INTRODUCTION
The apicomplexan parasite Toxoplasma gondii is widespread and zoonotic. It has been found in every warm-blooded vertebrate so far studied (Dubey, Reference Dubey2008). Its definitive host is any felid, where it reproduces sexually, leading to the excretion of oocysts in cat feces. Oocysts environmentally resistant and can be transported in substrates such as soil, water and vegetation. Any non-felid warm-blooded vertebrate can be an intermediate host, where T. gondii tachyzoites can cause acute toxoplasmosis. Thereafter T. gondii persists as bradyzoites in tissue cysts. Hosts can be infected prenatally by tachyzoite transfer, or postnatally by bradyzoite or oocyst ingestion.
Asymptomatic T. gondii infections have been reported both in wild and captive rabbits, and worldwide reports were summarized previously (Dubey and Beattie, Reference Dubey and Beattie1988; Dubey, Reference Dubey2010) Rabbits are considered an important intermediate hosts because cats that prey on them can excrete T. gondii oocysts. The wild rabbit is also a source of T. gondii for wild carnivores worldwide, including in Scotland (Burrells et al. Reference Burrells, Bartley, Zimmer, Roy, Kitchener, Meredith, Wright, Innes and Katzer2013), carnivorous and omnivorous wild birds in Spain (Darwich et al. Reference Darwich, Cabezón, Echeverria, Pabón, Marco, Molina-López, Alarcia-Alejos, López-Gatius, Lavín and Almería2012), game trappers in Wales (Beverley et al. Reference Beverley, Beattie and Roseman1954) and liver patients in Mexico (Alvarado-Esquivel et al. Reference Alvarado-Esquivel, Torres-Berumen, Estrada-Martínez, Liesenfeld and Mercado-Suarez2011). The grazing and burrowing wild rabbit could be exploited as a sentinel of T. gondii. Associations have been reported between T. gondii seroprevalence in wild rabbits and environmental variables likely to affect oocyst exposure in Spain (Almería et al. Reference Almería, Calvete, Pagés, Gauss and Dubey2004) and Australia (Cox et al. Reference Cox, Edmonds and Shepherd1981).
Here we examined T. gondii seroprevalence in wild rabbits on the Pitroddie Estate in Perthshire, central Scotland, where hill and lowland fields are used for livestock and arable crops, and feral cats are occasionally seen. The wild rabbit population has been sampled by shooting nearly every week since 1977 (Boag et al. Reference Boag, Hernandez and Cattadori2013). These rabbits carry other apicomplexan parasites, including Eimeria stiedae, the cause of lapine hepatic coccidiosis. They are infected also by Myxomatosis cuniculi and by helminths, including the roundworms Trichostrongylus retortaefomis and Graphidium strigosum.
MATERIALS AND METHODS
Samples
Sera and data were collected from 548 rabbits shot between January 2007 and December 2008 at a study site in central Scotland. The sampling area is 12–286 m above sea level. It contains Pitroddie Farm buildings (PF) at ordnance grid reference NO 2148 2520 and Nether Durdie cottages at ordnance grid reference NO 2140 2450. Within the sampling area, Pitroddie Crags (northwest of PF) and PF land (north of PF) are mostly marginal farmland, while Nether Durdie (southwest of PF) is mostly arable farmland. A disused quarry separates Pitroddie Crags from PF. A burn (stream) flows west to east from the quarry and passes slightly south of PF. An access road runs mostly alongside the burn but passes slightly north of PF.
Global Positioning System data were collected for each shot rabbit and used by triangulation to calculate distance from PF. Those calculated distances were between five and 240 m. Vegetation surrounding each rabbit was classed as woody (coniferous trees, deciduous trees, gorse and hawthorn) or non-woody (bracken, garden, grass and nettles). The season of shooting was classed as spring (March–May), summer (June–August) or autumn/winter (September–February) because rabbits rarely breed in winter. Rabbits were sexed, weighed entire and classed as neonate (200–749 g), juvenile (750–1249 g) or adult (⩾1250 g) as described (Boag et al. Reference Boag, Lello, Fenton, Tomkins and Hudson2001).
Sample size did not differ between 2007 and 2008, but it was relatively small in autumn/winter (detail not shown). Sampled rabbits included 100 kittens, 134 juveniles and 314 adults. There was no sex bias in any age class, vegetation type or distance from PF (detail not shown).
Infections
Freshly shot rabbits were examined in the field for overt myxomatosis. In a laboratory, the liver was examined for gross lesions consistent with coccidiosis. As required for different analyses, myxomatosis and coccidiosis were considered as binary variables or classed as absent, light, moderate, heavy or very heavy as described (Boag et al. Reference Boag, Hernandez and Cattadori2013). A 2 g sample of each rabbit's feces (rectal contents) was examined for helminth eggs and coccidial oocysts. Due to laboratory limitations, egg counts [eggs per gram (epg)] were pooled for the roundworms T. retortaefomis and G. strigosum. Oocyst counts [oocysts per gram (opg)] were pooled without species identification. As required for different analyses, egg and oocyst counts were considered as binary variables, or natural log-transformed, or classed as zero, low (1–99), medium (100–999) or high (⩾1000).
Sera were tested for specific anti-T. gondii IgG using the modified agglutination test (MAT) as described (Dubey and Desmonts, Reference Dubey and Desmonts1987), end-titrating at twofold dilutions starting at 1:20. Transformations of the inverse MAT titre did not normalize variance, so the MAT result was considered as a binary variable. An inverse titre of 1:20 was considered sufficient to indicate infection. Rabbits were classed as low seropositive (inverse titre 20 or 40) or high seropositive (inverse titre ⩾80). A titre of 1:25 has been considered sufficient to indicate infection for wild rabbits in Spain (Almería et al. Reference Almería, Calvete, Pagés, Gauss and Dubey2004; García-Bocanegra et al. Reference Garcia-Bocanegra, Astorga, Napp, Casal, Huerta, Borge and Arenas2010a , Reference Garcia-Bocanegra, Dubey, Martínez, Vargas, Cabezón, Zorrilla, Arenas and Almería b ) and for domestic rabbits in Brazil (Dubey et al. Reference Dubey, Passos, Rajendran, Ferreira, Gennari and Su2011) and Mexico (Alvarado-Esquivel et al. Reference Alvarado-Esquivel, Alvarado-Esquivel, Villena and Dubey2013). No formal validation of MAT for rabbits has been reported but the test has been extensively for the detection of T. gondii antibodies in many species of animals, including rabbits (Dubey, Reference Dubey2010).
Statistical analysis
Seropositive rabbits were examined as case studies. Statistical analyses were performed in R v2.12.2 (Foundation for Statistical Computing, 2011). Counts of rabbits were compared using the χ 2 test or Fisher's Exact Test, and factors associated with seropositivity were sought using additive logistic regression with binomial errors. That model was fitted using the likelihood ratio test (LRT) by χ 2. For the whole dataset (n = 548) candidate explanatory factors were the rabbit's sex and age class, the year and season of shooting and the distance from PF, the intensity class of myxomatosis, and log-transformed counts of coccidial lesions and roundworm epg. The same model, with log-transformed coccidial opg as a further candidate explanatory factor, was fitted for the 499 rabbits whose feces had been examined.
RESULTS
Apicomplexa
Antibodies to T. gondii were found in 18/548 (3·3%) rabbits with titres of 1:20 in nine, 1:40 in three, 1:80 in two, 1:160, 1:10 240, 1:20 480 and 1:81 920 each rabbit. Figure 1 shows the distribution of shot rabbits and their T. gondii infection status. The six high seropositives were shot within 103 m of PF, between January 2007 and May 2008, five of them before the end of June 2007. Visual inspection of the map did not suggest that seropositive rabbits were associated with the burn but there might be a north–south linear pattern in those rabbits’ locations.

Fig. 1. Map showing rabbit locations and serological results. Seronegative, inverse titre <20. Low seropositive, inverse titre 20 or 40. High seropositive, inverse titre ⩾80 (© Crown Copyright/database right 2015). An Ordnance Survey/EDINA supplied service.
Logistic regression (Table 1) showed that T. gondii seropositivity was associated with female sex and relatively heavy coccidiosis. Univariate comparisons confirmed that T. gondii seroprevalence was relatively high in females (P = 0·001 by Fisher's Exact Test; Fig. 2) that it varied with the severity of coccidiosis (P = 0·002 by Fisher's Exact Test; Fig. 2) and showed that it did not depend on age, year, season or vegetation type (detail not shown).

Fig. 2. Toxoplasma gondii seroprevalence in relation to sex, roundworms and Eimeria steidae. Univariate comparisons are shown.
Table 1. Factors associated with Toxoplasma gondii infection in wild rabbits (n = 548 unless otherwise stated)
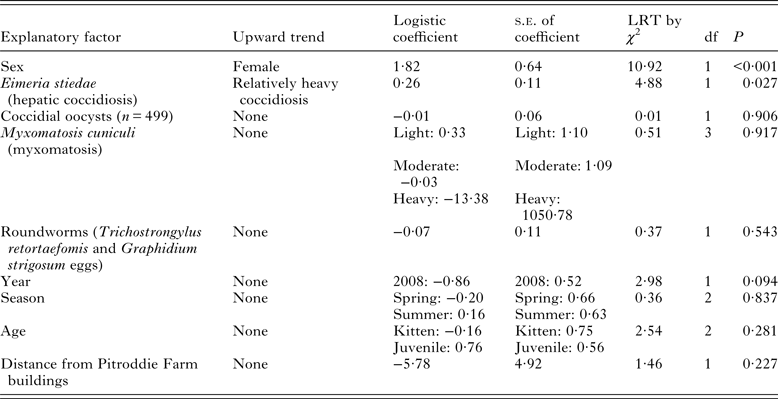
The prevalence of hepatic coccidiosis was 179/548 (32·7%). Coccidiosis was light in 148 rabbits (11 seropositive for T. gondii at titres between 1:20 and 1:20 480), moderate in ten (all seronegative for T. gondii), heavy in 17 (one seropositive for T. gondii at titre 1:81 920) and very heavy in four (one seropositive for T. gondii at titre 1:80). The prevalence of coccidiosis did not differ between sexes but it was relatively high in kittens and juveniles (detail not shown). It was relatively high in 2007 and in summer but it did not depend on vegetation type (detail not shown).
The prevalence of coccidial oocyst excretion was 368/499 (73·7%). Coccidial opg was low in ten rabbits (all seronegative for T. gondii), medium in 54 (one seropositive for T. gondii at titre 1:20) and high in 304 (11 seropositive for T. gondii at titres between 1:20 and 1:20 480). Toxoplasma gondii seropositivity was not associated with oocyst shedding (P > 0·999 by Fisher's Exact Test), a result also found for seroprevalence when multiple factors were considered (Table 1). The prevalence of coccidian oocysts did not differ between sexes but it was relatively high in kittens and juveniles (detail not shown). It was relatively high in spring and summer but it did not differ between years or between vegetation types (detail not shown). It was associated with coccidiosis (χ 2 = 17·39, df = 1, P < 0·001): oocysts were shed by 143/167 (85·6%) rabbits with coccidiosis and 225/332 (67·8%) without coccidiosis.
Myxomatosis
The prevalence of myxomatosis was 42/548 (7·7%). Myxomatosis was light in 18 rabbits (one seropositive for T. gondii at titre 1:81 920), moderate in 19 (one seropositive for T. gondii at titre 1:40) and heavy in five (all seronegative for T. gondii). No rabbit with very heavy myxomatosis was found. Toxoplasma gondii seropositivity was not associated with myxomatosis (P = 0·451 by Fisher's Exact Test), a result also found for seroprevalence when multiple factors were considered (Table 1). The prevalence of myxomatosis did not differ between sexes but it was relatively high in juveniles (detail not shown). It was relatively high in 2007 and in summer but it did not differ between vegetation types (detail not shown). Myxomatosis was associated with coccidiosis (χ 2 = 11·22, df = 1, P < 0·001): coccidiosis was observed in 24/42 (57·1%) rabbits with myxomatosis and 155/506 (30·6%) without myxomatosis. Coccidial oocyst shedding was not associated with myxomatosis (χ 2 = 0·86, df = 1, P = 0·3545): oocysts were shed by 34/42 (81·0%) rabbits with myxomatosis and 334/457 (73·1%) rabbits without myxomatosis.
Roundworms
The prevalence of roundworms was 521/548 (95·1%). Roundworm epg was 0 in 27 rabbits (all seronegative for T. gondii), low in 118 (six seropositive for T. gondii at titres between 1:20 and 1:102 40), medium in 227 (11 seropositive for T. gondii at titres between 1:20 and 1:81 920) and high in 176 (one seropositive for T. gondii at titre 1:20). Toxoplasma gondii infection was associated with roundworm burden (P = 0·034 by Fisher's Exact Test; Fig. 2) but that association was not found when multiple factors were considered (Table 1).
The prevalence of roundworms did not differ between sexes but it was relatively high in juveniles and adults (detail not shown). It was relatively high in summer and autumn/winter but it did not differ between years or vegetation types (detail not shown). Roundworms were not associated with coccidial oocyst shedding (χ 2 = 0·00, df = 1, P = 0·995): 353/478 (73·8%) rabbits with roundworms shed oocysts while from 15/21 (81·0%) rabbits without a report of roundworms, oocysts were not observed.
DISCUSSION
The T. gondii seroprevalence in rabbits at Pitroddie is the lowest yet reported in wild rabbits. Previous authors found seroprevalences of 5% in the Czech Republic (Hejlícek et al. Reference Hejlícek, Literák and Nezval1997), 11·9 and 14·2% in Spain (Almería et al. Reference Almería, Calvete, Pagés, Gauss and Dubey2004; García-Bocanegra et al. Reference Garcia-Bocanegra, Astorga, Napp, Casal, Huerta, Borge and Arenas2010a , Reference Garcia-Bocanegra, Dubey, Martínez, Vargas, Cabezón, Zorrilla, Arenas and Almería b ), 17·4% in Australia (Cox et al. Reference Cox, Edmonds and Shepherd1981), 21% in Norway and Sweden (Kapperud, Reference Kapperud1978), 50% in Wales (Beverley et al. Reference Beverley, Beattie and Roseman1954), and a PCR prevalence of 68·4% in England (Hughes et al. Reference Hughes, Thomasson, Craig, Georgin, Pickles and Hide2008).
The absence of acute toxoplasmosis agreed with previous surveys of wild rabbits. That could imply that acutely ill wild rabbits remain underground or that they are vulnerable to predation. Very low seroprevalence at Pitroddie may be due to few feral cats being present. Previously, T. gondii oocysts or infections have been reported where cats were present (Sroka et al. Reference Sroka, Wojcik-Fatla and Dutkiewicz2006) and where traces of their feces could have been carried on straw (Faull et al. Reference Faull, Clarkson and Winter1986) or in water (Cox et al. Reference Cox, Edmonds and Shepherd1981; Aramini et al. Reference Aramini, Stephen, Dubey, Engelstoft, Schwantje and Ribble1999; Conrad et al. Reference Conrad, Miller, Kreuder, James, Mazet, Dabritz, Jessup, Gulland and Grigg2005), but rarely where cats were absent (Dubey et al. Reference Dubey, Rollor, Smith, Kwok and Thulliez1997). Straw is not routinely imported to Pitroddie and waterborne T. gondii has not been reported there.
Wild rabbits’ frequent breeding could explain the associations between T. gondii and female sex, and heavy coccidiosis. In mammals with haemochorial placentation such as rabbits, mice and humans, gestational immunosuppression prevents rejection of the foetus (Moffett and Loke, Reference Moffett and Loke2006). The T helper cell type 1 (Th1) response to immune challenge is downregulated relative to the type 2 (Th2) response. This change impairs the pregnant female's defence against intracellular coccidia such as T. gondii and E. stiedae, and viruses such as M. cuniculi, while enhancing her defence against extracellular parasites such as helminths (Cox, Reference Cox2001). Therefore pregnant rabbits could be relatively susceptible to infection by T. gondii, E. stiedae and M. cuniculi. In managed wild rabbits in Spain, new coccidial infections were relatively frequent during the breeding season (Bertó-Moran et al. Reference Bertó-Moran, Pacios, Serrano, Moreno and Rouco2013).
Toxoplasma gondii infections usually progress to a chronic state (bradyzoite cysts) during which the host remains seropositive for life (Dubey, Reference Dubey2008). Therefore new T. gondii infections during pregnancy might have led to the non-seasonal but female-biased seroprevalence reported here. Sex-biased T. gondii seroprevalence was not observed in wild rabbits in Spain (Almería et al. Reference Almería, Calvete, Pagés, Gauss and Dubey2004) or domestic rabbits in Mexico (Alvarado-Esquivel et al. Reference Alvarado-Esquivel, Alvarado-Esquivel, Villena and Dubey2013). Neither of those reports mentioned E. stiedae. Ours is the first report of T. gondii coinfecting with E. stiedae except in one captive rabbit (Dubey et al. Reference Dubey, Brown, Carpenter and Moore1992). Unlike T. gondii, E. stiedae is cleared within weeks (Barriga and Arnoni, Reference Barriga and Arnoni1981). It was detected in the present study by transient evidence: hepatic lesions and fecal oocysts. That transience may explain the lack of sex bias in those observations.
Once inside host cells, apicomplexan parasites subvert the immune response. They enhance their own survival and their chance of transmission by mechanisms which increase the Th1:Th2 ratio (Frölich et al. Reference Fröhlich, Entzeroth and Wallach2012; Graham et al. Reference Graham, Cattadori, Lloyd-Smith, Ferrari and Biømstad2007). Subversion of the immune response by E. stiedae could limit any effect of pregnancy on rabbits’ susceptibility to new T. gondii infections. That may have contributed to the observed lack of variation in T. gondii seroprevalence between age classes, which contrasted with a tendency for this parasite's prevalence to raise with age in many hosts (Dubey, Reference Dubey2008). On most farms oocyst exposure is inferred to be near-continuous for grazing animals, leading to an accumulation of chronic infections (Kijlstra and Jongert, Reference Kijlstra and Jongert2009).
Association between oocyst excretion and hepatic coccidiosis indicated that many wild rabbits with overt coccidiosis at Pitroddie shed oocysts likely to represent the same parasite. Previously, experimental E. stiedae infection revealed its time course (Barriga and Arnoni, Reference Barriga and Arnoni1981). Those laboratory rabbits developed hepatic lesions in the third week post-infection, and they shed oocysts rapidly in the fourth and fifth weeks. But they shed some oocysts within the first 3 weeks, perhaps resembling wild rabbits at Pitroddie in which oocyst shedding and hepatic coccidiosis coincided. Those which shed oocysts without having overt coccidiosis might have cleared E. stiedae infection before being shot, or the oocysts in their feces might have been from other coccidia.
This is the first report of sex-independent coccidiosis in this population. The reduction in its prevalence with age and its seasonality were previously reported (Boag et al. Reference Boag, Hernandez and Cattadori2013). For E. stiedae oocyst shedding, an Australian study of wild rabbits found sex-independence, falling prevalence with age and seasonality (Hobbs et al. Reference Hobbs, Twigg, Elliot and Wheeler1999).
The MAT is a trusted method for many species and tissue types, measuring specific anti-T. gondii IgG (Dubey and Desmonts, Reference Dubey and Desmonts1987). However since Th2 cells are important in antibody responses, gestational reduction in the Th1:Th2 ratio might enhance females’ production of specific IgG, leading to false positive MAT results. Future studies could use IgM serology and other methods, not dependent on antibodies, to detect new infections.
We speculate that the observation of six high-seropositive rabbits, close to one another in time and space, could have arisen from a sudden influx or release of oocysts near PF in late 2006. It is noteworthy that transformations of the inverse MAT titre did not normalize variance, reflecting an unusual distribution in that variable. The lack of statistical association between seropositivity for T. gondii and year, season, age or distance from PF did not disprove the hypothesis of oocyst influx or release. Oocysts could have been introduced by a cat or by passive transport, perhaps on a vehicle. Thereafter oocysts could have been transported throughout the site, and they could have been released from soil by changes in temperature or moisture. It is noteworthy also that relatively high prevalence in 2007 was observed for clinical signs of coccidiosis but not for coccidial oocyst shedding. These observations concerning E. stiedae arose within a few months of the putative influx or release of T. gondii oocysts, perhaps reflecting weather conditions. Future studies could seek T. gondii oocysts in wild rabbits’ environment, and they could consider genetic relationships between rabbits.
Another infectious agent subverting the lapine immune response is M. cuniculi. That virus is immunosuppressive, decreasing all T cells (García-Bocanegra et al. Reference Garcia-Bocanegra, Astorga, Napp, Casal, Huerta, Borge and Arenas2010a , Reference Garcia-Bocanegra, Dubey, Martínez, Vargas, Cabezón, Zorrilla, Arenas and Almería b ) especially Th1 cells (Jeklova et al. Reference Jeklova, Leva, Matiasovic, Kovarcik, Kudlackova, Nevorankova, Psikal and Falyna2008). Therefore a rabbit with myxomatosis might resemble a pregnant rabbit in having a low Th1:Th2 cell ratio and hence being vulnerable to new T. gondii infections. If that is so, seropositivity for T. gondii could be associated with seropositivity for M. cuniculi. However, the present study did not test that hypothesis, examining M. cuniculi infection only as clinical myxomatosis which is transient.
Apicomplexan subversion of the immune response, increasing the Th1:Th2 cell ratio contrary to female rabbits’ gestational Th1 downregulation, could affect rabbits’ immune defences against nematodes (Boag et al. Reference Boag, Hernandez and Cattadori2013). The present study did not distinguish biological effects of T. gondii and E. stiedae. It also did not distinguish between roundworm species, perhaps explaining the lack of sex bias in roundworm prevalence. Trichostrongylus retortaefomis epg is higher in breeding females than in non-breeding females and males in this population (Cattadori et al. Reference Cattadori, Boag, Bjørnstad, Cornell and Hudson2005) and in a wild rabbit population in Spain (Molina et al. Reference Molina, Casanova and Feliu1999), and it was higher in intact females than in sterilized females in a managed wild rabbit population in Australia (Hobbs et al. Reference Hobbs, Twigg, Elliot and Wheeler1999). In rabbits at Pitroddie, a biological interaction between T. gondii and roundworms remains plausible.
Future studies of rabbit populations with more abundant T. gondii could examine coinfections between it, E. stiedae and other parasites in this host. Molecular detection could be integrated with high-resolution genetic characterization (Su et al. Reference Su, Shwab, Zhou, Zhu and Dubey2010). Screening of wild rabbit populations could be complemented by experimental infections, including pregnant females. The use of body weight to estimate age could be reconsidered because coccidiosis and myxomatosis are known to slow growth in the rabbits at Pitroddie (Lello et al. Reference Lello, Boag and Hudson2005; Cattadori et al. Reference Cattadori, Boag and Hudson2008) and such an effect has been described also for coccidiosis in laboratory rabbits (Barriga and Arnoni, Reference Barriga and Arnoni1981).
People eating wild rabbit meat, or feeding it to their cats and other pets, are sometimes unaware of the need to cook or freeze meat thoroughly. Human T. gondii infections were reported in rabbit trappers in Wales (Beverley et al. Reference Beverley, Beattie and Roseman1954) and liver patients who had eaten rabbit meat in Mexico (Alvarado-Esquivel et al. Reference Alvarado-Esquivel, Torres-Berumen, Estrada-Martínez, Liesenfeld and Mercado-Suarez2011). A risk of infection was noted for workers handling farmed rabbits in Poland (Sroka et al. Reference Sroka, Zwolinski, Dutkiewicz, Tos-Luty and Latuszynska2003) and for consumers of wild rabbit meat (Almería et al. Reference Almería, Calvete, Pagés, Gauss and Dubey2004). Wild rabbit meat could be screened using ELISA as suggested for meat from farmed rabbits (Mecca et al. Reference Mecca, Meireles and de Andrade2011) and consumers could be better informed through leaflets and advertisements.
The present study complements previous evidence that the wild rabbit can be used as an indicator species for T. gondii on land (Cox et al. Reference Cox, Edmonds and Shepherd1981), as can wild carnivores (Burrells et al. Reference Burrells, Bartley, Zimmer, Roy, Kitchener, Meredith, Wright, Innes and Katzer2013). It provides preliminary evidence that T. gondii in wild rabbits in Scotland behaves mostly as in other mammalian hosts. Gestational Th1 downregulation in the rabbit is probably involved. However in some rabbit populations, including the one studied here, outcomes are affected by another endemic apicomplexan parasite: E. stiedae. We suggest that T. gondii resembles Eimeria spp. in some of its effects on the immune system, including a rise in the Th1:Th2 ratio. Differences between the life cycles of T. gondii and E. stiedae may have led to different interactions with M. cuniculi and roundworms.
ACKNOWLEDGEMENTS
We thank T. Cameron and I. Cattadori for advice on the study design, I. Bradter and C. Polce for the map using data from EDINA (http://edina.ac.uk/), D. Allen and J. Steele for permission to collect rabbits from the study area.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.






