest stratum, and then species (M. bisulcata vs. T. bifoliolata), and their interaction, as fixed terms. The model's random term was the ‘block’ of each pair of gap and understorey locations, and all variance components were estimated using restricted maximum likelihood (REML) with the default Schall-fitting algorithm in Genstat v. 16.2 (VSN International Ltd, UK). Model assumptions were checked using graphic diagnostics.
Tissue traits
To determine whether the species differed in stem tissue density, a paired t-test was used to control for any environmental influences on this trait. To test for nutrient and phenolic differences between species and between tissue types (bark vs. wood), or their interaction, an analysis of covariance (ANCOVA) was used. In these six ANCOVAs the covariate was seedling height, which in turn should reflect some of the major variation in light and soil resources among the gap locations. Indeed, re-analyses with linear mixed models gave near identical results (not shown): indeed, in all cases the ‘block’ variance component was less than its standard error so long as height was included in the models.
Non-volatiles and volatiles
To reduce, in an unbiased way, the many peaks detected in the data, a principal component analysis (PCA) was used. If a modest separation appeared in the PCA score plots, as found in all cases here, then a partial least squares discriminate analysis (PLS-DA) was performed. In this ‘supervised’, follow-up approach, the apparent separation is enhanced using a priori knowledge, or class predictors – in this case, species identity. However, because PLS-DA is prone to ‘over-fitting’, models were checked using cross-validation with R2 and Q2 metrics: the former should not greatly exceed the latter (Worley & Powers Reference WORLEY and POWERS2013) and values of Q2 > 0.4–0.5 are considering reliably safe (Westerhuis et al. Reference WESTERHUIS, HOEFSLOOT, SMIT, VIS, SMILDE, VAN VELZEN, VAN DUIJNHOVEN and VAN DORSTEN2008). PCA and PLS-DA analyses were performed using Simca 13.0. After data processing, the most discriminating volatile organic compounds between species and tissues (i.e. wood vs. bark) were tentatively identified with the NIST11 mass spectral library (U.S. Department of Commerce), as well as the PBM search format (Agilent Technologies, Inc.).
In these wood analyses two samples – one M. bisulcata, and one T. bifoliolata, each from different locations – were accidentally cross-contaminated. These were removed and replaced with two spare samples, nearest to their location, to balance the analysis and presentation. One additional bark sample for M. bisulcata (the first processed) was manually cleaned of chemical contaminants not present in the other M. bisulcata samples.
It is worth highlighting here three aspects of our analysis which could inform future studies. Firstly, heating at 250°C for 6 min did not burn the stem tissue samples, and so should have revealed the most interesting and relevant species differences in volatile compounds in bark and wood. This is important because preliminary analyses at 320°C resulted in ashes after 6 min and a lot of irrelevant volatiles. Secondly, the insertion of an internal standard for quantification is strongly recommended because it is a robust way to evaluate the consistency of the GC-MS analyses (which were very accurate here). Thirdly, while the identified volatiles cannot be confirmed with 100% certainty, we are confident about their names since we used two databases, subtracted the noise of the chromatogram in each case, and considered only those matching between 80% and 99%.
RESULTS
Seedling predation
After 2 y, the percentage of the weakly shade-tolerant M. bisulcata seedlings that had died in the understorey (68%) was three times greater than that for the strongly shade-tolerant T. bifoliolata (23%) (Figure 2). In gaps, however, this species difference was more than four-fold, as very few seedlings of T. bifoliolata died there (47% vs. 11% respectively; Figure 2). However, only a single case of stem-cutting was recorded in the understorey, on T. bifoliolata, whereas in gaps it was responsible for 83% of M. bisulcata deaths, as it evidently killed 39% of all seedlings that had established there (Figure 2). In stark contrast, just two seedlings of T. bifoliolata were killed by stem-cutting in gaps (4.3%) of all that had established there. Accordingly, the likelihood of a stem being cut was significantly different between the two canopy types (GLMM, F1, 280.8 = 8.37, P = 0.004) and between species (F1, 238.2 = 25.7, P < 0.001). When examined on a monthly basis, the rate of stem-cutting in the first full wet season was 2.89% mo−1; it was 2.03% mo−1 in the second dry season; and it was 3.17% mo−1 in the second wet season after the 2007 mast ended.

Figure 2. Stacked bars showing the absolute proportions of naturally established seedlings that died from stem-cutting vs. other causes in 40 paired canopy gap and understorey areas over the c. 2-y study period at Korup, Cameroon. The total sample sizes for the tree species Microberlinia bisulcata and Tetraberlinia bifoliolata under each canopy type are given above each bar (these include any ‘replacement’ seedlings). Because of ballistic dispersal limitations, only 12 of the 40 gap-understorey areas had both species present for monitoring.
In addition, the random ‘block’ variance component had a value much greater than its SE (2.99 vs. 0.99): this revealed that some gap locations were more prone to stem-cutting than others in this forest (Figure 3). There was no circumstantial evidence that seedling stems of other tree species around cut M. bisulcata stems were also likewise predated. Might the degree of canopy disturbance, i.e. gap size, or proximity to one or more conspecific adult trees, explain this spatial pattern? A single, ad hoc analysis revealed that the proportion of cut stems did not increase significantly with percentage canopy openness measured in November 2008 when used as a proxy for gap size (generalized linear model, GLM, F1, 27 = 0.95, P = 0.34), even after first accounting for gap's distance to the nearest adult — which itself was a negative, albeit not significant, predictor of cut stems (F1, 27 = 2.20, P = 0.15). The final term in the model, adult basal area within a 50-m radius of gaps, was not significant either (F1, 27 = 0.03, P = 0.86). Like the GLMMs for seedling survival, this GLM accounted for any over-dispersion in the data, but complete predictor data were available for only n = 33 (of the 34 gaps with M. bisulcata).

Figure 3. Spatial variation in the proportion of Microberlinia bisulcata seedlings (1–9 per gap area) that had their stems cut by small mammals during the c. 2-y monitoring period in the 82.5-ha P-plot at Korup, Cameroon. Each point represents a canopy gap location (n = 34 of 40 in total in the study; six gaps did not contain any naturally established M. bisulcata seedlings).
Tissue traits
Stem tissue density
The M. bisulcata seedlings had, on average, a 15% lower density than T. bifoliolata seedlings growing in the same gap locations (paired t-test, df = 13, t = −5.17, P < 0.001). Their mean values (± SE) (range) were, respectively, 0.551 ± 0.019 mg mm−3 (0.410–0.644 mg mm−3) and 0.650 ± 0.024 mg mm−3 (0.414–0.749 mg mm−3). These M. bisulcata seedlings had a mean height of 133 ± 22.8 cm and were slightly taller than their T. bifoliolata counterparts (120 ± 24.3 cm), but this difference was not significant (paired t-test, df = 13, t = 1.88, P = 0.082).
Nutrients and total phenolics
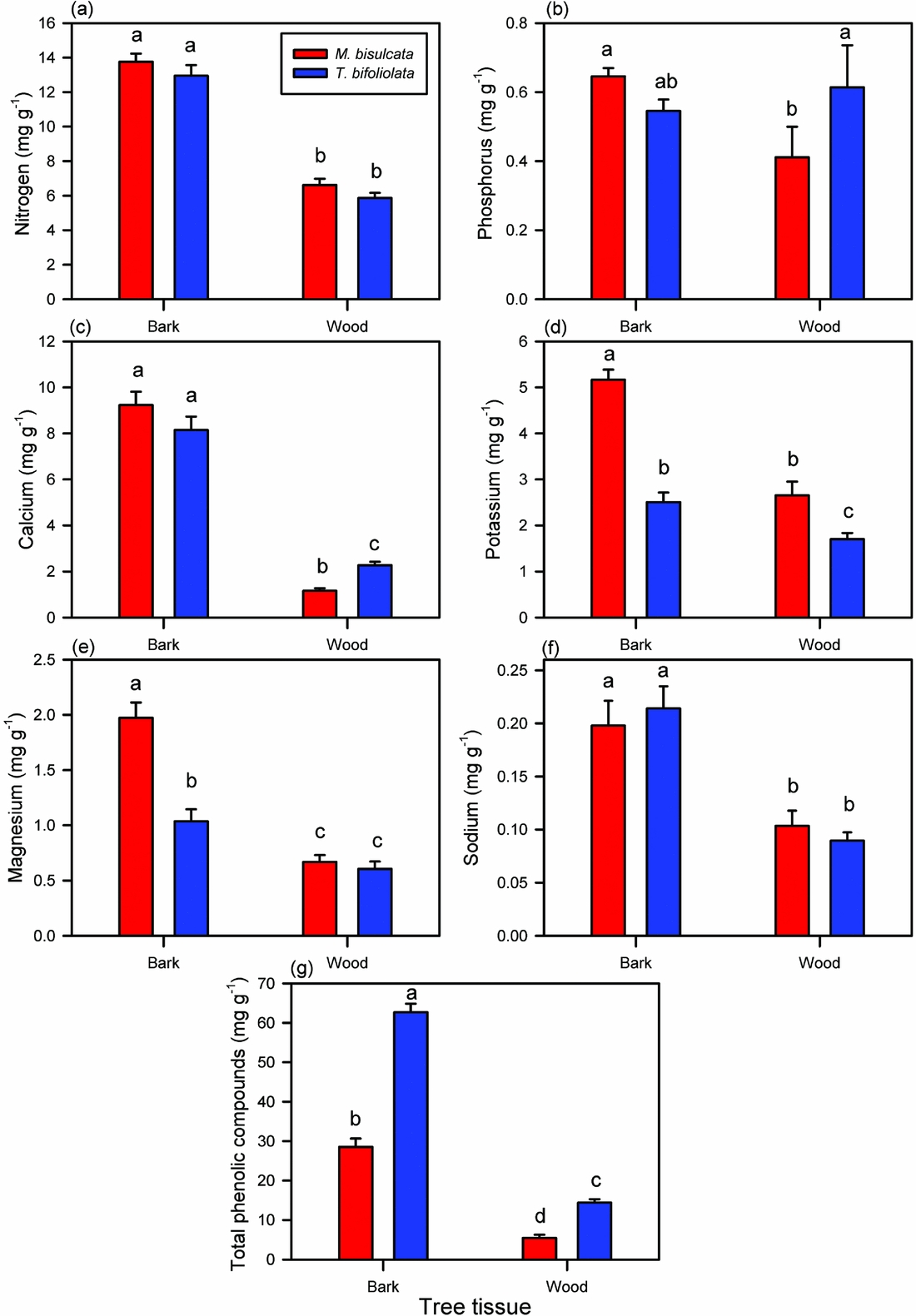
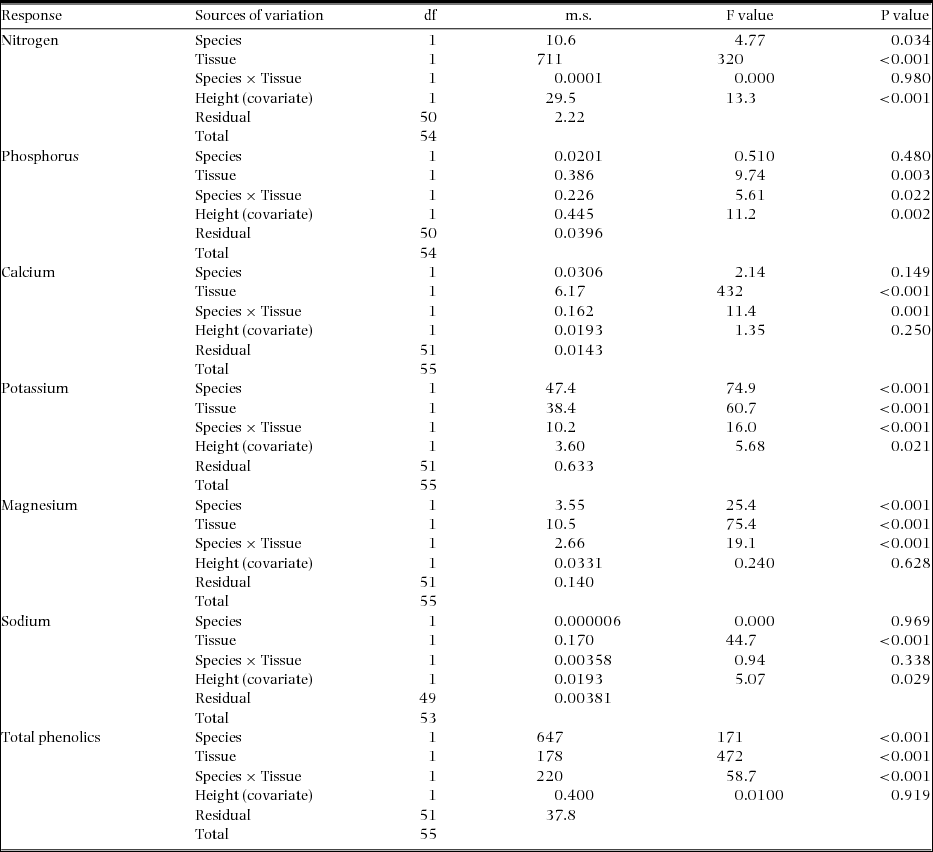
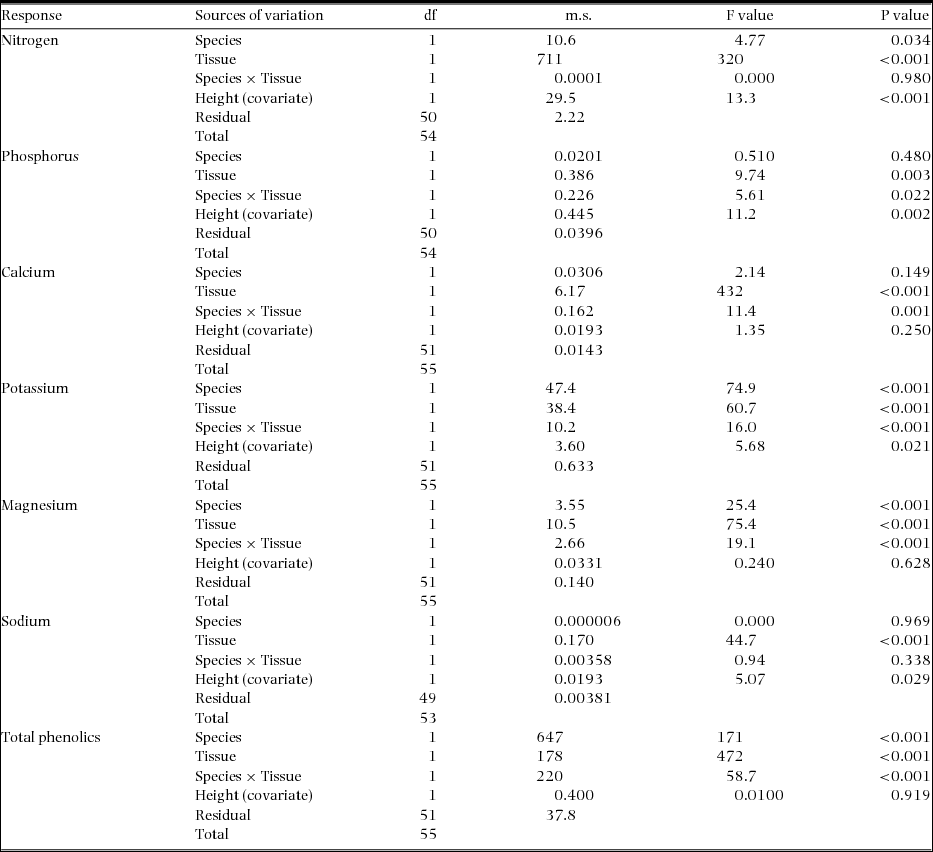
Bark concentrations of N and Na were similar between the two species, but twice that in wood (Figure 4a, f). For all other nutrients, whether or not the two species differed in their concentrations depended on the type of tissue (significant species × tissue interaction terms in ANCOVAs; Appendix 3). For instance, they differed in Ca and P only in their wood, but differed strongly in Mg and K in their bark (Figure 4b, c, e). In both tissue types, M. bisulcata contained more K than did T. bifoliolata seedlings, especially when bark was compared (Figure 4d). However, the seedling stems of T. bifoliolata contained more than two times the concentration of total phenolics than did M. bisulcata (Figure 4g). For M. bisulcata, all the nutrient and phenolic concentrations were much greater in its bark than wood. Though this tissue difference was not as pronounced for the nutrients in T. bifoliolata, it was more so for its phenolics. The seedling height of the samples was a significant covariate for explaining variation in N, P, K and Na but not Ca (P = 0.250), Mg (P = 0.628), or for total phenolics (0.919) (Appendix 3).

Figure 4. Concentrations of six nutrients (a–f) and that of total phenolic compounds (g) in the stem tissues of Microberlinia bisulcata and Tetraberlinia bifoliolata tree seedlings growing in canopy gap areas at Korup, Cameroon. Phenolic compounds are expressed in gallic acid equivalents (GA). Bars are the unadjusted, raw means (± SE); those with different letters are significantly different using a LSD test at 5% following ANCOVAs (full statistics in Appendix 2).
Non-volatiles
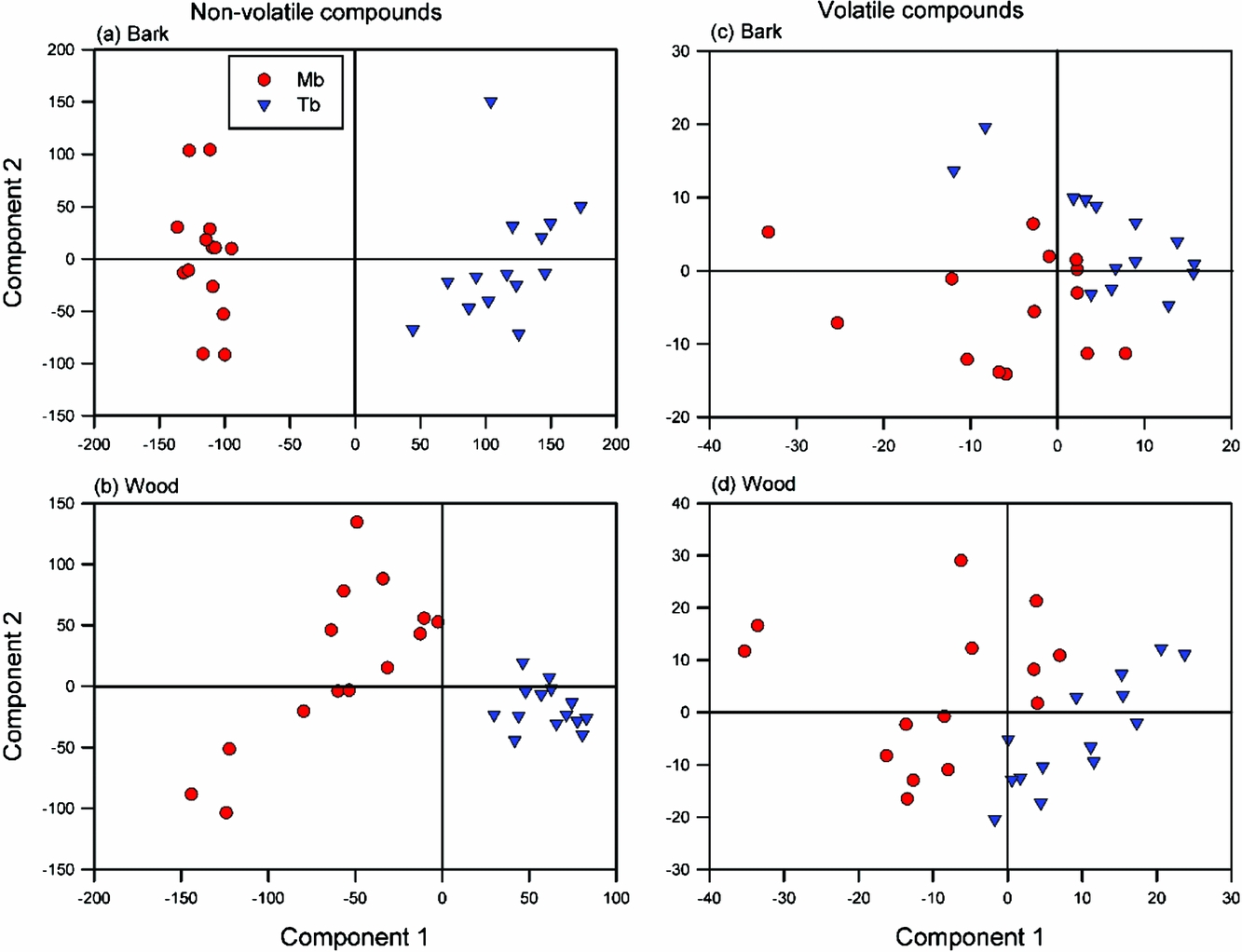
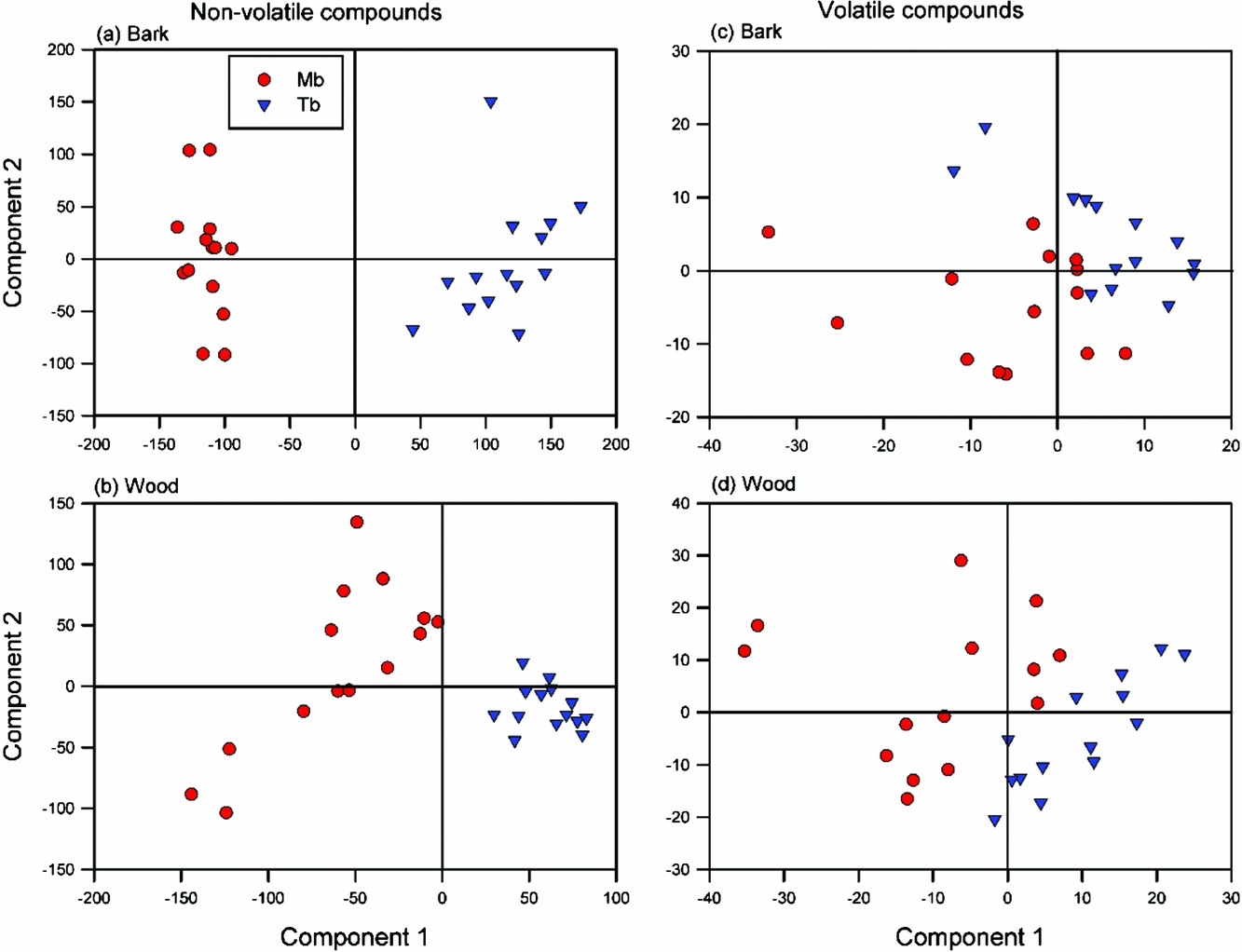
The PCA showed a clear separation between the two species, especially for bark (Appendix 4). The follow-up PLS-DA noticeably sharpened the species differences only for bark metabolites, especially along the first component axis (Figure 5a). Whereas the bark scores for M. bisulcata were more clustered than those of T. bifoliolata, this reversed for the wood scores (Figure 5b). Both PLS-DAs, however, were robust (bark, R2 = 0.99, Q2 = 0.97; wood, R2 = 0.96, Q2 = 0.91). However, upon closer inspection, only a few metabolites could be putatively identified as being typical of either species. For T. bifoliolata, these were: tetrahydroxyflavanone-rhamnopyranoside, tetrahydroxyflavan-pentahydroxyflavan (a dimer of flavonoid), dihydroyflavan-pentahydroxyflavan (another dimer), and pentahydroxyflavanone–rhamnopyranoside. For M. bisulcata, these were: hesperetin-7-O-sulphate (a sulphated flavonoid), and an unknown molecule (C19H26O12). In sum, flavonoids were more abundant in T. bifoliolata than M. bisulcata seedling stems; this result matches that for total phenolics.

Figure 5. Scores of follow-up PLS-DAs (partial least squares discriminate analysis) performed on the secondary metabolite data. Comparisons of the species-specific chemical profiles of non-volatile (a, b) and volatile compounds (c, d) detected in the stem tissues of Microberlinia bisulcata and Tetraberlinia bifoliolata tree seedlings grown together in canopy gaps at Korup, Cameroon. The percentage variance explained by components 1 and 2, respectively, were 37.3%, 5.0%; 22.0%, 11.3%; 25.7%, 17.4%; and 31.4%, 15.6%, respectively.
Volatiles
Species separation in the PCAs was evident, but weaker than that present for non-volatile metabolites (Appendix 4). For the bark samples the PLS-DA (Figure 5c) greatly improved the separation, and it was robust (R2 = 0.89, Q2 = 0.69). For the wood samples both species had less variation in their volatile profiles than they did for bark (Figure 5d) (R2 = 0.93, Q2 = 0.87). However, two M. bisulcata samples were outliers for unknown reasons, lying just outside the 95% confidence ellipse (Hotelling’s: not shown).
Closer inspection identified the chromatographic peaks containing the most discriminant ions responsible for the species separations in the PLS-DAs. These key volatiles are listed in Appendix 5, along with their ecological activity in other taxa. Generally, many more fatty acids were present in M. bisulcata than in T. bifoliolata which in turn was clearly richer in phenolics.
DISCUSSION
Canopy disturbance increases seedling susceptibility to stem-cutting by small mammals
Stem-cutting predation was restricted almost entirely to M. bisulcata in gaps, but was virtually absent in the forest understorey for either tree species. There are two plausible explanations for this habitat effect. The first is that the small mammals responsible are simply resident in these gap areas and rarely venture beyond them to harvest seedlings in this way because of predation risks. For example, in Japanese beech forest, rodents gnawed many more beech and oak seedling stems growing amidst Sasa grass vegetation than without it (Ida & Nakagoshi Reference IDA and NAKAGOSHI1996). Seeds of M. bisulcata, on the other hand, being nutritionally valuable and copious during masting events in this resource-poor forest (Newbery et al. Reference NEWBERY, CHUYONG and ZIMMERMANN2006), are rapidly removed in the understorey (Green & Newbery Reference GREEN and NEWBERY2002), albeit per-capita losses are still greater in gaps (Norghauer & Newbery Reference NORGHAUER and NEWBERY2011). The second explanation is that the small mammals responsible target gap areas because the resource quality of M. bisulcata is better there than in the understorey, where new seedlings cannot increase quickly in height and stem girth. Recall, that both tree species will remain stunted in the deep shade but they persist differently there (Green & Newbery Reference GREEN and NEWBERY2001, Newbery et al. Reference NEWBERY, CHUYONG and ZIMMERMANN2006, Norghauer & Newbery Reference NORGHAUER and NEWBERY2013). Preferential foraging in gap areas is not unheard of: several species of large browsing mammals do so in temperate forest (Kuijper et al. Reference KUIJPER, CROMSIGT, CHURSKI, ADAM, JEDRZEJEWSKA and JEDRZEJEWSKI2009). If plant size or quality determine risk of stem-cutting then it would be pertinent to know if surviving gap stems – now large relative to those suppressed in the understorey – are cut less often once gaps have closed up. To confirm the gap foraging/protective habitat preference suggested here, it would be necessary to survey small mammals in both habitats and to identify the agents of stem-cutting. This could be done via live- or camera-trapping small mammals, coupled to video footage of their behaviour (Jansen et al. Reference JANSEN, BONGERS and HEMERIK2004, Kuijper et al. Reference KUIJPER, CROMSIGT, CHURSKI, ADAM, JEDRZEJEWSKA and JEDRZEJEWSKI2009, Sato Reference SATO2000), to help to reveal the reason(s) stems are cut.
Indeed, it is unknown why small mammals would want to cut down growing seedlings in gaps. The most likely culprits are rodents, and in particular the widespread and large nocturnal giant pouched rat (Cricetomys emini) (Olayemi et al. Reference OLAYEMI, NICOLAS, HULSELMANS, MISSOUP, FICHET-CALVET, AMUNDALA, DUDU, DIERCKX, WENDELEN, LEIRS and VERHEYEN2012; body and tail length of c. 45 cm each weighing up to 1.4 kg, Kingdon Reference KINGDON1997). We can only speculate on the reasons, which include: (1) to simply gnaw on stems to wear down their growing incisors and thus keep them short, as suggested by Watt (Reference WATT1919, Reference WATT1923); (2) to ingest the bark/wood material to supplement their dietary needs (Bryant et al. Reference BRYANT, PROVENZA, PASTOR, REICHARDT, CLAUSEN and DUTOIT1991, Gill Reference GILL1992, Pigott Reference PIGOTT1985, Weeks & Kirkpatrick Reference WEEKS and KIRKPATRICK1978); (3) to use the stem portions cut and removed for nest-building in burrows (Watt Reference WATT1919, Reference WATT1923); and perhaps most interestingly, (4) to clean or disinfect their teeth or mouth, as suggested by the palmitic acid in M. bisulcata bark (Appendix 5 reference therein). This last might be the most plausible because if M. bisulcata stems are not actually ingested, rodents should prefer the tougher stems of T. bifoliolata to wear down their incisors. That they do not, coupled to the very wet environment at Korup favouring bacteria and fungi, instead suggests that M. bisulcata stems may provide a unique, yet easily found and chewable source of anti-microbial protection for their mouth (or nests).
The significant spatial variation in stem-cutting of M. bisulcata seedlings (Figure 3) could not be explained by either gap size or the neighbourhood of conspecific adult trees. Therefore, other unstudied factors may play a role in determining where sapling recruitment is ‘safer’ than others: this may include individual gap location relative to that of carnivores of small mammals, swamp areas, or other food resources used by small mammals. The results also suggest that following masting events, established seedlings are not killed in a distance- or density-responsive way by small mammals (Janzen Reference JANZEN1970); this is perhaps not surprising given the generalist diet of terrestrial vertebrate herbivores (Clark et al. Reference CLARK, POULSEN and LEVEY2012, Struhsaker Reference STRUHSAKER1997, Theimer et al. Reference THEIMER, GEHRING, GREEN and CONNELL2011). However, on a very coarse spatial scale, no stems were cut in gaps outside the adult M. bisulcata grove – this would make sense if the small mammals responsible were territorial, preferring to live within the M. bisulcata grove where seed-food inputs are frequent and large in masting events every 2–3 y.
Linking tree species traits to seedling stem-cutting
The stark contrast in stem-cutting between the tree species was mirrored by differences in some of their key physical and chemical traits. A 20% higher stem tissue density in the more shade-tolerant T. bifoliolata than in the light-demanding M. bisulcata is not surprising: this fits with prior work on both species’ resistance traits (Norghauer et al. Reference NORGHAUER, GLAUSER and NEWBERY2014), and with the general view of a growth-defence trade-off across species (Coley et al. Reference COLEY, BRYANT and CHAPIN1985). It has been shown experimentally that, although M. bisulcata persists poorly in the shade, it could grow faster in height than T. bifoliolata in gaps were it not so susceptible there to leaf herbivory from insects (Green & Newbery Reference GREEN and NEWBERY2001, Norghauer & Newbery Reference NORGHAUER and NEWBERY2013). Stem tissue density was very strongly correlated with early seedling survival at 7 mo for eight tree species studied in Panama (Alvarez-Clare & Kitajima Reference ALVAREZ-CLARE and KITAJIMA2007); it is also correlated with species’ leaf density and lifespans as well (Kitajima et al. Reference KITAJIMA, CORDERO and JOSEPH WRIGHT2013). At Korup, the stem tissue density of seedlings and saplings may be especially important for surviving the wet season when thunderstorms cause much debris to fall from the canopy, and for resisting further breakage and trampling from large-bodied mammalian herbivores typical of African forest fauna. Clearly, stem lignification did not protect M. bisulcata seedlings from being cut, however (Figure 1), nor did it protect 1–2-y-old temperate beech (Fagus crenata) seedlings from rodents after masting events (Ida & Nakagoshi Reference IDA and NAKAGOSHI1996). Nevertheless, there must be a threshold in stem girth after which the risk of stem-cutting in M. bisulcata becomes negligible because it is physically impossible, or inefficient energetically, for small mammals to do it. To confirm such an ontogenic shift in resistance will require long-term field study.
The strongest effects seen for species’ nutrient differences were for K and Mg concentrations in their bark (Figure 4). Sodium (Na), known to be preferred by wood-gnawing temperate rodents (Hansson Reference HANSSON1991, Weeks & Kirkpatrick Reference WEEKS and KIRKPATRICK1978), cannot explain the preference of M. bisulcata over T. bisulcata, nor can calcium (Ca), phosphorus (P) or nitrogen (N). Likewise, Hjältén & Palo (Reference HJÄLTÉN and PALO1992) also concluded that N was not a deciding factor in the feeding preferences of voles and hares, which was better explained by species differences in the digestibility and phenolics of temperate tree branches and shoots. Thus, in this resource-poor forest, we cannot discount a nutritional role of K as well as one for Mg, which together might prompt small mammals to selectively forage for M. bisulcata seedlings at Korup. Unfortunately, there is generally little research into the relationship between bark minerals and consumption by rodents in native tree species, much less in tropical regions (Baxter & Hansson Reference BAXTER and HANSSON2008).
The results also strongly point to much better resistance against herbivores in T. bifoliolata – and a lack thereof in M. bisulcata. That T. bifoliolata stems had higher total phenolic concentrations than those of M. bisulcata (Figure 4g), in addition to a very different composition of non-volatile secondary metabolites (Figure 5a,b), matches trends already found in their leaf tissues (Green & Newbery Reference GREEN and NEWBERY2001, Norghauer et al. Reference NORGHAUER and NEWBERY2014). Many of the volatile compounds detected in T. bifoliolata are linked to anti-herbivore or anti-pathogenic effects in other taxa, mainly insects (Appendix 5), and there is a vast accumulated literature on the role of secondary metabolites in woody plant defence against mammalian herbivores (Bryant et al. Reference BRYANT, PROVENZA, PASTOR, REICHARDT, CLAUSEN and DUTOIT1991, Freeland & Janzen Reference FREELAND and JANZEN1974). For instance, experimental work has shown that two rodent species in Chile prefer food items low in both fibre and tannins (Bozinovic et al. Reference BOZINOVIC, NOVOA and SABAT1997). Moreover, eugenol and cinnamaldehyde exhibited moderate and potent activity respectively as repellents against gnawing by mice (Lee et al. Reference LEE, LEE and AHN1999), and both of these, or their variants (excluding methoxyeugonol), were present in the bark and wood, respectively, of T. bifoliolata seedlings only (table in Appendix 5; ‘sinapaldehyde’ is the name given to 3,5 dimethoxy-4-hydroxycinnamaldehyde).
The tree species’ volatile profiles are particularly intriguing in this context (Appendix 5). If small mammals combine taste and odour to learn to avoid toxic woody plants (Bryant et al. Reference BRYANT, PROVENZA, PASTOR, REICHARDT, CLAUSEN and DUTOIT1991), then, when foraging for T. bifoliolata stems, their initial encounter with bark seems to be of utmost importance. It is noteworthy that while T. bifoliolata bark is characterized by terpenes and phenols, likely making them unpalatable to herbivores, M. bisulcata lacked any of these putative protective compounds; conversely, the fatty substances in its bark may attract rodents because of their nutritive value (Hansson Reference HANSSON1973). Finally, if M. bisulcata is preyed upon at night, as suggested here, sight becomes less important than smell and taste cues for small mammals. When the bark of M. bisulcata gap seedlings is scratched, a conspicuous scent is elicited that smells like topical menthol/methyl salicylate ointment (Norghauer, pers. obs.) – we hypothesize that the volatile 3-methypentanal found in the bark of M. bisulcata may act as an olfactory cue for their location by small mammals. A plant volatile need not only be elicited at high temperature (Bryant et al. Reference BRYANT, PROVENZA, PASTOR, REICHARDT, CLAUSEN and DUTOIT1991), or following herbivore attack, but may also serve as a reliable foraging cue for mammalian herbivores if it is ubiquitous even at very low concentrations (Bedoya-Perez et al. Reference BEDOYA-PEREZ, ISLER, BANKS and MCARTHUR2014).
Whether it is one trait primarily, which is doubtful, or rather a synergistic combination of stem tissue density and their chemical content that determined the avoidance of T. bisulcata, and preference for M. bisulcata, by small mammals cannot be known without experiments and in situ bioassays that explore the main and interactive effects of these putative defensive compounds.
Implications for Microberlinia bisulcata populations
We have no data on the abundance of rodents inside or outside the M. bisulcata grove, or how it might fluctuate over years. It is unlikely that their populations are stable, however (Struhsaker Reference STRUHSAKER1997) – they may spike soon after masting by M. bisulcata (which happens every 2–3 y, Newbery et al. Reference NEWBERY, CHUYONG and ZIMMERMANN2006). By removing M. bisulcata from gaps, which are those very habitats essential for its growth into larger-size classes, these predators function to diminish the abundance and frequency of its seedlings able to become saplings, and therefore should curb recruitment rates. Over time, this should limit the sapling bank further than it already is by the canopy disturbance regime. As such, we suggest that this rodent behaviour can make an important contribution to species coexistence beyond the seedling stage by pre-empting competition between the dominant, fast-growing M. bisulcata and other tree species for winning space and other resources in gaps. A prominent role for small mammals limiting the recruitment of seedlings from seed was recently found in another African forest, in the Congo Basin (Clark et al. Reference CLARK, POULSEN and LEVEY2012, also see Struhsaker Reference STRUHSAKER1997). In the case of M. bisulcata it would appear that stem-cutting impinges upon the fitness benefits associated with a masting reproduction strategy, if the aim is to satiate its predators after dispersal and not before. An expected increased in rodent abundance after masting may increase predator pressure on those established seedlings that escaped seed predation via successful satiation in gaps (Norghauer & Newbery Reference NORGHAUER and NEWBERY2011).
We anticipate that regeneration of remnant M. bisulcata in forest logged of other target species is at further risk from stem-cutting because logging generally has a positive influence on rodent abundance and richness (Lambert et al. Reference LAMBERT, MALCOLM and ZIMMERMAN2006, Malcolm Reference MALCOLM, Lowman and Nadkarni1995, Malcolm & Ray Reference MALCOLM and RAY2000, Struhsaker Reference STRUHSAKER1997). That said, in heavily logged forest the giant pouched rat might not be common because of unsuitable habitat (Struhsaker Reference STRUHSAKER1997) or high hunting pressure (Fa et al. Reference FA, SEYMOUR, DUPAIN, AMIN, ALBRECHTSEN and MACDONALD2006). Finally, efforts at restoring this critically endangered tree species (IUCN category A1c + 2c) in secondary or otherwise disturbed forests should consider protecting newly planted seedlings with cages, and surrounding any small populations of M. bisulcata trees with logging-free buffer areas.
Stem-cutting, as reported here, is not well documented from tropical forests. In several field studies of vertebrate exclusion in the Neotropics and Australia, the impact on seedling survivorship was either short-lived, within the first 2 mo, or the main cause of death was uprooting of the seedling for seeds and/or cotyledons to eat (Osunkoya et al. Reference OSUNKOYA, ASH, GRAHAM and HOPKINS1993, Paine & Beck Reference PAINE and BECK2007, Sork Reference SORK1987, Theimer et al. Reference THEIMER, GEHRING, GREEN and CONNELL2011). Some stem-cutting was noted in seedlings of six Panamanian species, but these were studied only in the understorey (Alvarez-Clare & Kitajima Reference ALVAREZ-CLARE and KITAJIMA2009). Without more investigation, it is too early to know if the high prevalence of stem-cutting on M. bisulcata may not only reflect stem traits associated with its life-history but perhaps also a geographic association with small mammals, namely rodents, endemic to African rain forest.
CONCLUSIONS
Our results point to tree species trait differences and habitat use by small mammals, likely rodents, as determining the selective stem-cutting of M. bisulcata seedlings in forest gaps. As such, in addition to other biotic and abiotic factors, stem-cutting predation is another contributing factor that helps explain the dearth of M. bisulcata saplings at Korup. Hopefully these results will entice tropical ecologists, foresters and zoologists to view small mammals a little differently, the rodents particularly, and spur field investigations of their functional role as predators in the seedling-to-sapling stages of other co-occurring tree species. It would be highly germane to know if rodents attack other dominant canopy tree species that share wood and bark traits with the fast-growing, weakly shade-tolerant seedlings of M. bisulcata – and likewise avoid very shade-tolerant, highly resistant tree species like T. bifoliolata.
ACKNOWLEDGEMENTS
We thank both Sylvanus Njibile and Charles Okha for their outstanding field help in the forest with the data collection, and also thank David Newbery for his comments on the manuscript. We are grateful for support from previous Conservators of Korup National Park, A. Kembou and P. Ndongmo, and research permission from the Ministries of Forestry and Wildlife (MINFOF) and Scientific Research and Innovation (MINRESI) in Cameroon. George Chuyong and Reymond Kometa of the University of Buea provided invaluable logistic support in the field.
Appendix 1. Details on the collection of stems used in the trait analyses from a separate sample of seedlings of the two study tree species in gaps at Korup, Cameroon.
A seed addition experiment had used both study species to investigate their seed losses to small mammals inside and outside the main M. bisulcata grove (see Norghauer & Newbery Reference NORGHAUER and NEWBERY2011 for full details). Outside the grove there were no signs of stem-cutting at the 15 (formerly) ‘gap’ locations, from where the surviving stems in the paired control and fenced-exclusion quadrats 2.5 m apart (edge to edge) were measured for their heights (21–26 January 2014). From either of the two quadrats, two seedlings – one of each species – that matched closest in height were selected; a 15-cm-long portion of their stem (beginning at ≈5 cm height above ground) was removed, affixed with a tag, and stored in a perforated plastic bag in a waterproof box. This was possible at 13 of 15 gap locations where both species were still present (a spare set was taken at one location); at a 14th location, samples were instead obtained from the paired ‘understorey’ location where a tree had fallen to create a new gap. (A second set of spare samples was obtained here.) The samples were oven-dried at 40°C for 36–48 h (22–28 January 2014), then packaged with silica gel and flown to Bern, Switzerland, where they were oven-dried at 45°C for 24 h (17 February 2014), and stored with silica in a freezer at −20°C until later analyses. Because these tissue samples, unavoidably, came from gap stems older (≈6.5 y) than those on which clipping was actually recorded (≈2.5 y at most) we assumed that species trait differences did not vary substantial through their development up c. 130 cm height (on average) when still vulnerable to small-mammal stem-cutting.
Lacking electricity on the collection site, it was impossible to collect the samples using portable freezers in the rain forest of Korup. Therefore some very volatile organic compounds were likely lost during sample collection and preparation (i.e. modest drying). Nonetheless, in the absence of very high heat (>100°C) it was anticipated that each species retained its characteristic volatile fraction in the samples.
Appendix 2. Further details on the methods used to detect non-volatile and volatile compounds in the stems of Microberlinia bisulcata and Tetraberlinia bifoliolata seedlings at Korup, Cameroon.
Non-volatiles
Chemicals, water, acetonitrile and formic acid used for metabolite profiling were of UPLC-MS grade (Biosolve, Valkenswaard, the Netherlands).
Metabolite profiling
Untargeted metabolite profiling was performed on an Acquity UPLCTM from Waters (Milford, MA, USA) coupled to a Synapt G2 quadrupole time-of-flight mass spectrometer (Waters). The analytical conditions were as follows. Ultra-high-pressure liquid chromatography: column, Acquity BEH C18 50 × 2.1 mm i.d., 1.7-μm particle size (Waters); solvent system, A: water (0.05% formic acid), B: acetonitrile (0.05% formic acid); gradient program, 2%−35% B in 3 min, 35−100% B in 3 min, 100% B for 1.5 min, re-equilibration at 2% B for 1.5 min; flow rate, 600 μl min−1; column temperature, 40°C; injection volume, 2.5 μl. Mass spectrometer: positive ion electrospray nebulization, capillary voltage 2800 V, cone voltage 25 V, source temperature 120°C, desolvation gas flow 800 l h−1, desolvation gas temperature 400°C, acquisition in MSE mode over the range 85–1200 Da. The software used for data analysis was Masslynx 4.1 (Waters).
Metabolomic data were treated using Markerlynx XSTM (Waters) using the following parameters: initial and final retention times 0.0–7.6 min, mass range 100–1200 Da, mass tolerance 0.02 Da, retention time tolerance 0.06 min, intensity threshold 400 counts, automatic measure of peak width and peak-to-peak baseline noise, de-isotoping function applied. The obtained peak list was Pareto-scaled prior to multivariate analysis.
Volatiles
In the TDU (split-less mode), the samples were kept at 50°C for 0.2 min before an increase to 250°C at a rate of 640°C min−1 (hold time 6 min). The emitted volatiles were cryo-focused with liquid nitrogen (−80°C) in the CIS before being heated at 12°C s−1 to 270°C (hold time 6.5 min) and injected onto the GC column. The PTV inlet was operated in the solvent vent mode, with a vent pressure of 14 psi, a vent flow of 50 ml min−1, and a purge flow of 50 ml min−1. Compounds were separated on Agilent HP-1MS columns (30 m length × 0.25 mm i.d. and 0.25-μm film thickness). The helium carrier gas flow rate was 1.3 ml min−1 (constant flow mode). The temperature program of the GC operation was 50°C for 0.01 min, then increased to 260°C at a rate of 7°C min−1 (hold time 5 min), followed by a 2 min post run at 270°C. In all cases, the MSD transfer line temperature was set at 280°C and the ion source and quadrupole temperatures were set at 230°C and 150°C, respectively. Electron impact (EI) mode was used with a scanning over the mass range of 33–350 amu.
Appendix 3. The ANCOVA statistics for concentrations of nutrients and phenolics in the seedling stems of Microberlinia bisulcata and Tetraberlinia bifoliolata trees in gaps at Korup, Cameroon. One T. bifoliolata sample of bark tissue digest was insufficient for the phosphorus analyses; concentrations were log-transformed for calcium and phosphorus analyses (the latter, first multiplied by 100) and for which two bark samples of T. bifoliolata were unusable (one was accidently contaminated and the other too low in mass). Height is of each seedling sampled.
Appendix 4. PCAs for non-volatile and volatile organic compounds in seedling stems of Microberlinia bisulcata and Tetraberlinia bifoliolata trees in gaps at Korup, Cameroon.
Appendix 5. Tabulation of the key volatile organic compounds isolated from seedling bark and wood tissues of two rain-forest tree species, Microberlinia bisulcata and Tetraberlinia bifoliolata, at Korup, Cameroon. Grouped volatiles are sorted by ascending retention times (r/t). HIPV refers to a herbivore induced plant volatile. The cited numbered references follow the table.

1. DUKE, J. 2015. Dr. Duke's phytochemical and ethnobotanical databases. [Online Database] accessed 24–26 February 2015. http://www.ars-grin.gov/duke/.
2. FALODUN, A., SIRAJ, R. & CHOUDHARY, M. I. 2009. GC-MS analysis of insecticidal leaf essential oil of Pyrenacantha staudtii Hutch and Dalz (Icacinaceae). Tropical Journal of Pharmaceutical Research 8:139–143.
3. HORIKOSHI, M., TAKABAYASHI, J., YANO, S., YAMAOKA, R., OHSAKI, N. & SATO, Y. 1997. Cotesia glomerata female wasps use fatty acids from plant–herbivore complex in host searching. Journal of Chemical Ecology 23:1505–1515.
4. HUANG, C. B., GEORGE, B. & EBERSOLE, J. L. 2010. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Archives of Oral Biology 55:555–560.
5. HUANG, C. B., ALIMOVA, Y., MYERS, T. M. & EBERSOLE, J. L. 2011. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Archives of Oral Biology 56:650–654.
6. REINER, W. B., PETZINGER, C., POWER, M. L., HYEROBA, D. & ROTHMAN, J. M. 2014. Fatty acids in mountain gorilla diets: implications for primate nutrition and health. American Journal of Primatology 76:281–288.
7. BERDANIER, C. D., DWYER, J. & FELDMAN, E. B. 2007. Handbook of nutrition and food. (Second edition). CRC Press, Boca Raton. 1288 pp.
8. RAJASHEKAR, Y., RAGHAVENDRA, A. & BAKTHAVATSALAM, N. 2014. Acetylcholinesterase inhibition by biofumigant (Coumaran) from leaves of Lantana camara in stored grain and household insect pests. BioMed Research International 2014:187019–187019.
9. MORIMOTO, M., FUJII, Y. & KOMAI, K. 1999. Antifeedants in Cyperaceae: coumaran and quinones from Cyperus spp. Phytochemistry 51:605–608.
10. LEVIN, D. A. 1976. Chemical defenses of plants to pathogens and herbivores. Annual Review of Ecology and Systematics 7:121–159.
11. PINO, J. A., ALMORA, K. & MARBOT, R. 2003. Volatile components of papaya (Carica papaya L., Maradol variety) fruit. Flavour and Fragrance Journal 18:492–496.
12. EL-MOUGY, N. S., EL-GAMAL, N., MOHAMED, M. & ABDEL-KADER, M. 2008. Furfural approaches as control measures against root rot and root-knot incidence of tomato under greenhouse and field conditions. Journal of Plant Protection Research 48:93–105.
13. PING, L. Y., SHEN, Y. B., JIN, Y. J. & HAO, J. H. 2001. Leaf volatiles induced by mechanical damage from diverse taxonomic tree species. Acta Botanica Sinica 43:261–266.
14. WATERMAN, P. G. & MOLE, S. 1994. Analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford. 238 pp.
15. KAPPERS, I. F., HOOGERBRUGGE, H., BOUWMEESTER, H. J. & DICKE, M. 2011. Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. Journal of Chemical Ecology 37:150–160.
16. GROSSMAN, J. 1993. Botanical pesticides in Africa. Integrated Pest Management Programme Practice 15:1–9.
17. OBENGOFORI, D. & REICHMUTH, C. 1997. Bioactivity of eugenol, a major component of essential oil of Ocimum suave (Wild) against four species of stored-product Coleoptera. International Journal of Pest Management 43:89–94.
18. KOEDUKA, T., LOUIE, G. V., ORLOVA, I., KISH, C. M., IBDAH, M., WILKERSON, C. G., BOWMAN, M. E., BAIGA, T. J., NOEL, J. P., DUDAREVA, N. & PICHERSKY, E. 2008. The multiple phenylpropene synthases in both Clarkia breweri and Petunia hybrida represent two distinct protein lineages. Plant Journal 54:362–374.
19. CHAIEB, K., HAJLAOUI, H., ZMANTAR, T., BEN KAHLA-NAKBI, A., ROUABHIA, M., MAHDOUANI, K. & BAKHROUF, A. 2007. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. myrtaceae): a short review. Phytotherapy Research 21:501–506.
20. HUANG, Y., HO, S. H., LEE, H. C. & YAP, Y. L. 2002. Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch (Coleoptera : Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera : Tenebrionidae). Journal of Stored Products Research 38:403–412.
21. SUTFELD, R., PETEREIT, F. & NAHRSTEDT, A. 1996. Resorcinol in exudates of Nuphar lutea. Journal of Chemical Ecology 22:2221–2231.
22. GALLO, M. B. C. & SARACHINE, M. J. 2009. Biological activities of lupeol. International Journal of Biomedical and Pharmaceutical Sciences SI1:46–66.
23. DIAZ, M., CASTILLO, L., DIAZ, C. E., ALVAREZ, R. G., GONZALEZ-COLOMA, A. & ROSSINI, C. 2014. Differential deterrent activity of natural products isolated from Allophylus edulis (Sapindaceae). Advances in Biological Chemistry 4:168–179.
24. GUHLING, O., HOBL, B., YEATS, T. & JETTER, R. 2006. Cloning and characterization of a lupeol synthase involved in the synthesis of epicuticular wax crystals on stem and hypocotyl surfaces of Ricinus communis. Archives of Biochemistry and Biophysics 448:60–72.
25. THOISON, O., SEVENET, T., NIEMEYER, H. M. & RUSSELL, G. B. 2004. Insect antifeedant compounds from Nothofagus dombeyi and N-pumilio. Phytochemistry 65:2173–2176.
26. GUNALAN, G., KRISHMAMURTHY, V. & SARASWATHY, A. 2014. GC-MS and HPTLC fingerprinting of Bauhinia variegata leaves for anticancer activity. World Journal of Pharmaceutical Research 3:1313–1336.
27. LIN, S., BINDER, B. F. & HART, E. R. 1998. Insect feeding stimulants from the leaf surface of Populus. Journal of Chemical Ecology 24:1781–1790.
28. ESPELIE, K. E., BERNAYS, E. A. & BROWN, J. J. 1991. Plant and insect cuticular lipids serve as behavioral cues for insects. Archives of Insect Biochemistry and Physiology 17:223–233.
29. ARA, I., SHINWARI, M. M. A., RASHED, S. A. & BAKIR, M. A. 2013. Evaluation of antimicrobial properties of two different extracts of Juglans regia tree bark and search for their compounds using gas chromatography-mass spectrum. International Journal of Biology 5:92–102.
30. SALAUN, J. & BAIRD, M. S. 1995. Biologically active cyclopropanes and cyclopropenes. Current Medicinal Chemistry 2:511–542.
31. MAYBRIDGE. 2009. Safety data sheet: Cyclopropyl carbinol. Fisher Scientific, Fair Lawn. 8 pp.
32. JANES, D., KANTAR, D., KREFT, S. & PROSEN, H. 2009. Identification of buckwheat (Fagopyrum esculentum Moench) aroma compounds with GC-MS. Food Chemistry 112:120–124.
33. WALRADT, J. P., PITTET, A. O., KINLIN, T. E., MURALIDHARA, R. & SANDERSON, A. 1971. Volatile components of roasted peanuts. Journal of Agricultural and Food Chemistry 19:972–979.
34. NOGUEIRA, P. C. L., BITTRICH, V., SHEPHERD, G. J., LOPES, A. V. & MARSAIOLI, A. J. 2001. The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae). Phytochemistry 56:443–452.
35. RAVIKUMAR, V. R., GOPAL, V. & SUDHA, T. 2012. Analysis of phytochemical constituents of stem bark extracts of Zanthoxylum tetraspermum Wight & Arn. Research Journal of Pharmaceutical, Biological and Chemical Sciences 3:391–402.
36. JAKUBAS, W. J., SHAH, P. S., MASON, J. R. & NORMAN, D. M. 1992. Avian repellency of coniferyl and cinnamyl derivatives. Ecological Applications 2:147–156.
37. TRESSL, R. & DRAWERT, F. 1973. Biogenesis of banana volatiles. Journal of Agricultural and Food Chemistry 21:560–565.
38. TAN, K. H. & NISHIDA, R. 2012. Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. Journal of Insect Science 12:1–74.
39. SAENZ, M. T., TORNOS, M. P., ALVAREZ, A., FERNANDEZ, M. A. & GARCIA, M. D. 2004. Antibacterial activity of essential oils of Pimenta racemosa var. terebinthina and Pimenta racemosa var. grisea. Fitoterapia 75:599–602.
40. CUSTODIO, D. L., BURGO, R. P., MORIEL, B., BARBOSA, A. D. M., REZENDE, M. I., DE SOUZA DANIEL, J. F., PINTO, J. P., BIANCHINI, E. & FARIA, T. D. J. 2010. Antimicrobial activity of essential oils from Pimenta pseudocaryophyllus and Tynanthus micranthus. Brazilian Archives of Biology and Technology 53:1363–1369.
41. CAVALLI, J. F., FERNANDEZ, X., LIZZANI-CUVELIER, L. & LOISEAU, A. M. 2003. Comparison of static headspace, headspace solid phase microextraction, headspace sorptive extraction, and direct thermal desorption techniques on chemical composition of French olive oils. Journal of Agricultural and Food Chemistry 51:7709–7716.
42. KALUA, C. M., ALLEN, M. S., BEDGOOD, D. R., JR., BISHOP, A. G., PRENZLER, P. D. & ROBARDS, K. 2007. Olive oil volatile compounds, flavour development and quality: a critical review. Food Chemistry 100:273–286.
43. VAN POECKE, R. M. P., POSTHUMUS, M. A. & DICKE, M. 2001. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. Journal of Chemical Ecology 27:1911–1928.
44. POELMAN, E. H., BRUINSMA, M., ZHU, F., WELDEGERGIS, B. T., BOURSAULT, A. E., JONGEMA, Y., VAN LOON, J. J. A., VET, L. E. M., HARVEY, J. A. & DICKE, M. 2012. Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biology 10: e1001435.
45. PACZKOWSKI, S., PACZKOWSKA, M., DIPPEL, S., FLEMATTI, G. & SCHUETZ, S. 2014. Volatile combustion products of wood attract Acanthocnemus nigricans (Coleoptera: Acanthocnemidae). Journal of Insect Behavior 27:228–238.
46. NAIK, D. G., PUNTAMBEKAR, H. & ANANTPURE, P. 2010. Essential oil of Terminalia chebula fruits as a repellent for the Indian honeybee Apis florea. Chemistry & Biodiversity 7:1303–1310.
47. MORENO, J. & PEINADO, R. 2012. Enological chemistry. Academic Press, London. 442 pp.
48. FURSTENBERG-HAGG, J., ZAGROBELNY, M. & BAK, S. 2013. Plant defense against insect herbivores. International Journal of Molecular Sciences 14:10242–10297.
49. MICHALOWICZ, J. & DUDA, W. 2007. Phenols – sources and toxicity. Polish Journal of Environmental Studies 16:347–362.
50. AYASSE, M., SCHIESTL, F. P., PAULUS, H. F., IBARRA, F. & FRANCKE, W. 2003. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proceedings of the Royal Society B–Biological Sciences 270:517–522.
51. PATRICIO, E., LOPEZ, L. C., MAILE, R. & MORGAN, E. D. 2003. Secretions of stingless bees: the Dufour glands of some Frieseomelitta species (Apidae, Meliponinae). Apidologie 34:359–365.
52. JAKUBSKA-BUSSE, A., JASICKA-MISIAK, I., POLIWODA, A., SWIECZKOWSKA, E. & KAFARSKI, P. 2014. The chemical composition of the floral extract of Epipogium aphyllum SW. (ORCHIDACEAE): a clue for their pollination biology. Archives of Biological Sciences 66:989–998.
53. FAO/WHO. 2007. Evaluation of certain food additives and contaminants: sixty-eighth report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO technical report series 947.
54. KHANIZADEH, S. & BELANGER, A. 1995. Leaf essential oil composition of selected strawberry cultivars with different degrees of susceptibility to two-spotted spider mites. HortScience 30:831.
55. BOSMAN, A. A., COMBRINCK, S., ROUX-VAN DER MERWE, R., BOTHA, B. M. & MCCRINDLE, R. 2004. Isolation of an anthelmintic compound from Leucosidea sericea. South African Journal of Botany 70:509–511.
56. MEERUNGRUEANG, W. & PANICHAYUPAKARANANT, P. 2014. Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata. Pharmaceutical Biology 52:1104–1109.
57. MEDIMAGH-SAIDANA, S., DAAMI-REMADI, M., ABREU, P., HARZALLAH-SKHIRI, F., BEN JANNET, H. & HAMZA, M. A. 2014. Asterisulphoxide and asterisulphone: two new antibacterial and antifungal metabolites from the Tunisian Asteriscus maritimus (L.) Less. Natural Product Research 28:1418–1426.
58. LOURENCO, A., NEIVA, D. M., GOMINHO, J., MARQUES, A. V. & PEREIRA, H. 2015. Characterization of lignin in heartwood, sapwood and bark from Tectona grandis using Py-GC-MS/FID. Wood Science and Technology 49:159–175.
59. HANNINEN, T., KONTTURI, E. & VUORINEN, T. 2011. Distribution of lignin and its coniferyl alcohol and coniferyl aldehyde groups in Picea abies and Pinus sylvestris as observed by Raman imaging. Phytochemistry 72:1889–1895.
60. ZIEGENBEIN, F. C., KOENIG, W. A. & HANSSEN, H.-P. 2010. Volatile metabolites from the wood-inhabiting fungi Bjerkandera adusta, Ganoderma applanatum, and Stereum hirsutum. Journal of Essential Oil Research 22:116–118.
61. TAMULI, P., BORUAH, P., NATH, S. C. & LECLERCQ, P. 2005. Essential oil of eaglewood tree: a product of pathogenesis. Journal of Essential Oil Research 17:601–604.
62. EL-SHAZLY, A. M. & HUSSEIN, K. T. 2004. Chemical analysis and biological activities of the essential oil of Teucrium leucocladum Boiss. (Lamiaceae). Biochemical Systematics and Ecology 32:665–674.
63. D’ARCY, B. R., RINTOUL, G. B., ROWLAND, C. Y. & BLACKMAN, A. J. 1997. Composition of Australian honey extractives. 1. Norisoprenoids, monoterpenes, and other natural volatiles from blue gum (Eucalyptus leucoxylon) and yellow box (Eucalyptus melliodora) honeys. Journal of Agricultural and Food Chemistry 45:1834–1843.
64. RAVINSKAR, N., SIDDIQ, A., SEENI, S. & JOSEPH, J. 2015. GC-MS analysis and identification of bioactive constituents from flowers of Toddalia asiatica (Rutaceae). International Journal of Pharma and Bio Sciences 6:1246–1254.
65. VIJISARAL, E. D. & SUBRAMANIAN, A. 2014. GC–MS analysis of ethanol extract of Cyperus rotundus leaves. International Journal of Current Biotechnology 2:19–23.
66. MCGAW, L. J., JAGER, A. K. & VAN STADEN, J. 2002. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia 73:431–433.
67. SEIDEL, V. & TAYLOR, P. W. 2004. In vitro activity of extracts and constituents of Pelargonium against rapidly growing mycobacteria. International Journal of Antimicrobial Agents 23:613–619.
68. AGORAMOORTHY, G., CHANDRASEKARAN, M., VENKATESALU, V. & HSU, M. J. 2007. Antibacterial and antifungal activities of fatty acid methyl esters of the Blind-your-Eye mangrove from India. Brazilian Journal of Microbiology 38:739–742.
69. AMELUNG, W., BRODOWSKI, S., SANDHAGE-HOFMANN, A. & BOL, R. 2008. Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Advances in Agronomy 100:155–250.
70. NAGA, V. K. A., NADEEM, M. D., PARDHA, S. M., MAHENDRAN, B. & BHARATHI, S. 2014. Cumulative activity of the p-coumaric acid and syringaldehyde for antimicrobial activity of different microbial strains. European Journal of Experimental Biology 4:40–43.
71. IBRAHIM, M. N. A., BALAKRISHNAN R. B. S., SHAMSUDEEN, S. BAHWANI, S. A. & ADAM, F. 2012. A concise review of the natural existence, synthesis, properties, and applications of syringaldehyde. BioResources 7:4377–4399.
72. DAAYF, F. & LATTANZIO, V. 2008. Recent advances in polyphenol research. Wiley-Blackwell, Oxford. 416 pp.
73. SHREAZ, S., BHATIA, R., KHAN, N., MURALIDHAR, S., MANZOOR, N. & KHAN, L. A. 2013. Influences of cinnamic aldehydes on H+ extrusion activity and ultrastructure of Candida. Journal of Medical Microbiology 62:232–240.
74. DEV, V., QUINTANA, R. P. & LASSLO, A. 1966. Synthesis of grisan and coumaran-3–1 derivatives with potential insect-repellent properties. Journal of Medicinal Chemistry 9:242–244.
75. ZABETAKIS, I., GRAMSHAW, J. W. & ROBINSON, D. S. 1999. 2,5-dimethyl-4-hydroxy-2H-furan-3-one and its derivatives: analysis, synthesis and biosynthesis – a review. Food Chemistry 65:139–151.
76. LIN, D. Y., SHEA, S. D. & KATZ, L. C. 2006. Representation of natural stimuli in the rodent main olfactory bulb. Neuron 50:937–949.
77. ROTSTEIN, A., LIFSHITZ, A. & KASHMAN, Y. 1974. Isolation and antibacterial activity of acylphloroglucinols from Myrtus communis. Antimicrobial Agents and Chemotherapy 6:539–542.
78. LEE, H. K., LEE, H. S. & AHN, Y. J. 1999. Antignawing factor derived from Cinnamomum cassia bark against mice. Journal of Chemical Ecology 25:1131–1139.