Introduction
Mental disorders are a major contributor to the global burden of disease and one of the foremost healthcare challenges of this century. Every year, over a third (164.7 million persons) of the total EU population is affected by mental illness (Wittchen et al. Reference Wittchen, Jacobi, Rehm, Gustavsson, Svensson, Jonsson, Olesen, Allgulander, Alonso, Faravelli, Fratiglioni, Jennum, Lieb, Maercker, van Os, Preisig, Salvador-Carulla, Simon and Steinhausen2011). Given these issues, prevention strategies are needed. One potentially modifiable target for prevention is cardiovascular fitness, the overall capacity of the cardiovascular and respiratory systems and the ability to carry out prolonged vigorous exercise (Ortega et al. Reference Ortega, Ruiz, Castillo and Sjostrom2008). Cardiovascular fitness is a state that is in part genetically determined. It is also highly influenced by physical activity. Multiple aspects of brain function are positively affected by physical activity (Hillman et al. Reference Hillman, Erickson and Kramer2008) and both cross-sectional (De Moor et al. Reference De Moor, Beem, Stubbe, Boomsma and De Geus2006; Muhsen et al. Reference Muhsen, Lipsitz, Garty-Sandalon, Gross and Green2008; Harvey et al. Reference Harvey, Hotopf, Overland and Mykletun2010) and shorter term longitudinal epidemiological studies (Motl et al. Reference Motl, Birnbaum, Kubik and Dishman2004; Strohle et al. Reference Strohle, Hofler, Pfister, Muller, Hoyer, Wittchen and Lieb2007; Sanchez-Villegas et al. Reference Sanchez-Villegas, Ara, Guillen-Grima, Bes-Rastrollo, Varo-Cenarruzabeitia and Martinez-Gonzalez2008; Pasco et al. Reference Pasco, Williams, Jacka, Henry, Coulson, Brennan, Leslie, Nicholson, Kotowicz and Berk2011; Ten Have et al. Reference Ten Have, de Graaf and Monshouwer2011) provide growing evidence of a link between physical activity and mental health. Fewer studies focus on the relationship between fitness and mental disorders. The distinction between cardiovascular fitness and physical activity is important as there is robust evidence that low cardiovascular fitness is a strong predictor of all-cause mortality, cardiovascular disease, and general ill health, also after physical activity has been taken into consideration (Kodama et al. Reference Kodama, Saito, Tanaka, Maki, Yachi, Asumi, Sugawara, Totsuka, Shimano, Ohashi, Yamada and Sone2009; Bouchard et al. Reference Bouchard, Blair and Katzmarzyk2015).
It is also well known that most of the major mental disorders are impacted by familial influences, such as genetics, upbringing, and shared environment (Hettema et al. Reference Hettema, Neale and Kendler2001). It is yet unclear how potential associations of cardiovascular fitness and mental disorder are affected by familial influences, such as having a sibling with the disorder.
Low cardiovascular fitness at age 18 is associated with increased risk of serious depression and bipolar disorder in adulthood (Aberg et al. Reference Aberg, Waern, Nyberg, Pedersen, Bergh, Aberg, Nilsson, Kuhn and Toren2012) and the authors of a recent meta-analysis concluded that low cardiorespiratory fitness is associated with higher risk of subsequent depression (Schuch et al. Reference Schuch, Vancampfort, Sui, Rosenbaum, Firth, Richards, Ward and Stubbs2016). It remains to be determined whether cardiovascular fitness is prospectively associated with other types of mental disorders. A meta-analysis of 23 studies found that individuals with severe mental illness (schizophrenia, bipolar disorder, and major depression) have significantly lower cardiorespiratory fitness compared with controls (Vancampfort et al. Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst and Stubbs2017), and there is cross-sectional evidence that cardiovascular fitness is reduced already at the first episode of psychosis (Vancampfort et al. Reference Vancampfort, Rosenbaum, Probst, Soundy, Mitchell, De Hert and Stubbs2015). Fitness is also associated with decreased symptoms of anxiety and better coping in clinical samples (DiLorenzo et al. Reference DiLorenzo, Bargman, Stucky-Ropp, Brassington, Frensch and LaFontaine1999) and fitness can be improved with exercise (Vancampfort et al. Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst and Stubbs2017). However, the role of premorbid fitness needs to be elucidated. The aim of the study was to determine the risk of developing serious non-affective mental disorders in a national cohort of over one million men whose cardiovascular fitness was measured using a cycle ergometer test at age 18, during a follow-up time of 3–42 years. Given the known influence of familial factors on both cardiovascular fitness and mental disorders, we also carried out separate analyses utilizing data for full brother pairs.
Method
We performed a population-based prospective study of mentally healthy Swedish men enlisting for military service. Cardiovascular fitness was measured at conscription (baseline) and recorded in the Swedish Military Service Conscription Register. To investigate the influence of cardiovascular fitness and later risk of serious non-affective mental disorders, data from the conscription register were linked to the Swedish National Hospital Discharge Register, the Longitudinal Integration Database for Health Insurance and Labour Market Studies, the Swedish Cause of Death Register, and the Multi-Generation Register. Linkage of individual data was made possible by the unique personal identification number assigned to each person in Sweden. After linkage, all data were coded.
Study population
The source population of the study comprised all Swedish men (n = 1 694 121) who enlisted for military service during 1968–2005. It was then mandatory by Swedish law for all 18-year-old men to enlist. Only individuals with severe chronic medical or mental conditions, serious disabilities or incarcerated individuals were granted exemption (in all 2–3% of the male population per year). Enlistment consisted of a 2-day examination in which all men underwent extensive standardized physical and cognitive testing. Individuals were also assessed by a psychologist during a structured interview. If psychiatric symptoms were identified, they were further seen by a physician, who then diagnosed psychiatric disorders in accordance with the International Classification of Diseases (ICD). Men with prior or ongoing psychiatric disorder were identified through the Swedish National Hospital Discharge Register and/or the Swedish Military Service Conscription Register and excluded from the study population. After excluding men with prior or ongoing psychiatric disorder (n = 56 343) as well as men lacking complete information from the military conscription (e.g. men who could not perform the cardiovascular fitness test due to current infectious disease or other causes, n = 527 992), the final study cohort included 1 109 786 individuals.
Exposure
Cardiovascular fitness was assessed using a bicycle ergometer maximal test where heart rate was constantly measured. The men were instructed to maintain pedal cadence between 60 and 70 rpm. After a normal resting electrocardiogram, the test started with 5 min of submaximal exercise at work rates of 75–175 W (depending on body weight). Work rate was then continuously increased by 25 W/min until volitional exhaustion. The final work rate (Wmax) was recorded and divided by body weight. This measure was used due to a stronger correlation with maximum oxygen consumption (V O2max) (correlation coefficient ~0.9). The resulting value (Wmax/kg) was transformed into stanine scores, with 1 as the lowest and 9 as the maximal performance. As the frequency distribution of cardiovascular fitness was right-shifted, cardiovascular fitness categories were trichotomized as score 1–4 (low), score 5–7 (medium) and score 8–9 (high). The test has been shown to have good reliability and validity (Nordesjö & Schéle, Reference Nordesjö and Schéle1974; Aberg et al. Reference Aberg, Pedersen, Toren, Svartengren, Backstrand, Johnsson, Cooper-Kuhn, Aberg, Nilsson and Kuhn2009).
Outcome
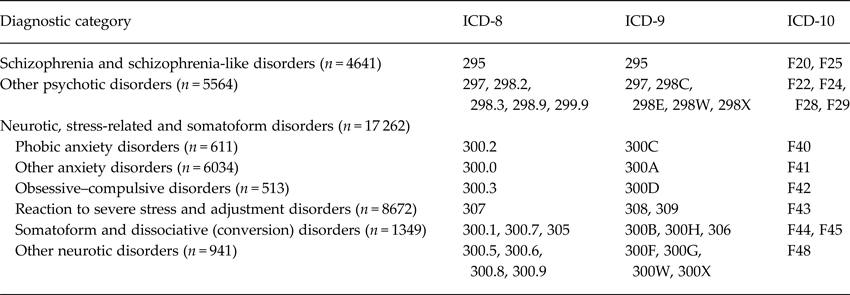
Incident cases of serious non-affective mental disorders during the follow-up period were identified through the Swedish National Hospital Discharge Register. It is mandatory for all hospitals in Sweden, private and publicly funded, to register one principal discharge diagnosis and up to five contributory diagnoses in the Swedish National Hospital Discharge Register (Ludvigsson et al. Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim, Reuterwall, Heurgren and Olausson2011). Register coverage for all inpatient care increased gradually during 1968–1986, and is complete for inpatient psychiatric care since 1973. We use the term serious when referring to the non-affective mental disorders identified in the register. The diagnoses are coded in accordance with ICD versions 8, 9, or 10 (WHO, 1967, 1978, 1992). The specific ICD codes within the diagnostic categories used in the current study are shown in Table 1. To reduce the risk of possible reverse causation, we restricted the analyses to men with first-time diagnoses recorded 3 or more years after conscription. An individual could be included in more than one diagnostic group during the observation period, which lasted from 1 January 1971 to 31 December 2010.
Table 1. Diagnostic categories, frequencies (with 3 years latency from conscription date) and ICD codes, of male conscripts followed up to 42 years in the National Hospital Register
Confounders
Measures of cognitive performance, body mass index (BMI), region, and year for enlistment were obtained from the Swedish Military Service Conscription Register. Cognitive tests measured performance in four cognitive domains: verbal, visuospatial, logical, and technical cognition. A measure of general cognitive performance (IQ) was obtained by combining the results from all four tests with equal weight in order to calculate the mean score. All test results were standardized against data from previous years to follow a Gaussian distribution, resulting in scores from 1 to 9. The normally distributed IQ stanine scores were then trichotomized as scores 1–3 (low), 4–6 (medium), and 7–9 (high). The cognitive performance tests have been employed in other studies (David et al. Reference David, Malmberg, Brandt, Allebeck and Lewis1997; Aberg et al. Reference Aberg, Pedersen, Toren, Svartengren, Backstrand, Johnsson, Cooper-Kuhn, Aberg, Nilsson and Kuhn2009). Information on emigration and parental education (80% coverage) was collected from the Longitudinal Integration Database for Health Insurance and Labour Market Studies (Swedish acronym LISA; http://www.scb.se/Pages/List____257743.aspx) at Statistics Sweden. The LISA database includes data from all Swedish residents aged 16 years and older and is annually updated. Mother's and father's education levels were graded in six levels: from <9 years of pre-high school as the lowest to 2 or more years of college and postgraduate training as the highest. Date of death was obtained from the Swedish Cause of Death Register, which covers virtually all deaths since 1961. Identification of full brothers was performed using the Multi-Generation Register (http://www.scb.se/Pages/List____257501.aspx) at Statistics Sweden.
Statistical analyses
Cox proportional hazards models with 95% confidence interval (CI) were used in order to assess the influence of cardiovascular fitness in mentally healthy 18-year-old males on the first occurrence of the disorders specified in Table 1. The statistical calculations were performed with SAS version 8.1 (SAS Institute, Cary, North Carolina, USA). Cardiovascular fitness was graded as low, medium, and high, with high fitness as the reference group. The follow-up period began at conscription (baseline) and subjects were censored at time of (1) first onset of outcome diagnosis, (2) death, (3) emigration, or (4) at the end of follow-up, i.e. on 31 December 2010 (minimum 3 years and maximum 42 years follow-up). We excluded individuals who fulfilled criteria for any previous or ongoing psychiatric disorders or symptoms at or before conscription (ICD-8 and 9 codes 290–319; ICD-10 codes F00–99), as well as those with outcome diagnoses registered during the first 3 years after conscription. Furthermore, we performed separate analyses excluding individuals diagnosed with depression (ICD-8: 296.0, 296.2, 298.0, 300.4; ICD-9: 296B, 300E, 311, 298A; ICD-10: F32, F33, F34, F38, F39) at or before the time of the specified outcome diagnosis. To minimize possible effects of variation in diagnosis rate and differences in conscription procedures depending on what year the subject enlisted, we adjusted for calendar year by stratifying the Cox model by decade (60s, 70s, etc.). The men enlisted at one of nine different regional test centers and differences among regions and test centers could introduce bias. Therefore, conscription test center was considered a possible confounder and adjusted for. BMI is positively associated with anxiety disorders in adults (Gariepy et al. Reference Gariepy, Nitka and Schmitz2010) and was also included as a confounder, as were both mother's and father's education levels separately. To examine how IQ at age 18 might affect the associations between cardiovascular fitness and future risk of serious non-affective mental disorders, we also performed separate analyses including IQ as a confounder. To assess potential effects of familial factors, subanalyses were performed within full brother pairs. Doing this, many of the early childhood risk factors could be accounted for, including genetic makeup and shared environment such as parental treatment and upbringing. If associations between cardiovascular fitness and the specified disorders were entirely explained by familial conditions, associations should be substantially reduced or even disappear within full brother pairs. A Cox proportional hazard model was used for a subpopulation consisting of all men that had one or more brothers who had enlisted (instead of the population containing all enlisting men), to investigate the influence of cardiovascular fitness in late adolescence on risk of future mental disorders. Presence of a specified non-affective mental disorder in one or more male siblings at any time during the observation period was then included as an additional explanatory variable in these analyses. We also conducted separate sensitivity analyses for all models, excluding all twins, taking the interdependence of observations within twins into account. Due to the large number of observations, p values were very small (in all analyses in which the 95% CI did not include 1, the p values were <0.0001). Therefore, p values are not reported and the risk for type-I errors is considered to be very low. The Ethics Committee of the University of Gothenburg, as well as Confidentiality Clearance at Statistics Sweden approved the study.
Results
During the follow-up period, 4641 men were diagnosed with schizophrenia and schizophrenia-like disorders, 5564 with other psychotic disorders and 17 262 with neurotic, stress-related, and somatoform disorders (Table 1). Within the latter group, over 6000 had an anxiety disorder diagnosis in the hospital register, and there were over 8000 incident cases with reaction to severe stress and adjustment disorders.
Baseline characteristics and proportions of serious non-affective mental disorders by cardiovascular fitness levels are shown in Table 2. The mean follow-up time was 24.9 years [standard deviation (s.d.): 9.6] and the median was 26.0 years. In total, 27 528 903 person-years of follow-up were included. During the follow-up period, 55 697 individuals died or emigrated. Of the total number of individuals diagnosed with schizophrenia and schizophrenia-like disorders, 46.6% also had 1–4 diagnoses within the category other psychotic disorders and 17.4% had 1–4 diagnoses within neurotic, stress-related, and somatoform disorders. Corresponding figures for individuals diagnosed with other psychotic disorders were 38.8% within schizophrenia and schizophrenia-like disorders and 18.9% within neurotic, stress-related, and somatoform disorders. Among individuals with neurotic, stress-related, and somatoform disorders, 4.7% also had a registered diagnosis within schizophrenia and schizophrenia-like disorders, and 6.1% within other psychotic disorders (Table 3).
Table 2. Population baseline characteristics of male conscripts and numbers and proportions with serious non-affective mental disorders during a 3–42 years follow-up period, shown by level of cardiovascular fitness
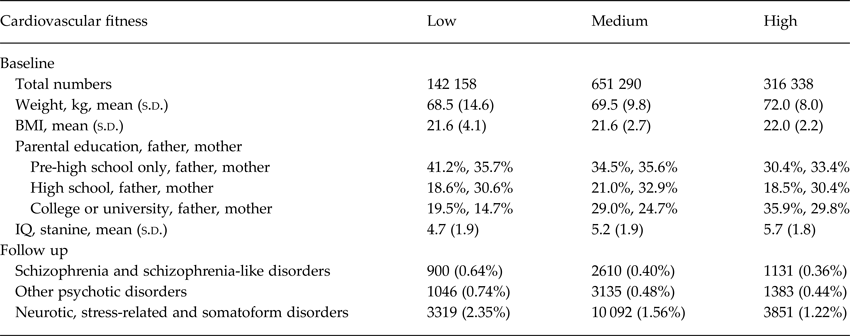
Table 3. Numbers (proportions) with multiple diagnoses, by main diagnostic categories of serious non-affective mental disorders in male conscripts followed up to 42 years

Cardiovascular fitness and risk of serious non-affective mental disorders
There was a graded association for cardiovascular fitness with schizophrenia and schizophrenia-like disorders with a higher risk for individuals with low cardiovascular fitness compared with high (Table 4). Low fitness resulted in a hazard ratio (HR) of 1.44, 95% CI 1.29–1.61 and medium fitness with a HR of 1.13, 95% CI 1.04–1.23 when adjusting for calendar year, conscription test center, BMI, parental education, and cognitive performance (IQ) at age 18. Similar associations were observed for low fitness and other psychotic disorders (HR 1.41, 95% CI 1.27–1.56). Associations remained significant in models that also controlled for cognitive performance (IQ). Little or no change in HRs was observed when individuals with a current or previous diagnosis of depression were excluded (data not shown). No difference was found in the HRs for cardiovascular fitness and non-affective mental disorders in the sensitivity analyses excluding all twins (monozygotic and dizygotic, n = 3076). Dose–response relationships with cardiovascular fitness were also observed for men diagnosed with neurotic or stress-related and somatoform disorders where HR for low fitness was 1.45, 95% CI 1.37–1.54. Again, excluding individuals with previous or ongoing depression had little or no effect on the HRs (data not shown). Table 4 also shows results of analyses for specified groups of disorders within the large category of neurotic or stress-related and somatoform disorders. Low cardiovascular fitness was associated with a near 50% increase in risk of developing anxiety disorders, obsessive-compulsive disorder, and other neurotic disorders. Most of the men in this category had non-phobic anxiety disorders (n = 6034), and an almost identical risk estimate was seen for the fully adjusted model for this subgroup and low fitness (HR 1.48, 95% CI 1.35–1.61). While similar HRs were observed for the association between low cardiovascular fitness and reaction to severe stress and adjustment disorders (HR 1.51, 95% CI 1.39–1.63), the relationship did not remain significant in fully adjusted models for somatoform and dissociative disorders (HR 1.03, 95% CI 0.81–1.31) (Table 4).
Table 4. Hazard ratios for serious non-affective mental disorders in relation to cardiovascular fitness in male conscripts
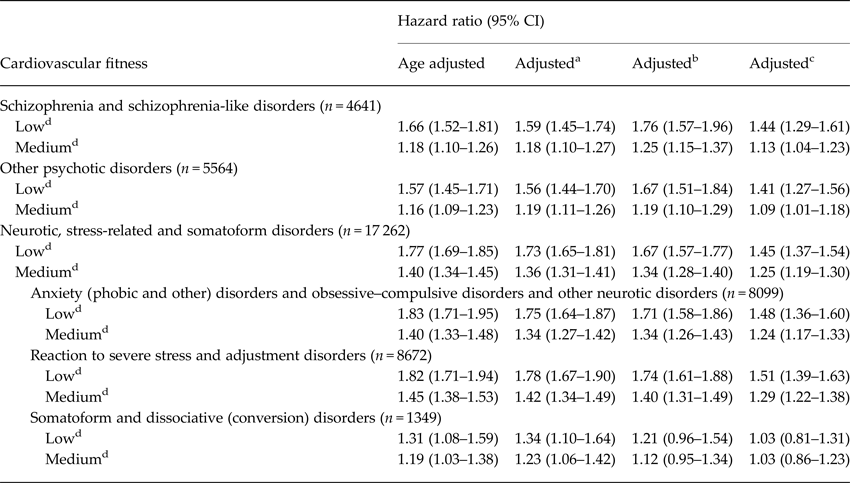
a Adjusted for calendar year, BMI, region.
b Adjusted for calendar year, BMI, region, parental education.
c Adjusted for calendar year, BMI, region, parental education, IQ.
d Reference category: high.
Familial influence on the associations of cardiovascular fitness and serious non-affective mental disorder
There were 492 070 individuals with one or more full brothers in the cohort. Among these, 2308 individuals developed schizophrenia and schizophrenia-like disorders; 2790 developed other psychotic disorders; and 7848 developed neurotic, stress-related, and somatoform disorders during the observation period. The association of low cardiovascular fitness and risk for specified disorder remained significant for all diagnostic categories when the presence of the outcome diagnosis in one or more brothers was included as an additional explanatory variable (Table 5). While having a brother with schizophrenia and schizophrenia-like disorder was associated with an almost 10-fold increase in risk for this disease category, adolescent cardiovascular fitness remained a significant risk modifier in the brother model (HR 1.52, 95% CI 1.34–1.72). The association of having a diagnosed brother and risk for other psychotic disorders was a more than sixfold increase in risk, and the risk of low fitness was still significant (HR 1.56, 95% CI 1.39–1.75). The familial influence reached a smaller magnitude (2.5-fold increase) in the neurotic, stress-related, and somatoform disorders. However, the risk associated with low fitness when adjusted for disorder in a brother remained significant (HR 2.58, 95% CI 2.37–2.82). Among all mental disorder categories included in the current study, the association between low cardiovascular fitness and future risk for reaction to severe stress and adjustment disorders was attenuated the least when afflicted brothers were included in the model.
Table 5. Subanalyses for conscripts (n = 492 070) with one or more brothers: hazard ratios for serious non-affective mental disorders in relation to cardiovascular fitness when adding presence of the specified disorder in a brother as an additional explanatory variable
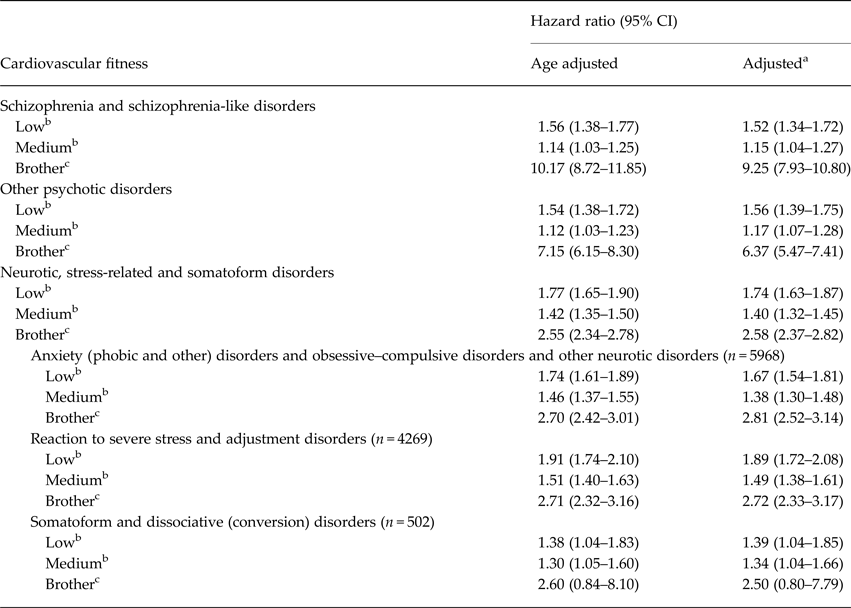
a Adjusted for calendar year, BMI, region.
b Reference category: high.
c One or more brothers with specified mental disorder.
Discussion
To our knowledge, this large population-based study is the first to provide evidence that low premorbid cardiovascular fitness in late adolescence is associated with an increased risk of schizophrenia and other psychotic disorders as well as anxiety and stress-related conditions during long-term follow-up of up to more than four decades. Associations persisted after adjustment for confounders, including familial influences.
Low and medium (compared with high) cardiovascular fitness increases the risk for future schizophrenia and schizophrenia-like disorders as well as for other psychotic disorders. HRs were very similar for these two diagnostic categories, which in part can be explained by a high degree of diagnostic overlap. While previous longitudinal studies on the relationship between objectively measured cardiovascular fitness and schizophrenia incidence are lacking, meta-analyses clearly demonstrate that people with schizophrenia have a significantly impaired cardiorespiratory fitness compared with age- and gender-matched controls (Vancampfort et al. Reference Vancampfort, Rosenbaum, Probst, Soundy, Mitchell, De Hert and Stubbs2015, Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst and Stubbs2017). Evidence from clinical research supports a positive effect of physical activity in reducing symptom severity and improving quality of life (Rosenbaum et al. Reference Rosenbaum, Tiedemann, Sherrington, Curtis and Ward2014) and results from a meta-analysis show that exercise improves global cognition (Firth et al. Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein and Yung2017) in persons with schizophrenia. Brain imaging studies have shown that individuals with schizophrenia have a smaller volume of the amygdala–hippocampal complex, smaller thalamus, and greater ventricular volumes than healthy controls. Further, individuals having a biological relative with schizophrenia had a volume midway between the two (Niemi et al. Reference Niemi, Suvisaari, Tuulio-Henriksson and Lonnqvist2003). Given the positive effects of physical activity on neuroplasticity, neuroprotection, and hippocampal (Christie et al. Reference Christie, Eadie, Kannangara, Robillard, Shin and Titterness2008) as well as prefrontal cortex (Weinstein et al. Reference Weinstein, Voss, Prakash, Chaddock, Szabo, White, Wojcicki, Mailey, McAuley, Kramer and Erickson2012) volume, cardiovascular fitness in late adolescence might affect the risk of schizophrenia through such mechanisms.
We demonstrate an inverse association between cardiovascular fitness and risk for future neurotic disorders. While not directly comparable since our study focuses on fitness as opposed to activity, our results expand on previously published population-based findings demonstrating shorter term (3–6 years) risk of developing anxiety disorders in persons with self-reported low physical activity. Our results regarding reaction to severe stress and adjustment disorders show similarities to the increased risk for post-traumatic stress disorders in young people with low physical activity (Strohle et al. Reference Strohle, Hofler, Pfister, Muller, Hoyer, Wittchen and Lieb2007). A short-term prospective relationship has been shown for lower physical activity and somatoform disorder (Strohle et al. Reference Strohle, Hofler, Pfister, Muller, Hoyer, Wittchen and Lieb2007). Low cardiovascular fitness was not associated with increased risk for future somatoform and dissociative (conversion) disorders in the fully adjusted models in our study, indicating that confounders might explain the association.
The associations between cardiovascular fitness and serious non-affective mental disorders remained significant also after considering familial influence by adding presence of the specific disorder in a brother as an additional explanatory variable. When doing so, the HRs were attenuated the least for reaction to severe stress and adjustment disorders. The results therefore indicate that modifiable factors, such as physical fitness, may reduce vulnerability to stress and hence might be particularly protective for patients in this diagnostic category. Although familial factors had a large influence on risk of future schizophrenia and schizophrenia-like disorders (HR = 9.25) as well as other psychotic disorders (HR = 6.37), the associations with cardiovascular fitness still remained significant. This was not the case when we examined familial influence in bipolar disorder (Aberg et al. Reference Aberg, Waern, Nyberg, Pedersen, Bergh, Aberg, Nilsson, Kuhn and Toren2012), suggesting that cardiovascular fitness is more strongly related to future risk of schizophrenia and psychotic disorders than to future risk of bipolar disorder.
Dysregulation of stress response systems may be involved in the pathogenesis and progress of mental disorders (Zschucke et al. Reference Zschucke, Renneberg, Dimeo, Wustenberg and Strohle2015). Our current results are supported by other studies reporting that both a higher physical activity (Tsatsoulis & Fountoulakis, Reference Tsatsoulis and Fountoulakis2006; Gerber & Puhse, Reference Gerber and Puhse2009; Jonsdottir et al. Reference Jonsdottir, Rodjer, Hadzibajramovic, Borjesson and Ahlborg2010) and cardiovascular fitness (Gerber et al. Reference Gerber, Lindwall, Lindegard, Borjesson and Jonsdottir2013) are associated with lower self-reported symptoms of stress and a better stress resilience. There is a clear relationship between stress, the hypothalamic-pituitary-adrenal axis and the immune system; both acute and chronic stresses are known inducers of pro-inflammatory states (Moylan et al. Reference Moylan, Eyre, Maes, Baune, Jacka and Berk2013). Therefore, one mechanism by which cardiovascular fitness might affect the pathogenesis of mental disorders is through modulating the level of pro-inflammatory immune responses. Indeed, it has been concluded that the anxiolytic effects of physical activity are mediated at least in part via anti-inflammatory and anti-oxidant mechanisms (Moylan et al. Reference Moylan, Eyre, Maes, Baune, Jacka and Berk2013). Moreover, cardiovascular fitness might also influence the risk of mental disorders through mechanisms involving vascular risk factors, neurotrophic factors, neuroprotection, and neuroplasticity (Christie et al. Reference Christie, Eadie, Kannangara, Robillard, Shin and Titterness2008). Interventions that improve fitness may potentially increase stress resilience and/or control inflammation, thus having the potential to contribute to a reduction in the incidence of mental ill health in a broad range of disorders. They could also be targeted towards specific high-risk groups of younger individuals in order to prevent onset of severe mental illness later on. Our results support a role for cardiovascular fitness level in risk of subsequent mental disorders, a globally increasing problem in the population. One way of increasing cardiovascular fitness is to increase physical activity. Currently, there are public health recommendations for physical activity in order to prevent cardiovascular disease (Piepoli et al. Reference Piepoli, Hoes, Agewall, Albus, Brotons, Catapano, Cooney, Corra, Cosyns, Deaton, Graham, Hall, Hobbs, Lochen, Lollgen, Marques-Vidal, Perk, Prescott, Redon, Richter, Sattar, Smulders, Tiberi, van der Worp, van Dis and Verschuren2016), and these may also be of relevance for the prevention of mental illness in the population.
Strengths and limitations
The size of the cohort (over one million individuals), the use of an objective measure of cardiovascular fitness, and a long follow-up covering up to 42 years are important strengths of the study. Symptoms of mental ill health were identified by psychologists, and mental disorders were diagnosed by physicians. Risk of reverse causation was reduced by the exclusion of individuals with previous psychiatric disorders or symptoms at baseline, as well as those with any outcome diagnosis reported during the 3 years following conscription. The degree of validity of the diagnosis for schizophrenic disorders in the Swedish National Hospital Discharge Register has been shown to be high (Dalman et al. Reference Dalman, Broms, Cullberg and Allebeck2002). While not all mental diagnoses have been individually validated, the positive predictive values for most ICD diagnoses in the Swedish National Hospital Discharge Register are reported to be 85–95% (Ludvigsson et al. Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim, Reuterwall, Heurgren and Olausson2011).
An important consideration is that incidence figures are likely to be underestimated for neurotic, stress-related, and somatoform disorders since many persons with these conditions will not be identified through the hospital register. This is not a concern for estimates for schizophrenia and other psychotic disorders, as most persons with these disorders will come to the attention of psychiatric services at some point in time. Other confounders which we were not able to control for, such as drug abuse, might affect both cardiovascular performance at conscription and future mental health. Our findings are not explanatory regarding causal chains leading to the onset of mental disorders. Whether low cardiovascular fitness at conscription actually affects disorder onset needs further investigation. For example, poor motor function is an early sign of schizophrenia (Fryers & Brugha, Reference Fryers and Brugha2013) that might have contributed to a lower cardiovascular performance. Moreover, adolescents later diagnosed with schizophrenia are less physically active in the premorbid phase compared with healthy controls (Okkenhaug et al. Reference Okkenhaug, Tanem, Johansen, Romild, Nordahl and Gjervan2016). Another limitation is that we were not able to adjust for degree of social adjustment at conscription. Social functioning might be compromised in teens who will in time develop serious mental disorders, and this could affect participation in physical activities and the level of physical fitness at conscription. Long-term intervention studies based on randomized controlled trials are needed to fully elucidate the associations between adolescent cardiovascular fitness and future risk of non-affective mental disorders. An important consideration is that fitness data were measured at baseline only. However, recording observational data at several sampling points would still not suffice for making conclusions about causality. The results of our study cannot be directly extrapolated to women. Our inability to examine possible gender differences is an important limitation as there is some evidence that physical activity could be more protective in men (Bhui & Fletcher, Reference Bhui and Fletcher2000).
Overall, our data demonstrate significant associations between low cardiovascular fitness in early adolescence and later risk of non-affective mental disorders, also after adjusting for familial influences. The results of this study overcome many of the methodological constraints of previous work with its large sample size, long follow-up, and objective measures and contributes to the evidence for a prospective relationship between fitness and mental health.
Acknowledgements
The authors would like to thank Dr Tommy Johnson for statistical help and advice. This study was supported by grants from Stiftelsen Handlanden Hjalmar Svenssons forskningsfond, Stiftelsen Peter Erikssons minnesfond för hjärnforskning, Stiftelsen Lars Hiertas Minne, the Swedish Research Council (2013-2699, 2014-3224, 2016-01590), Swedish Brain Research Foundation (Hjärnfonden), and the Swedish government under the LUA/ALF agreement for biomedical research.
Declaration of Interest
None.







