Introduction
Apathy is a common syndrome present in a wide range of neurologic and neuropsychiatric conditions (Levy & Dubois, Reference Levy and Dubois2006; Marin, Reference Marin1991; Starkstein et al., Reference Starkstein, Merello, Jorge, Brockman, Bruce and Power2009). Clinically, apathy is defined as a state of diminished emotions, interests, or motivations, manifested as a quantifiable reduction of self-generated voluntary and purposeful behaviors (Levy & Dubois, Reference Levy and Dubois2006; Marin, Reference Marin1991). Prevalence ranging from 17% to 70% reveals apathetic symptoms as one of the most common neuropsychiatric features in Parkinson's disease (PD) (Aarsland et al., Reference Aarsland, Bronnick, Alves, Tysnes, Pedersen, Ehrt and Larsen2009; Llebaria et al., Reference Llebaria, Pagonabarraga, Kulisevsky, Garcia-Sanchez, Pascual-Sedano, Gironell and Martinez-Corral2008; Pluck & Brown, Reference Pluck and Brown2002). Nevertheless, the mechanisms underlying the expression of apathy in PD remain unclear (Reijnders et al., Reference Reijnders, Scholtissen, Weber, Aalten, Verhey and Leentjens2010; Starkstein, Reference Starkstein2009; Starkstein et al., Reference Starkstein, Merello, Jorge, Brockman, Bruce and Power2009), in part due to the confounding influence of commonly associated non-motor conditions and the absence of studies focusing on apathy in isolation (Aarsland et al., Reference Aarsland, Bronnick, Alves, Tysnes, Pedersen, Ehrt and Larsen2009; Kirsch-Darrow, Fernandez, Marsiske, Okun, & Bowers, Reference Kirsch-Darrow, Fernandez, Marsiske, Okun and Bowers2006; Llebaria et al., Reference Llebaria, Pagonabarraga, Kulisevsky, Garcia-Sanchez, Pascual-Sedano, Gironell and Martinez-Corral2008; Starkstein, Reference Starkstein2009).
Previous studies have established a strong relationship between apathy and executive dysfunction, depression, concurrent progression of cognitive impairment during the course of the disease, and dementia. Apathetic symptoms coexisted with depression and dementia in up to 11% of a sample of PD patients with apathy. Ten percent exhibited apathy and depression without dementia; 6.5% apathy and dementia without depression, and 9% apathy without dementia and depression (Dujardin, Sockeel, Delliaux, Destee, & Defebvre, Reference Dujardin, Sockeel, Delliaux, Destee and Defebvre2009; Dujardin et al., Reference Dujardin, Sockeel, Devos, Delliaux, Krystkowiak, Destee and Defebvre2007; Pedersen, Larsen, Alves, & Aarsland, Reference Pedersen, Larsen, Alves and Aarsland2009). In addition, apathy has been suggested to be a potential predictor of mild cognitive impairment (MCI) and dementia in PD (Dujardin et al., Reference Dujardin, Sockeel, Delliaux, Destee and Defebvre2009). Moreover, a relationship between apathy and more severe motor symptoms has also been proposed for early untreated PD patients (Pedersen et al., Reference Pedersen, Alves, Bronnick, Aarsland, Tysnes and Larsen2010), suggesting the possible involvement of the same frontal-striatal circuits in the development of both symptoms caused by dopaminergic-dependent degeneration of the thalamic projections of the caudate nucleus to the dorsal-lateral prefrontal cortex (DLPFC) (Dujardin et al., Reference Dujardin, Sockeel, Delliaux, Destee and Defebvre2009). However, the presence of apathy during all the stages of the disease in patients without apparent associated cognitive deterioration or other neuropsychiatric features (Aarsland et al., Reference Aarsland, Bronnick, Alves, Tysnes, Pedersen, Ehrt and Larsen2009; Pedersen et al., Reference Pedersen, Alves, Bronnick, Aarsland, Tysnes and Larsen2010; Starkstein, Reference Starkstein2009) and the unclear response of apathy to dopaminergic drugs point toward other possible underlying mechanisms (Dujardin et al., Reference Dujardin, Sockeel, Delliaux, Destee and Defebvre2009; Kulisevsky et al., Reference Kulisevsky, Avila, Barbanoj, Antonijoan, Berthier and Gironell1996; Levy & Dubois, Reference Levy and Dubois2006).
Recent findings have shown impaired facial emotion recognition in cognitively intact PD patients with isolated apathy (Martinez-Corral et al., Reference Martinez-Corral, Pagonabarraga, Llebaria, Pascual-Sedano, Garcia-Sanchez, Gironell and Kulisevsky2010) and a blunted response to monetary rewards in ventro-medial prefrontal cortex (vmPFC), amygdale, striatum, and midbrain (Lawrence, Goerendt, & Brooks, Reference Lawrence, Goerendt and Brooks2011). Moreover, the role of hypodopaminergic stimulation of the basal ganglia-orbitofrontal cortical circuit in apathetic patients with PD has been recently discussed as a possible ethiopathogenic mechanism (Poletti, De Rosa, & Bonuccelli, Reference Poletti, De Rosa and Bonuccelli2012). Accordingly, the participation of structures conforming the frontal-striatal limbic pathway and involving the ventral striatum, have been suggested to participate in the etiology of apathy in association with abnormalities of circuits underlying executive dysfunction (Lawrence et al., Reference Lawrence, Goerendt and Brooks2011).
The comprehension of the cognitive correlates of apathy in PD may help us delineate the underlying neural mechanisms. The aim of this study was to clarify the relationship between apathy and executive functions in PD as well as with other possible cognitive domains. We studied a sample of non-demented PD patients exhibiting no other neuropsychiatric symptom than apathy. We assessed the functionality of distinct prefrontal-subcortical systems using a comprehensive neuropsychological battery assessing global cognitive functioning, frontal-related behaviors, set-shifting, and decision making. Due to the influence that effort may exert over cognitive functioning, we also assessed measures of effort-related cognitive performance.
Methods
Participants
Thirty-seven outpatients regularly attending the Movement Disorders Unit at Sant Pau Hospital and fulfilling diagnostic criteria for PD (Daniel & Lees, Reference Daniel and Lees1993) participated in the study. All the procedures were previously approved by the ethics committee of our center.
Sample selection
The sample was carefully selected based on a preliminary screening visit for the presence of apathetic symptoms as assessed by the Starkstein's Apathy Scale (SAS ≥ 14) (Starkstein et al., Reference Starkstein, Merello, Jorge, Brockman, Bruce and Power2009) during pharmacological “on” condition. This measure allowed us to differentiate those patients presenting clear symptoms of apathy—but not necessarily an apathetic syndrome—to those without clinical signs of apathy. The SAS was specifically developed for PD and consists of 14 items phrased as questions to be answered in a four-point Likert scale and demonstrated good psychometric properties. It is recommended by the Movement Disorders Society for the screening and the assessment of severity of apathy in PD. Based on the prolonged duration of the long-term response of L-Dopa during the early stages of the disease (up to 3 weeks in some patients without motor fluctuations), patients were not tested in the pharmacological “off” condition (Anderson & Nutt, Reference Anderson and Nutt2011).
The presence of depression, anxiety, or impairment on global cognitive functions constituted the main exclusion criteria. Screening for anxiety and depression was assessed using a cutoff score ≥11 in the Anxiety and Depression subscores of the Hospital Anxiety and Depression Scale (HADS) (Mumford, Reference Mumford1991). More accurate assessment for the presence of anxiety or depression was assessed using a clinical interview based on the Diagnostic and Statistical Manual of Mental Disorders, Revision IV (DSM-IV), criteria for affective disorders.
Absence of clinically relevant cognitive impairment was initially screened using the Clinical Dementia Rating scale (CDR = 0) (Morris, Reference Morris1993) and a score >26 in the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975). The Mattis Dementia Rating scale (MDRS) was also administered in the screening visit to more precisely assess global cognitive functioning (Llebaria et al., Reference Llebaria, Pagonabarraga, Kulisevsky, Garcia-Sanchez, Pascual-Sedano, Gironell and Martinez-Corral2008). Absence of visual hallucinations was assessed using Part I of the Hallucinations and Psychosis item of the MDS-UPDRS (Goetz et al., Reference Goetz, Tilley, Shaftman, Stebbins, Fahn, Martinez-Martin and LaPelle2008).
Each patient was interviewed regarding disease onset and medication history, type of motor response to L-Dopa (LD), and current medication and dosage (LD daily dose, dopaminergic agonists - LD equivalent daily dose [DA-LEDD]). All the participants were taking stable doses of dopaminergic drugs with stable response in the 12 weeks before the study. Any patient was taking antipsychotic/antidepressant drugs at the time of study. Motor status and severity of disease were assessed by neurologists with extensive experience in movement disorders (J.P. and J.K.) using the Unified Parkinson's Disease Rating Scale (UPDRS) and the Hoehn and Yahr classification (H&Y) (Hoehn & Yahr, Reference Hoehn and Yahr1967).
We also excluded patients with abnormalities in neuroimaging studies, blood tests, and non-compensated systemic diseases.
Twenty patients with apathy and 17 without apathy were selected to participate in the study. This sample is not representative of the prevalence of apathy in PD; this group was specifically selected to participate in this study to allow the presence of a sufficient number of apathetic patients, accomplishing the pre-established enrollment criteria.
Materials And Procedures
Neuropsychological Assessment
Cognition and behavior were assessed by an experienced neuropsychologist in movement disorders (S.M.H.). Testing followed a fixed sequence and was completed over a period of 90 min. All patients were assessed during the pharmacological “on” condition.
1. Global cognitive functioning
The Parkinson's Disease - Cognitive Rating Scale (PD-CRS) (Pagonabarraga et al., Reference Pagonabarraga, Kulisevsky, Llebaria, Garcia-Sanchez, Pascual-Sedano and Gironell2008) was used to assess a wide range of cognitive domains (memory, attention, visuospatial-visuoconstructive skills, and frontal functions). The PD-CRS is a brief cognitive battery specifically designed to assess cognition in PD. The analysis of the PD-CRS provides a total score of global cognitive functioning and also a frontal-subcortical and cortical composite score (Pagonabarraga et al., Reference Pagonabarraga, Kulisevsky, Llebaria, Garcia-Sanchez, Pascual-Sedano and Gironell2008).
2. Frontal-related behavior (FrSBe)
The “family rating form” of the Frontal Systems Behavior Scale (FrSBe) was completed by a familiar companion of the patient to assess the presence of frontal-related behavioral symptoms such as apathy, disinhibition, and executive dysfunction (Grace & Malloy, Reference Grace and Malloy2001). The FrSBe has demonstrated excellent psychometric properties and validity to assess fronto-striatal–dependent behavioral changes in PD (Zgaljardic, Borod, Foldi, & Mattis, Reference Zgaljardic, Borod, Foldi and Mattis2003).
3. Set-shifting (WCST)
The computerized versions of the WCST in their original format of 128 cards (Heaton, Reference Heaton1981) were administered. The WCST constitutes a common tool used to assess executive functions. Neuroimaging studies associated set-shifting processes with dorsal-lateral prefrontal cortex (DLPFC) (Nagahama et al., Reference Nagahama, Fukuyama, Yamauchi, Matsuzaki, Konishi, Shibasaki and Kimura1996). In addition, based on the frontal-executive defects mainly characterizing PD patients, the WCST has been proved to be sensible detecting initial signs of cognitive impairment in PD (Lees & Smith, Reference Lees and Smith1983).
In the WCST, subjects are asked to place a total number of 128 cards on below four different stimulus cards. Every card had one to four symbols (circle, triangle, square, or star) in different colors (red, blue, green, or yellow). Subjects have to learn possible sorting rules according to number, color, or shape by using the feedback (correct or incorrect) of the previous trial. After an unpredictable change of the sorting rule, subject must learn the new rule. The WCST assesses categorization, set-shifting, cognitive flexibility, perseveration, and ability to use feedback (Heaton, Reference Heaton1981).
4. Decision making (IGT)
The IGT is a useful tool to assess decision making, risk-taking, and reward-related learning in ambiguous situations (Bechara, Damasio, Damasio, & Anderson, Reference Bechara, Damasio, Damasio and Anderson1994). Neuroimaging studies mainly involved limbic system structures, the vmPFC, and the orbital prefrontal cortex (OPFC) in tasks like the IGT (Bechara, Tranel, & Damasio, Reference Bechara, Tranel and Damasio2000; Poletti et al., Reference Poletti, Frosini, Lucetti, Del Dotto, Ceravolo and Bonuccelli2010).
The IGT is based on the selection of one card of four possible selections (A, B, C, and D) for every trial during 100 trials. After card selection, feedback is immediately provided, indicating related gains and/or related gains and losses. Cards A and B are related to higher immediate gains but unpredictable major losses, while cards C and D are related to minor immediate gains but also minor unpredictable losses. Accordingly, cards A and B are defined as “disadvantageous or risky” and D and C as “advantageous or secure.” Participants were instructed to gain as much money as possible and to avoid losing as much money as possible. Task performance was analyzed by subtracting the total number of disadvantageous selections (A and B) from the total number of advantageous choices (C and D). This was computed for the total net scores and for the scores obtained every 20 selections to analyze decision making during performance. The higher the net scores, the better participants performed the task.
Additionally, we also analyzed the use of negative feedback during the gambling task (Brand et al., Reference Brand, Kalbe, Labudda, Fujiwara, Kessler and Markowitsch2005; Euteneuer et al., Reference Euteneuer, Schaefer, Stuermer, Boucsein, Timmermann, Barbe and Kalbe2009). The “used” or “non-used” negative feedback was computed based on the decision made immediately after the previous outcome. When participants selected a disadvantageous card (A or B) and received a negative feedback (losses ranging between 150€ and 1250€), if they selected again a disadvantageous card, this was defined as “non-used negative feedback.” Conversely, if they selected advantageous cards (C or D), it was computed as “used negative feedback.”
5. Cognitive effort
The influence that lack of effort exerts over cognitive performance should be considered to take into account motivation and cognitive effort during neuropsychological assessment (Lange, Iverson, Brooks, & Rennison, Reference Lange, Iverson, Brooks and Rennison2010; O'Bryant et al., Reference O'Bryant, Gavett, McCaffrey, O'Jile, Huerkamp, Smitherman and Humphreys2008). Based on the clinical characteristics of apathy (Marin, Reference Marin1991), we included the assessment of cognitive effort using the Trial 1 of the Test of Memory Malingering (TOMM). The Trial 1 of the TOMM appeared as a proper validated measure to assess cognitive effort (Bauer, O'Bryant, Lynch, McCaffrey, & Fisher, Reference Bauer, O'Bryant, Lynch, McCaffrey and Fisher2007; O'Bryant et al., Reference O'Bryant, Gavett, McCaffrey, O'Jile, Huerkamp, Smitherman and Humphreys2008). The TOMM test is a forced choice visual recognition memory test for adults, consisting of two learning trials and a 15-min delayed retention trial. During the learning trials, 50 line drawings of common objects are shown for a period of 3 s each. Following the learning trials, 50 recognition trials—with two possibilities on each where just one is correct—are presented. The subject is asked to select which possibility appeared in the previous learning trial. Despite that TOMM is usually administered to detect malingering, numerous studies demonstrated the validity of the Trial 1 of the TOMM to assess cognitive effort (O'Bryant et al., Reference O'Bryant, Gavett, McCaffrey, O'Jile, Huerkamp, Smitherman and Humphreys2008; Teichner & Wagner, Reference Teichner and Wagner2004) and is the most commonly administered “symptom validity test” to assess cognitive effort when performing a neuropsychological assessment (Bauer et al., Reference Bauer, O'Bryant, Lynch, McCaffrey and Fisher2007; Slick, Tan, Strauss, & Hultsch, Reference Slick, Tan, Strauss and Hultsch2004). Moreover, the TOMM is relatively unaffected by age, education, anxiety, and depression, or by cognitive impairment due to most forms of neuropathology (Ashendorf, Constantinou, & McCaffrey, Reference Ashendorf, Constantinou and McCaffrey2004; Rees, Tombaugh, & Boulay, Reference Rees, Tombaugh and Boulay2001; Teichner & Wagner, Reference Teichner and Wagner2004).
Statistical Analysis
Data are expressed as means ± standard deviation (SD) for the continuous variables, as percentage for the categorical variables and as mean range for the ordinal variables. Group differences in demographic, clinical, cognitive, and behavioral characteristics between groups were analyzed with independent two-tailed t tests for continuous variables, Mann-Whitney test for ordinal data, and the χ2 test for categorical variables. Significance was set at p < .05. On the FrSBe, raw scores were corrected for age, sex, and educational level and transformed to typified scores (TS) using the existing normative data for Spanish population. Correlation analysis was performed controlling for age, medication, and disease duration. Multiple stepwise regression analysis was performed to evaluate the correlation between different scores and to examine the relationship between apathy and other variables. IGT Total and WCST Number of Categories were separately used as dependent variable. We also evaluated the influence of these scores over the PD-CRS subtests and the FrSBe scale. Socio-demographic and neuropsychiatric measures (age, education level, PD evolution, UPDRS-III, HADS, and Starkstein Apathy Scale score) were included as independent variables of adjustment.
Results
Thirty-seven non-depressed PD patients with preserved global cognitive function, with (n = 20; age = 68.2; disease duration = 6.7) and without apathy (n = 17; age 65.06; disease duration = 5.38) were included in the study. As shown in Table 1, groups were carefully matched for age, sex, educational level, and main clinical characteristics.
Table 1 Clinical and socio-demographic data of apathetic versus non-apathetic PD patients

UPDRS = Unified Parkinson's Disease Rating Scale; DA = dopamine agonist; MMSE = Mini-Mental State Examination; MDRS = Mattis Dementia Rating Scale; HADS = Hospital Anxiety and Depression Scale; SAS = Starkstein's Apathy Scale.
Screening for apathy based on the SAS in our sample was supported by a significant positive correlation between SAS scores and the Apathy subscore of the FrSBe (rho = 0.727; p < .0001). According to the apathy scores on the SAS (21.75 ± 5.35) and the FrSBe (69.15 ± 15.9), the severity of apathy was rated as moderate in our sample of apathetic patients.
No statistically significant differences where found between groups when compared the clinical and socio-demographic characteristics. Despite no raised significance, a tendency was found for the DA-LEDD comparison showing that apathetic patients were taking higher doses of DA (176.7 ± 128 vs. 105 ± 83; t, p = .05).
Neuropsychological Assessment
Global cognitive functioning (PD-CRS)
Significant differences were found between groups in different subtests of the PD-CRS. Apathetic patients performed worse on verbal memory (7.15 ± 1.1 vs. 8.13 ± 1.2; t, p = .01), confrontation naming (18.4 ± 1.5 vs. 19.3 ± 0.7; t, p = .03), delayed free recall (4.05 ± 2.13 vs. 6.31 ± 1.51; t, p < .001), alternating verbal fluency (9.15 ± 2.8 vs. 10.7 ± 1.6; t, p = .05), and action verbal fluency (12.50 ± 4.19 vs. 16.88 ± 3.71: t, p = .002). Differences were also found on the Frontal-Subcortical composite score (55.1 ± 9.7 vs. 65.5 ± 7.9; t, p = .001), Cortical composite score (27.7 ± 1.5 vs. 28.4 ± 0.8; t, p = .001), as well as in the Total Score (84.5 ± 11.1 vs. 92.6 ± 9.5; t, p = .02).
Despite these differences, both groups scored within the normal range in the PD-CRS CRS Total score (Table 2). Accordingly to the proposed criteria for MCI and dementia in PD (Emre et al., Reference Emre, Aarsland, Brown, Burn, Duyckaerts, Mizuno and Dubois2007; Litvan et al., Reference Litvan, Aarsland, Adler, Goldman, Kulisevsky, Mollenhauer and Weintraub2011), the sample was classified as cognitively intact.
Table 2 Neuropsychological performance of apathetic and non-apathetic patients
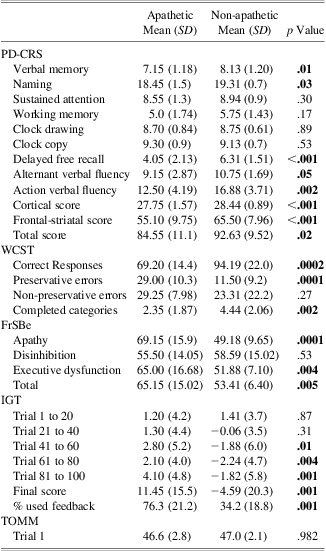
PD-CRS = Parkinson's Disease Cognitive Rating Scale; WCST = Wisconsin card sorting test; FrSBe = Frontal systems behavior scale; IGT = Iowa Gambling Task; TOMM = Test of Memory Malingering.
Frontal-related behavior (FrSBe)
The analysis of the FrSBe (Figure 1) showed significant differences for the apathetic patients on apathy (69.15 ± 15 vs. 49.18 ± 9; t, p < .001), executive dysfunction (65 ± 16 vs. 51.8 ± 7; t, p = .004), and total scores (65.1 ± 15 vs. 53.4 ± 6; t, p = .005). In addition, while in non-apathetic the FrSBe scores remained under the proposed clinically significant cutoff score, apathy, executive dysfunction, and total scores raised the clinical significance in the apathetic group (Grace & Malloy, Reference Grace and Malloy2001).

Fig. 1 Frontal Systems Behavior Scale profiles for apathetic and non-apathetic patients. Mean typified scores corrected by age, educational level and sex. Dashed line indicates the proposed cut-off score.
Set-shifting (WCST)
Based on normative data (Heaton, Reference Heaton1981), both groups performed the WCST in a pathological range as exhibited by the number of completed categories and perseverative errors (Table 2). However, apathetic patients performed significantly worse on both measures (Perseverative errors: 29 ± 10.3 vs. 11.5 ± 9.2; t, p < .0001; Completed categories: 2.34 ± 1.87 vs. 4.44 ± 2.06; t, p = .002).
Decision making (IGT)
Notably, as shown in Figures 2 and 3, apathetic patients performed significantly better on the IGT than non-apathetic patients. While apathetic patients scored within the normal range, non-apathetic patients progressively performed worse, making risky selections along the task. Final score, provided by the total amount of advantageous choices ([C+D]-[A+B]), was significantly better in apathetic patients (11.4 ± 15 vs. −4.5 ± 20; t, p = .001). Apathetic patients made less disadvantageous choices from the third block of trials (trials 41 to 60; t, p = .01), and progressively chose more safety during the rest of the task (trials 61 to 80; t, p = .004 and trials 81 to 100; t, p = .001).

Fig. 2 Title: Iowa Gambling Task performance across trials. Mean scores were presented every 20 selections by subtracting highly risky choices (A+B) to low risky choices (C+D) in both groups.

Fig. 3 Iowa Gambling Task final scores for apathetic and non-apathetic patients. Bars represents total scores for both groups computed by subtracting total disadvantageous choices to total advantageous choices.
In addition, the analysis of the rate of feedback use, demonstrated significant differences between groups. Comparison of the percentage of used and non-used negative feedback raised the statistical significance, exhibiting that apathetic patients used the negative feedback in up to 76.3% of the trials, while non-apathetic patients used it in up to 34.2% of them (χ2, p < .001) (Figure 4). In addition, correlation analysis showed a strong positive relationship between apathy scores and use of negative feedback (r = 0.716; p < .001) (Figure 5).

Fig. 4 Percentage of used negative feedback. Bars represents mean percentage of used negative feedback.

Fig. 5 Correlations between severity of apathy, cognition, and behavior. Relationship between (a) severity of apathy and FrSBe executive dysfunction subscore, (b) preservative errors on the WCST, (c) IGT final score and (d) PD-CRS subcortical score.
Cognitive effort
As proposed in previous studies (Bauer et al., Reference Bauer, O'Bryant, Lynch, McCaffrey and Fisher2007; O'Bryant et al., Reference O'Bryant, Gavett, McCaffrey, O'Jile, Huerkamp, Smitherman and Humphreys2008), data obtained from Trial 1 of the TOMM test were used to assess cognitive effort. No significant differences appeared when apathetic and non-apathetic patients were compared (see Table 2).
Regression analysis
Multiple stepwise regressions showed apathy as the major factor related to the performance on the different tasks. IGT total score (p = .043), WCST correct responses (p = .003), preservative errors (p < .0001), and completed categories (p = .004) showed significant correlation with apathy but not with any other factor (Table 3). The same analysis was calculated for the PD-CRS subscores, revealing the influence of apathy over the PD-CRS subtests. A significant correlation appeared between SAS score and verbal learning (p = .003), naming (p = .020), delayed free recall (p < .0001), and alternating (p = .048) and action verbal fluency (p = .002). Similar significant correlations were found between SAS and the PD-CRS subcortical (p < .0001) and total scores (p = .017) (Table 4).
Table 3 Multivariate stepwise regression analysis between IGT and WCST and other independent variables

Table 4 Multivariate stepwise regression analysis between PD-CRS subscales and other independent variables

Interestingly, the PD-CRS cortical composite score was found significantly related to disease duration (p = .018), also when SAS score was included into the analysis (p = .034). For the FrSBe scale, a significant relationship was found between the SAS and executive dysfunction (p = .001), apathy (p < .0001), and total scores (p < .0001) (Table 5).
Table 5 Multivariate stepwise regression analysis between FrSBe and other independent variables

Discussion
In the present study, we used a comprehensive neuropsychological assessment battery to further clarify the relationship between apathy and cognition in PD. Compared to previous studies, the main strengths are methodological. Highly selected PD patients presenting significant apathetic symptoms as isolated neuropsychiatric features were compared to a carefully matched sample of non-apathetic patients. Moreover, both groups scored within normal ranges in PD-validated global cognitive scales (MDRS and PD-CRS). We propose that our study is one of the few addressing apathy in PD as the unique non-motor feature, avoiding the overlapping effect of other variables, such cognitive impairment, cognitive effort, anxiety, or depression. The main findings of our study are that apathetic patients (i) performed significantly worse and below normal ranges in provided measures of executive functioning in terms of behavior (FrSBe) and cognitive performance (WCST); (ii) obtained significant worse scores, both in composite and total scores of a PD specific cognitive battery (PD-CRS); and (iii) performed significantly better and within normal ranges in a decision-making task (IGT) in which non-apathetic patients exhibited an abnormal performance. Moreover, this pattern of poorer cognitive performance was not linked to lack of effort. Our results consistently support the construct of apathy as an isolated syndrome that can be present in the early and middle stages of PD and as a clinically meaningful symptom, also in the absence of significant cognitive deterioration but with a clear involvement of executive dysfunction.
A significant pattern of executive dysfunction differentiated between apathetic and non-apathetic PD patients. As measured by the PD-CRS, both groups scored as cognitively intact. However, significant differences between groups in the composite frontal-subcortical, cortical, and total score supported a relationship between some degree of cognitive impairment and apathy in PD (Levy & Dubois, Reference Levy and Dubois2006). Moreover, the clinically significant scores obtained for the executive dysfunction subscore in the FrSBe and in the WCST accounted for a relationship between executive dysfunction and apathy in PD.
From the very beginning of the disease, executive dysfunction (i.e., set-shifting, and attention and working memory deficits) characterizes the initial signs of cognitive impairment in PD (Muslimovic, Post, Speelman, & Schmand, Reference Muslimovic, Post, Speelman and Schmand2005). The characteristic dopaminergic denervation of early and mid-stages of PD, mainly involving the nigrostriatal projections to the dorsal caudate nucleus and DLPFC (Yeterian & Pandya, Reference Yeterian and Pandya1991), explain the pattern of worse executive performance of PD patients when they are compared to normal controls (Kulisevsky et al., Reference Kulisevsky, Avila, Barbanoj, Antonijoan, Berthier and Gironell1996). Accordingly, dopaminergic replacement therapy addressed to restore motor symptoms is also associated with an incomplete but clinically significant improvement of the dysexecutive syndrome (Cools, Stefanova, Barker, Robbins, & Owen, Reference Cools, Stefanova, Barker, Robbins and Owen2002; Kulisevsky et al., Reference Kulisevsky, Avila, Barbanoj, Antonijoan, Berthier and Gironell1996; Pascual-Sedano et al., Reference Pascual-Sedano, Kulisevsky, Barbanoj, Garcia-Sanchez, Campolongo, Gironell and Gich2008). Based on the pathophysiology of executive dysfunction in PD, more impaired executive functioning in apathetic PD patients account for the involvement of a greater degeneration of the nigrostriatal pathway in these patients (Braak, Bohl, et al., Reference Braak, Bohl, Muller, Rub, de Vos and Del Tredici2006; Braak, Muller, et al., Reference Braak, Muller, Rub, Ackermann, Bratzke, de Vos and Del Tredici2006). Previous studies linked apathy, executive dysfunction, and the degeneration of the nigrostriatal pathway showing worse UPDRS motor scores for apathetic patients (Pedersen et al., Reference Pedersen, Alves, Bronnick, Aarsland, Tysnes and Larsen2010). Despite that our sample does not exhibited differences in the UPDRS motor scores, the tendency of apathetic patients to be under higher doses of DA during the study might suggest a compensatory mechanism with apathetic patients suffering a more severe form of the disease than non-apathetic patients. However, assessing our patients while pharmacological “on” prevented the capture of possible significant differences in basal motor scores. Nevertheless, a more aggressive degeneration in apathetic PD patients of the frontal-subcortical circuits involving both motor and executive functions might account for the proposed hypodopaminergic stimulation of the basal ganglionic-orbitofrontal cortical circuit in apathetic patients with PD (Poletti et al., Reference Poletti, De Rosa and Bonuccelli2012). All this circuitry has been consistently associated to cognitive functions such working memory, generative behaviors, and set-shifting (Cummings, Reference Cummings1993, Reference Cummings1998), the same processes that we observed more disturbed in our sample of apathetic patients.
Regression analysis controlling for clinical and demographic variables confirmed a strong relationship between apathy and executive dysfunction as measured using the WCST, the FrSBe, and the frontal-subcortical composite score of the PD-CRS. As previously reported, our data support a relationship between apathy and executive dysfunction in PD (Dujardin et al., Reference Dujardin, Sockeel, Devos, Delliaux, Krystkowiak, Destee and Defebvre2007; Levy & Dubois, Reference Levy and Dubois2006; Pluck & Brown, Reference Pluck and Brown2002). Apathetic patients also exhibited problems in verbal memory, both for immediate learning and delayed free recall, confrontation naming, and alternating and action verbal fluency. Interestingly, regression analysis also revealed a significant negative correlation between apathy scores and confrontation naming.
Along this line, whereas PD executive dysfunction may show a progressive linear decrease along the course of disease but not necessarily occurring in dementia, the addition of cortical defects has been proposed as strong predictors for conversion to dementia (Aarsland, Muniz, & Matthews, Reference Aarsland, Muniz and Matthews2011; Williams-Gray et al., Reference Williams-Gray, Evans, Goris, Foltynie, Ban, Robbins and Barker2009). In PD, the involvement of cortical defects has been explained by the inclusion of non-dopaminergic mechanisms such cholinergic alterations and alpha-synuclein depositions (Masliah et al., Reference Masliah, Rockenstein, Adame, Alford, Crews, Hashimoto and Schenk2005; Starkstein, Reference Starkstein2010). Accordingly, our results linking cortical defects and apathy, suggest that apathy occurs beside a subclinical pattern of cognitive impairment, not just characterized as executive dysfunction, where subtle, but significant cognitive defects are present. It should explain in part, the construct of apathy as a heralding sign of MCI and dementia and why apathetic symptoms are more severe in cognitively impaired and demented PD patients. Additionally, it may propose an initial explanation concerning the poor response of apathy to dopaminergic replacement therapy by linking the expression of this syndrome to non-dopaminergic mechanisms.
We also observed that apathetic patients performed significantly better on a decision-making task based on risk-taking (IGT) in terms of final net score and use of negative feedback. A good performance on the IGT requires the integrity of sensitivity to reward and punishment as well as the avoidance to generate risky behaviors (Brand et al., Reference Brand, Kalbe, Labudda, Fujiwara, Kessler and Markowitsch2005). Based on outcomes after card selections, healthy subjects progressively learn to select less disadvantageous decks to avoid major punishments. This process mediated by reward-based learning has been consistently linked to the functionality of the mesocortico-limbic pathway and related structures (i.e., ventral striatum, OPFC) (Euteneuer et al., Reference Euteneuer, Schaefer, Stuermer, Boucsein, Timmermann, Barbe and Kalbe2009). Impulsive patients such as those characterizing addictive behaviors or obsessive-compulsive disorders exhibited a bad performance in this task (Brand et al., Reference Brand, Kalbe, Labudda, Fujiwara, Kessler and Markowitsch2005; da Rocha, Alvarenga, Malloy-Diniz, & Correa, Reference da Rocha, Alvarenga, Malloy-Diniz and Correa2011).
However, in PD, the performance on the IGT and other reward-related tasks has been shown to be modulated by the dopaminergic drugs (Frank, Seeberger, & O'Reilly, Reference Frank, Seeberger and O'Reilly2004; Poletti et al., Reference Poletti, Frosini, Lucetti, Del Dotto, Ceravolo and Bonuccelli2010). Due to the relatively integrity of the mesocortico-limbic pathway and related structures in the early and middle stages of the disease, PD patients “on” medication exhibited an abnormal performance on these tasks resulting from the “overdosing” effects that dopaminergic drugs exerts over non-depleted circuitry (Aarts et al., Reference Aarts, Helmich, Janssen, Oyen, Bloem and Cools2012; Cools, Altamirano, & D'Esposito, Reference Cools, Altamirano and D'Esposito2006). This singularity depends on the inverted U-shape relationship between optimal levels of dopamine and cognition (Gotham, Brown, & Marsden, Reference Gotham, Brown and Marsden1986).
Numerous studies demonstrated the deleterious effect of dopaminergic replacement therapy over decision-making and reward-related tasks (i.e., reversal learning task) in PD patients “on” medication (Cools et al., Reference Cools, Stefanova, Barker, Robbins and Owen2002; Frank et al., Reference Frank, Seeberger and O'Reilly2004; Poletti et al., Reference Poletti, Frosini, Lucetti, Del Dotto, Ceravolo and Bonuccelli2010). Accordingly, the poor performance observed in the sample of non-apathetic patients over decision making and the poor use of negative feedback might be explained as a result of this overdosing status. However, based on the clinical characteristics of apathy and the prominent nature of the IGT as a risk-taking task, the observed performance might also depend on the absence of risk-taking mediated by apathetic symptoms.
Other possible explanations link previous observations regarding a high sensitivity to reward and low sensitivity to punishment exhibited by PD patients in pharmacological “on” condition (Frank et al., Reference Frank, Seeberger and O'Reilly2004). From this approach, the prospect of a large gain outweighs any prospect of loss, as indicated by the exhibited impairment on the IGT. This mechanism of hypersensitivity to reward observed in other studies and in our sample of non-apathetic PD patients has served as an argument to explain the risk for developing impulse control disorders (ICD) in PD under dopaminergic replacement therapy.
Along this line, patients who developed ICD or any other condition linked to disinhibition consistently performed wrong the IGT and other reward-related tasks (Rossi et al., Reference Rossi, Gerschcovich, de Achaval, Perez-Lloret, Cerquetti, Cammarota and Leiguarda2010). Conversely, our sample of apathetic PD patients exhibited the opposite pattern, showing a better performance on the IGT as well as a significant higher use of negative feedback. If we consider apathy as behaviorally the opposite of disinhibition, results highly interesting that the performance pattern on a task highly sensible to disinhibition also resulted as the opposite. Taking into account our results, the higher disinhibited, less apathetic the patient was, the worse the patient performed on the IGT; whereas the higher apathetic, less disinhibited the patient was, the better the patient performed on the IGT.
Based on the overdose hypothesis, it may be conceptualized as resulting from an absence of overdosing effects. The ventral striatum and the mesocortico-limbic circuitries have been consistently linked to motivation and reward processing (Burke, Tobler, Schultz, & Baddeley, Reference Burke, Tobler, Schultz and Baddeley2010; Schultz, Dayan, & Montague, Reference Schultz, Dayan and Montague1997). Despite assumed that these structures are not specifically affected by dopamine depletion in PD, some degree of degeneration of them may serve as an explanation for the absence of deleterious effects of dopaminergic replacement treatment over related cognitive functions. As observed in other studies, the effects of dopaminergic therapy on ventral striatum function depend on the duration of the disease (Macdonald et al., Reference Macdonald, Monchi, Seergobin, Ganjavi, Tamjeedi and Macdonald2012). In more advanced stages, the eventual deleterious effects of dopamine over reward-based learning appear less pronounced (Macdonald et al., Reference Macdonald, Monchi, Seergobin, Ganjavi, Tamjeedi and Macdonald2012). On the basis of this model, a more diffuse pattern of neurodegeneration in apathetic patients with early involvement of the ventral striatum might partially explain the observed IGT pattern. It may indicate that some degree of degeneration along the mesocortico-limbic circuitry is present in—and/or underlies—the behavioral expression of apathy in PD. Accordingly, better performance on the IGT should be explained as a consequence of repositioning the curve to the optimal or better levels to perform the task.
Moreover, the relationship between apathy, absence of disinhibition, and normalized reward-related processing may indicate that apathy can act as “protective” to the development of ICD. However, a single explanation for this performance pattern based on the avoidance of risk-taking mediated by the clinical characteristics of apathy could not be refused.
Some limitations of the present design should be taken into account in future studies. Motor, cognitive, and neuropsychiatric assessment of the same sample, both in pharmacological “on” and “off” conditions, may serve to prove the dissociated effects of dopaminergic therapy over decision making as a function of the presence or not of apathy. In addition, neuroimaging studies are needed to prove the existence of the more severe degeneration here proposed. Nevertheless, this study is the first one addressing the issue of apathy in PD controlling for all the other variables that may act as confounding factors (i.e., other neuropsychiatric symptoms, cognitive impairment, or dementia).
In conclusion, apathy may be present as an isolated syndrome also during the early and middle stages of PD. This syndrome is different than depression and does not necessarily appear in cognitively impaired or demented patients. Apathy appeared linked to a more severe motor affectation and is accompanied by a pattern of behavioral and cognitive impairment mainly involving executive dysfunction as well as defects that have been associated with cortical malfunctioning (i.e., confrontation naming). Interestingly, the performance of apathetic PD patients in decision making may involve the degeneration of the mesocortico-limbic and/or related structures in this syndrome. Further studies focused on neuroimaging, as well as controlling for pharmacological “on” and “off” conditions are needed to consistently clarify the underlying mechanisms of apathy in PD.
Acknowledgments
This study was funded in part by Fundació La Marató de TV3 (060310) and by public research grants from CIBERNED (Fundación CIEN, Instituto de Salud Carlos III, Spain). The authors report no conflicts of interest.












