Introduction
Adolescence is often regarded as a time of defiance, rule breaking, and drug and alcohol experimentation. But in truth there is great variability in how this period of life is experienced. Some adolescents become mired in deviant behavior and then ensnared by its consequences, while others sail through these years without so much as sampling a cigarette. What accounts for these considerable individual differences?
The behaviors that seem to worry parents most, such as an unwillingness to conform to rules and expectations, a general lack of behavioral constraint, and compulsive substance use, are typically referred to as externalizing behaviors. These behaviors tend to hang together such that if an adolescent does one, he or she is likely to do another (Krueger, Reference Krueger1999; Young et al. Reference Young, Stallings, Corley, Krauter and Hewitt2000). In fact, they predict each other. For example, early trouble with the police predicts adult alcohol problems just as well as does early alcohol experimentation (McGue & Iacono, Reference McGue and Iacono2005). This co-occurrence is largely a consequence of the behaviors having the same genetic root. Disinhibited, antisocial behavior and substance use seem to be variable expressions of a common, general vulnerability (Hicks et al. Reference Hicks, Krueger, Iacono, McGue and Patrick2004), and a vulnerability that is highly heritable (Young et al. Reference Young, Stallings, Corley, Krauter and Hewitt2000; Krueger et al. Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002; Kendler et al. Reference Kendler, Prescott, Myers and Neale2003). Most of the genetic risk for each, individual externalizing disorder is then explained by this general, latent risk factor.
Thus, genes appear to play a significant role in determining who is most predisposed to this clustering of behaviors. Yet genes are, of course, only one part of the equation. Research consistently shows important but largely unknown shared environmental influences on the individual disorders (e.g. Han et al. Reference Han, McGue and Iacono1999; Jacobson et al. Reference Jacobson, Prescott and Kendler2002), as well as non-shared environmental influences on both the disorders and the general externalizing factor (e.g. Young et al. Reference Young, Stallings, Corley, Krauter and Hewitt2000; Krueger et al. Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002). Biometric analyses are, however, not necessary to know that such behaviors are sensitive to environmental conditions. The historically low rates of both substance use and rule breaking within communities such as the Amish demonstrate the essential role played by community and cultural norms.
An underexploited strength of twin studies is their ability to identify such non-genetic factors. One innovative method postulates that genetic influences on a phenotype become attenuated whenever external factors limit personal choice, as personal choice is believed to be a reflection of innate characteristics and personality (Heath et al. Reference Heath, Berg, Eaves, Solaas, Corey, Sundet, Magnus and Nance1985). As a result, a trait that is heritable at the population level may not be heritable when measured only under conditions of limited choice. Alternatively, certain environments may elicit genetically influenced behaviors by providing a greater range of opportunities for their expression. By using twins to obtain heritability estimates across two or more sets of circumstances, researchers can thus learn about the strength, and effects, of particular social pressures.
For example, educational attainment's heritability rises as societies become more egalitarian and educational opportunities more widespread (e.g. Heath et al. Reference Heath, Berg, Eaves, Solaas, Corey, Sundet, Magnus and Nance1985). That is, when social standing no longer restricts access, education level increasingly comes to reflect innate ability. Even more striking is the finding that while measured IQ is heritable at the population level, conditions of extreme poverty can negate this effect (Turkheimer et al. Reference Turkheimer, Haley, Waldron, D'Onofrio and Gottesman2003). Under such strong environmental pressures, genes no longer play an important role in determining individual differences in children's IQ. This demonstrates that even highly heritable traits may be modified by external factors such as poverty and its accompanying deprivation.
These statistical interactions are generally referred to as gene–environment interactions, as the effect of the genes is dependent upon the environmental condition. It is, however, not necessarily clear a priori which environments will constrain the genetic influence on a particular behavior and which will allow for its more full expression. Precocious menarche has been shown to reduce the heritability of conduct problems (Burt et al. Reference Burt, McGue, DeMarte, Krueger and Iacono2006), low socio-economic status to reduce the heritability of illegal activities (Tuvblad et al. Reference Tuvblad, Grann and Lichtenstein2006), and family dysfunction to reduce the heritability of females' smoking (Kendler et al. Reference Kendler, Aggen, Prescott, Jacobson and Neale2004). It can be reasoned post hoc that these environments limit personal choice or, as one theorist has put it, that environments like this provide so much of a ‘social push’ (Raine, Reference Raine2002), encouraging problematic behavior, that the importance of genetic factors diminish in comparison. But it is not clear that all these results could have been predicted.
Kendler et al. (Reference Kendler, Aggen, Prescott, Jacobson and Neale2004), in fact, predicted the opposite. Their presupposition was that heritability would increase in the presence of an adversity such as family dysfunction. This hypothesis was backed by both the diathesis-stress model of mental disorders and by some earlier research. For instance, marriage and religiosity could be conceptualized as stress buffering and, for women, being single rather than in a marriage-like relationship magnifies the impact of inherited tendencies toward both depression (Heath et al. Reference Heath, Eaves and Martin1998) and alcohol consumption (Heath et al. Reference Heath, Jardine and Martin1989). For males, receiving a non-religious rather than a religious upbringing amplifies the genetic influences on the personality trait of disinhibition (Boomsma et al. Reference Boomsma, de Geus, van Baal and Koopmans1999). Likewise, the molecular–genetic interaction research has consistently supported the diathesis-stress model, whereby genetic vulnerabilities are most often expressed under stressful circumstances (e.g. Caspi et al. Reference Caspi, Sugden, Moffitt, Taylor, Craig, Harrington, McClay, Mill, Martin, Braithwaite and Poulton2003, Reference Caspi, Moffitt, Cannon, McClay, Murray, Harrington, Taylor, Arseneault, Williams and Braithwaite2005; Kahn et al. Reference Kahn, Khoury, Nichols and Lanphear2003; Eley et al. Reference Eley, Sugden, Corsico, Gregory, Sham, McGuffin, Plomin and Craig2004).
There is therefore, at present, no one formula that adequately captures how nature and nurture interact to produce complex behaviors. As mentioned, low socio-economic status sometimes constrains genetic effects, as seen in the reduced heritability of adaptive traits such as intelligence (e.g. Turkheimer et al. Reference Turkheimer, Haley, Waldron, D'Onofrio and Gottesman2003) and also non-adaptive traits such as delinquency (Tuvblad et al. Reference Tuvblad, Grann and Lichtenstein2006). At other times, this same stress encourages the expression of a genetic susceptibility, as seen in the increased heritability of chronic illness within the lower socio-economic statuses (Johnson & Krueger, Reference Johnson and Krueger2005). Clearly, much remains to be learned about the dynamic nature of the heritability statistic and how group-specific environmental pressures moderate genetic effects for particular phenotypes.
In this paper, we examine one potentially constraining environment, that of rural Minnesotan communities. We hypothesize that genetic predisposition will be less important in shaping individual differences in externalizing behavior in these sparsely populated areas; rather, family- or community-level influences (i.e. shared environmental influences) will take on more importance. Conversely, urban environments, with their wider variety of social niches, will allow for a more complete expression of genetically influenced traits. Whether someone's genes nudge them toward substance use and rule breaking, or abstinence and obedience, there will be more opportunities to express these genetic tendencies in an urban setting. Note that these same results would also be expected under the diathesis-stress model, as there is some suggestion that urban environments are more stressful and that this stress can elicit psychological disorders (e.g. Paykel et al. Reference Paykel, Abbott, Jenkins, Brugha and Meltzer2000). We are not pitting these two theories (constraining/eliciting versus diathesis-stress) against each other; we believe they both have explanatory power. Rather, we propose a particular hypothesis, backed by both theories, while acknowledging that prior gene–environment interaction research in the behavioral sciences suggests caution when advancing any particular prediction.
We also have reason to believe that rural settings can modify the genetic effects on externalizing psychopathology because Rose et al. (Reference Rose, Dick, Viken and Kaprio2001) have demonstrated that the etiological influences on one externalizing behavior, drinking frequency, varies by regional residency. For both males and females, living in rural rather than urban Finland reduced the genetic influence on adolescent drinking frequency. Because alcohol misuse is a central component of the externalizing spectrum, it seems likely that urban–rural environments will interact with genetic risk for the entire latent factor. Here, we include a broad array of markers of disinhibition to ask whether a gene–environment interaction previously observed at the individual-variable level extends to the entire spectrum of related disorders. That is, are individual differences in adolescents' externalizing behaviors, behaviors that are immensely costly to both families and communities, influenced by where their parents have chosen to raise them?
Method
Participants
The 608 same-sex twin pairs [male: 184 monozygotic (MZ), 97 dizygotic (DZ); female: 213 MZ, 114 DZ] were born in the state of Minnesota between 1972 and 1979 and, at the time of their assessment (average age 17.47 years, range 16.55–18.52, s.d.=0.46), continued to reside there. Seventeen is an age at which adolescents have begun to express the phenotypes of interest but still remain in the family environment. (See Iacono et al. Reference Iacono, Carlson, Taylor, Elkins and McGue1999, for additional information about the Minnesota Twin Family Study's design and sample.)
Measures
Urban–rural classification
We used the US 2000 census Rural–Urban Commuting Area (RUCA) system to classify the adolescents as urban or rural. Classification was based on their school zip code; these data were available and considered appropriate, given the salience of the school environment to adolescent development. The RUCA system uses measures of urbanization, population density, and daily commuting patterns. There are four general population classifications: urban (>49 999), large town (10 000–49 999), small town (2500–9999) and isolated rural (<2500). For our analyses, ‘rural’ individuals go to school in towns of less than 10 000 and towns in which the primary commuting pattern is within that town or to a town of equally small size. Alternatively, they go to school in an area that is classified as isolated rural and there is no primary flow to a larger area. The remainder was classified as ‘urban’.
Zip-code areas receive a higher classification than would be expected by population alone whenever the primary commuting pattern is >30% to a town or city of greater size (e.g. a small town is classified as ‘urban’ when there is sufficient commuting to a nearby large town). Approximately 9% of our sample received a higher classification in this manner. Zip-code areas with only 5–30% of the primary commuting population going to a nearby area are similarly reclassified; however, in our sample, <0.5% were classified based on such low levels of commuting. This classification system created a 60.5% urban, 39.5% rural division and ensured that our rural group was very rural. Its fairly substantial size reflects Minnesota's largely agricultural landscape.
Externalizing behaviors
We used the Diagnostic Interview for Children and Adolescents – Revised (DICA-R; Reich & Welner, Reference Reich and Welner1988) to interview separately the twins and their mothers regarding the twins' conduct disorder (CD) and oppositional defiant disorder (ODD) symptomatology. The DICA-R addresses lifetime disorders under DSM-III-R, the current diagnostic system when these data were collected. Nine criterion-A ODD symptoms and 12 criterion-A CD symptoms were assessed (the forced sexual activity symptom was omitted). We did not enforce the DSM-III-R stipulation that ODD symptoms not be assigned in the presence of a CD diagnosis. Adult antisocial behavior (AAB) was similarly assessed, except mothers did not report. AAB is the post-age-15 portion of the antisocial personality disorder criteria; we relaxed the DSM requirement that only adults be assessed for personality disorders.
A team of doctoral students reviewed each file, considered both the twin's and mother's report, and assigned symptoms for each diagnosis. When the described behavior fell short of our severity or frequency criteria, but was nonetheless judged significant, that symptom was assigned at the subthreshold level. In the symptom-count scales used in the analyses, these were weighted half (0.5) of those judged fully present.
The adolescents reported on their alcohol (ALC) and drug (DRUG) use through the Substance Abuse Module (Robins et al. Reference Robins, Babor and Cottler1987) of the Composite International Diagnostic Interview (Robins et al. Reference Robins, Wing, Wittchen, Helzer, Babor, Burke, Famer, Jablenski, Pickens, Regier, Sartorious and Towle1988). For ALC, we summed DSM-III-R Alcohol Dependence symptoms plus nine items assessing non-criterion behaviors, ranging from simply using alcohol to consuming 20+ drinks on a single occasion, because using only psychiatric criteria with this community-based, adolescent sample produced a restricted range and highly skewed distribution. Previous research supports the validity of this alcohol-problems continuum (Krueger et al. Reference Krueger, Nichol, Hicks, Markon, Patrick, Iacono and McGue2004). DRUG was simply a tally of the classes of substances tried from: tobacco, alcohol, marijuana, amphetamines, tranquilizers, quaaludes/barbiturates, cocaine, heroin/opiates, PCP/psychedelics and inhalants.
Statistical analyses
We examined externalizing behaviors separately and as a factor construct. Support for our contention that they may appropriately be conceptualized as a single, broad risk factor comes from several sources. First, they are commonly co-morbid, and this appears to be due to genetic overlap among them (Young et al. Reference Young, Stallings, Corley, Krauter and Hewitt2000; Krueger et al. Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002). Second, their familial transmission appears to be general, meaning that what is passed from parent to child is a vulnerability to a spectrum of disorders rather than a disorder-specific risk (Hicks et al. Reference Hicks, Krueger, Iacono, McGue and Patrick2004). Finally, they predict each other (McGue & Iacono, Reference McGue and Iacono2005).
Data were analyzed using SPSS version 11.0.1 (SPSS Inc., Chicago, IL, USA), SAS (Littell et al. Reference Littell, Milliken, Stroup and Wolfinger1996) and Mx (Neale et al. Reference Neale, Boker, Xie and Maes2002). SAS was used for the hierarchical linear models, which took the clustered nature of the twin sample into account. Mx, a structural-equation modeling program, was used for the biometric analyses. Mx uses maximum-likelihood techniques that maximize fit between the model and the data, thus providing parameter estimates that offer the smallest discrepancies from the data. We estimated the means, variances and covariances using raw data. Initially, we fit a series of five-variable Cholesky models (Neale & Cardon, Reference Neale and Cardon1992), which allowed us to estimate simultaneously the genetic and environmental contributions to each variable and test whether these estimates could be constrained across gender and urban–rural residency.
Next, we fit a single factor (or common-pathway) model to the male sample, in which each variable's variance was partitioned into that which is common to them all (i.e. attributable to the factor) and that which is specific to each. In the base factor model, all parameters were free to vary across the urban/rural division. The fit of this model was then compared to a number of more restrictive models that constrained non-standardized parameter estimates to be equal across the urban/rural groups. Model fit was evaluated by taking the difference in minus twice the log-likelihood values (−2ln L), which is distributed as a χ2 random variable under the null hypothesis of the more restrictive model. Akaike's Information Criterion (AIC; Akaike, Reference Akaike1987) was also used to compare the fit of alternative models. AIC is a fit index conventionally used in behavioral-genetic analyses. It is the model's χ2 minus twice its degrees of freedom; it thus considers both parsimony and goodness of fit. As a general aim of model fitting is to explain the data as parsimoniously as possible, the model with the smallest AIC is generally considered best.
Results
Table 1 presents the means and standard deviations for each of the five variables separately by gender and urban–rural classification. As would be expected with a population-based sample, the symptom counts and substance-use measures were all positively skewed; thus, to better approximate normality, they were log transformed for all analyses. Hierarchical linear modeling determined the effects of gender and residency on externalizing measures while simultaneously controlling for the non-independence of the twins. In both urban and rural environments, males demonstrated significantly more symptoms of CD [F(1, 366)=86.61, p<0.0001 urban; F(1, 238)=59.37, p<0.0001 rural], AAB [F(1, 365)=23.27, p<0.0001 urban; F(1, 238)=21.85, p<0.0001 rural] and ALC [F(1, 366)=3.96, p<0.05 urban; F(1, 238)=4.35, p<0.05 rural]. Within gender, there was a trend toward more externalizing behavior in urban areas. However, the only variable to reach statistical significance was ODD in the male sample, with more symptomatology exhibited in urban than in rural settings (p<0.05). There were no gender x residency interactions; all F values were less than 0.65 and all p values greater than 0.40. Thus, overall, the level of externalizing behavior is similar across urban and rural environments.
Table 1. Descriptive statistics and intra-class correlations for the five externalizing variables, by gender and by urban–rural classification

CD, Conduct disorder symptom count; ODD, oppositional defiant disorder symptom count; AAB, adult antisocial behavior symptom count; ALC, alcohol use; DRUG, substance use; s.d., standard deviation; MZ, monozygotic; DZ, dizygotic.
Within both the urban and rural environments, males demonstrated significantly (p<0.05) more CD, AAB and ALC than females. Across urban–rural settings, only males' ODD differed significantly (p<0.05). Sample sizes for the males are: urban MZs (n=230–232), urban DZs (n=130), rural MZs (n=136), rural DZs (n=64). Sample sizes for the females are: urban MZs (n=228–232), urban DZs (n=140–142), rural MZs (n=194), rural DZs (n=86).
Table 1 also presents the intra-class twin correlations separately by gender and urban–rural classification. The larger differences in correlation size between MZ and DZ twins in urban environments, as opposed to MZ and DZ twins in rural environments, implies that the factors influencing externalizing behavior vary by environment. Genetic influences appear to take on more importance in urban settings, while shared environmental influences appear to be more important in rural settings.
A Cholesky multivariate model, in which no constraints were placed on the genetic and environmental parameter estimates, fit the data reasonably well, as measured by AIC, when compared to the fully saturated model [Δχ2(260)=306.99, p<0.05, AIC=−213.01]. Constraining model parameters to be equal across gender resulted in a significant increase in χ2 (p<0.001), indicating that males and females differ from one another. Constraining model parameters to be equal across urban–rural also resulted in a significant increase in χ2 (p<0.01), indicating an overall effect for residency. To investigate this more fully, we estimated the model parameters individually for each of the five indicators of externalizing behavior separately for males and females. Estimates for the proportion of variance attributable to additive genetic (a 2), shared or common environmental (c 2), and non-shared or unique environmental (e 2) influences are presented in Table 2. With ODD as the only exception, genetic influences appear to be relatively more important, and shared environmental influences relatively less important, in urban than in rural settings. We formally tested for residency effects by constraining the non-standardized genetic and shared environmental variance estimates to be equal across urban–rural for each of the externalizing variables. In males, the urban–rural difference reached significance for three of the five variables, but in females, the effect was non-significant for all variables (see Table 2 for model-fitting statistics). Thus, an urban–rural effect appears limited to the male sample.
Table 2. Parameter estimates and 95% confidence intervals for additive genetic (a2), shared environmental (c2) and non-shared environmental (e2) components of variance plus fit statistics for urban–rural comparisons

df, Degrees of freedom; AIC, Akaike's Information Criterion; n.s., not significant; CD, conduct disorder symptom count; ODD, oppositional defiant disorder symptom count; AAB, adult antisocial behavior symptom count; ALC, alcohol use; DRUG, substance use.
Fit statistics: genetic and shared environmental variance estimates were constrained to be equal across urban–rural for each of the externalizing variables.
To further explore this urban–rural externalizing effect in males, the five variables were fit to a latent externalizing factor to obtain a summary estimate of urban–rural differences. Relative to the fully saturated model, the male factor model fit well [Δχ2 (176)=−158.7, n.s., AIC=−510.7]. The base factor model, in which all parameters were free to vary by urban–rural residency (model 1), was then compared to nested models in which non-standardized parameter estimates were systematically constrained across urban–rural residency. Fit statistics are presented in Table 3. Constraining both the factor loadings (model 2) and the residual variance estimates (model 3) to be equal across urban/rural environments produced non-significant changes in χ2 and improvements in fit as measured by AICs, suggesting that the externalizing factor was similarly defined in the two environments. Model 4 tests for the presence of an interaction by asking whether the A, C and E variance that is common to the factor could be constrained equally across urban–rural settings; this produced a significant decrement in fit and positive AIC. These results suggest a gene–environment interaction by regional residency, as genes and the environment are differentially contributing to the latent externalizing factor in the two settings.
Table 3. Biometric factor model-fitting results for the male sample
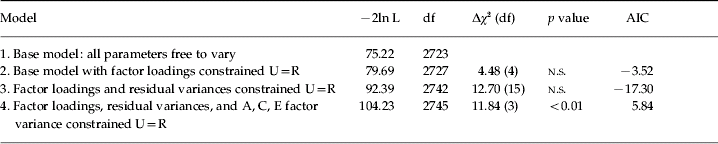
U, Urban; R, rural; df, degrees of freedom, n.s., not significant; AIC, Akaike's Information Criterion.
Each model is nested within and compared to the previous model.
Neither the A factor variance alone [Δχ2(1)=10.7, p<0.005, AIC=8.7] nor the C factor variance alone [Δχ2(1)=4.6, p<0.05, AIC=2.6] could be set equal across urban–rural environments; however, the E factor variance could be [Δχ2(1)=1.9, n.s., AIC=−0.1]. This latter constraint suggests that the observed effect was not due to heteroscedasticity, as differences in error variance would be likely to lead to disparate estimates of the non-shared environment. Fig. 1 presents a model in which the non-standardized factor loadings, residual variance estimates, and non-shared environmental (E) factor variance were all constrained to be equal across urban and rural environments, but the genetic (A) and shared environmental (C) factor variance estimates were allowed to differ by environmental circumstance. To facilitate interpretation, standardized estimates for the A, C and E factor variances are also presented in boxes above the non-standardized estimates. Note that although the standardized estimates for the E factor variance vary slightly by residency, the non-standardized parameter estimates were constrained to be equal across environments. There is a clear and significant moderating influence of urban–rural residency. In urban environments, genetic factors accounted for 64% of the factor's variance and shared environmental influences for 25% of the factor's variance. In rural environments, genetic influences dropped to 0% and shared environmental influences increased to 86%.

Fig. 1. Biometric factor model of males' externalizing psychopathology. Urban (U), rural (R) and urban and rural together (U/R) non-standardized estimates are presented. In boxes, the standardized estimates and 95% confidence intervals are shown for the genetic (A), shared environmental (C) and non-shared environmental (E) contributions to the factor's variance. For males, the A and C influences on the factor's variance differ significantly by region, indicating a gene–environment interaction. CD, Conduct disorder; ODD, oppositional defiant disorder; AAB, adult antisocial behavior; ALC, alcohol use; DRUG, substance use.
Discussion
We hypothesized a gene–environment interaction in the development of adolescent externalizing psychopathology. Specifically, we thought externalizing behavior would be more heritable in urban environments, where greater personal choice allows for a more complete expression of genetically influenced individual differences. The male sample supported our hypothesis. For three of the five variables selected to measure externalizing behavior, constraining the genetic and shared environmental variance to be equal across urban–rural residency resulted in a significant decrement in fit. Biometric factor modeling then confirmed that genetic and shared environmental parameter estimates varied, for males' externalizing, by urban–rural residency. Genetic influences were greater in urban environments while shared environmental influences were more pronounced in rural settings. For our female sample, however, genetic and shared environmental parameters estimates were similar across urban–rural residency for all five of the externalizing variables.
These findings from the male sample fit with an emergent literature suggesting that certain environments may restrict the expression of particular genetic tendencies. Even in the face of high heritability estimates, many psychologists had been unwilling to relinquish their belief that the environment plays a tremendously important role in development. Now, as evidence such as ours for gene–environment interactions accumulates, it seems possible that these ‘nurture’ proponents will be vindicated.
Our results highlight the contextual nature of the heritability statistic: heritability is not a fixed quality of a trait, but is instead sensitive to environmental conditions. For males in urban environments, heritable factors are primarily responsible for individual differences in substance use and rule-breaking behavior, explaining 64% of the variance. But for males in rural environments, it is shared environmental factors that are most influential, explaining 86% of the variance. Thus, the generally substantial heritability of externalizing behavior (e.g. Iacono et al. Reference Iacono, Carlson, Taylor, Elkins and McGue1999; Krueger et al. Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002; Rhee & Waldman, Reference Rhee and Waldman2002; Kendler et al. Reference Kendler, Prescott, Myers and Neale2003) was not evident for male adolescents raised in towns of less than 10 000 that were isolated from areas of greater population density.
However, while rural environments constrained the genetic effects on externalizing, they did not constrain the overall behavioral expression. The level of externalizing behavior appeared greater in urban settings, but this difference only reached statistical significance for one disorder, ODD, and only in males. This is not surprising because contemporary studies generally suggest that adolescent substance use is equally, if not more, prevalent in rural compared with urban settings (e.g. Cronk & Sarvela, Reference Cronk and Sarvela1997; CASA, 2000; Levine & Coupey, Reference Levine and Coupey2003). Moreover, while juvenile delinquency has historically been associated with urban areas (e.g. Shaw & McKay, Reference Shaw and McKay1969), we could find no modern, American replication. Rather, an urban–rural difference in conduct disorder/delinquency was either not detected (Offord et al. Reference Offord, Boyle, Szatmari, Rae-Grant, Links, Cadman, Byles, Crawford, Blum, Byrne, Thomas and Woodward1987), or showed up only for females, only under parents' (not teachers') report (Zahner et al. Reference Zahner, Jacobs, Freeman and Trainor1993), and thus was not a robust effect.
It is not unprecedented to have found a gene–environment interaction in the absence of an environmental main effect. Rose et al. (Reference Rose, Dick, Viken and Kaprio2001) found that regional residency moderated adolescent alcohol use, but they did not find an urban–rural difference in the abstinence rates, or a mean regional difference in drinking frequency among the non-abstinent. Others, similarly, have found interactions without environmental main effects (Wahlberg et al. Reference Wahlberg, Wynne, Oja, Keskitalo, Pykalainen, Lahti, Moring, Naarala, Sorri, Seitamaa, Laksy, Kolassa and Tienari1997; Koopmans et al. Reference Koopmans, Slutske, van Baal and Boomsma1999; Ozkaragoz & Noble, Reference Ozkaragoz and Noble2000; Silberg et al. Reference Silberg, Rutter, Neale and Eaves2001; Kahn et al. Reference Kahn, Khoury, Nichols and Lanphear2003; O'Connor et al. Reference O'Connor, Caspi, DeFries and Plomin2003; Eley et al. Reference Eley, Sugden, Corsico, Gregory, Sham, McGuffin, Plomin and Craig2004). Not only do these studies demonstrate the prevalence of this phenomenon but they also serve as valuable reminders that, without knowledge of the interaction, key environmental variables could go unrecognized.
There is also precedence for an interactive effect reaching statistical significance for one gender but not the other (e.g. Boomsma et al. Reference Boomsma, de Geus, van Baal and Koopmans1999; Grabe et al. Reference Grabe, Lange, Wolff, Volzke, Lucht, Freyberger, John and Cascorbi2005; Tuvblad et al. Reference Tuvblad, Grann and Lichtenstein2006). Several papers examining environments' constraining influences on genetic effects have shown this. Moreover, to the extent that environmental or social pressures vary by gender, this might in fact be anticipated. To illustrate, secular changes, which weakened the social taboo surrounding women's smoking, led to a significant increase in the heritability of tobacco use for women in recent years (Kendler et al. Reference Kendler, Thornton and Pedersen2000). This effect was not seen for men over the same time period, presumably because the social disapproval surrounding men's smoking was always less pronounced. Similarly, while a religious upbringing suppressed the heritability of alcohol-use initiation for both males and females, the disparity in heritability across the two environments only reached statistical significance for the female sample (Koopmans et al. Reference Koopmans, Slutske, van Baal and Boomsma1999). Other environmental changes seemed to reduce the constraining influences only for males. For instance, societal changes in Norway resulted in males' but not females' educational attainment becoming more heritable in recent years (Heath et al. Reference Heath, Berg, Eaves, Solaas, Corey, Sundet, Magnus and Nance1985). Therefore, not only is there a power issue when attempting to replicate an interaction across gender, but any time social pressures might limit one gender more than the other, non-replication could be expected. In our sample, rural environments appear slightly less genetically constraining for females than for males, especially when it comes to the non-substance-related behaviors (see Table 2). Simultaneously, urban environments are somewhat less eliciting of genetic tendencies for females than males, creating a smaller heritability differential and thus a lack of any significant interaction in the female sample.
The present study has a number of strengths, including our use of diagnostic criteria and our application of a factor model. A growing body of research suggests that there is value in thinking beyond single behavioral variables, in conceptualizing certain types of behaviors and disorders as inter-related (e.g. Krueger, Reference Krueger1999). Another advantage to our work is that our externalizing measures, while not based on direct observations, were obtained from in-person interviews by trained staff of both the parent and the child. These interviews were later reviewed by a separate team of doctoral students in clinical psychology who assigned the behavioral symptoms. However, although our sample was representative of Minnesota at the time it was ascertained, an evident limitation is its lack of racial and ethnic diversity. This restricts the generalizability of our results, as does the relatively small number of subjects living in abject poverty (Iacono et al. Reference Iacono, Carlson, Taylor, Elkins and McGue1999). Whether the findings hold for other racial and ethnic groups, and whether they hold for urban areas characterized by concentrations of extreme poverty, is left for future researchers to determine. We also acknowledge that the studied behaviors may not be completely comparable across males and females, as externalizing behavior is more rarely expressed in females (e.g. Romano et al. Reference Romano, Tremblay and Vitaro2001), and the lower rates of behavioral expression could have contributed to our lack of a significant interactive effect. A further limitation is that any interaction, unless disordinal, will depend on measurement scale. It is thus possible that there are non-linear transformations of our variables that would eliminate the evidence in support of the existence of an interaction (Eaves, Reference Eaves2006). Nevertheless, the robustness of our findings across multiple measures (see Tables 1 and 2) gives us confidence in the reliability of our results. One final critique would be to ask whether families that choose to live in rural environments differ, genetically, from those that choose to reside in urban areas. Although it is true that if such a gene–environment correlation existed it would affect the interpretation of our results, the lack of an environmental main effect in our sample argues against its existence.
In summary, our results provide evidence for a specific, identifiable environmental effect on externalizing behavior. Rural environments appeared to dampen the expression of genetic differences for externalizing, replacing them with familial differences. These findings underscore the importance of refraining from assuming that population-level results will extend to all subpopulations. It must not be forgotten that traits that are highly heritable in one situation may be much less so when measured in another population or setting. Perhaps community norms are stronger in small towns. Or perhaps, for rural parents who choose to do so, it is easier to monitor and thus control their adolescents' activities. These are important areas of investigation that, unfortunately, cannot be directly tested with the current dataset. In the future, it will be of interest for researchers to investigate exactly what the operative environments might be. What is it about very rural, Midwest America that allows families and communities more influence over their children? In the meantime, when looking for ways to increase influence over adolescents' substance use or rule-breaking behavior, any variable that varies by urban–rural residency would be a good place to start.
Acknowledgements
This research was supported in part by NIH grants DA05147 and AA09367. It represents a portion of the doctoral thesis submitted by Lisa N. Legrand toward fulfillment of degree requirements under the mentorship of Matt McGue and William Iacono. We are grateful to MCTFR research associates Brian Hicks, Steve Malone and Rob Vatalaro for their assistance with this project.
Declaration of Interest
None.