Introduction
The quality of livestock semen is a main determinant of fertilization success, therefore only semen of high quality is required for breeding programmes and conservation of animal genetic resources in specialized gene banks (Chrenek et al., Reference Chrenek, Kubovicova and Makarevich2017). Motility and the concentration of spermatozoa are the primary indicators of semen quality (Baláži et al., Reference Baláži, Vašíček, Svoradová, Macháč, Jurčík, Huba, Pavlík and Chrenek2020). A deeper analysis of spermatozoa quality by flow cytometry, however, could reveal cell defects that are not visible using basic microscopic assessment. Over recent years, flow cytometry has become a standard laboratory method for the evaluation of specific spermatozoa attributes (Svoradová et al., Reference Svoradová, Kuželová, Vašíček, Olexíková, Baláži, Kulíková, Hrnčár, Ostro, Bednarczyk and Chrenek2018).
There are five Slovak national breeds of sheep that are valuable for breeders and for the preservation of genetic resources (Chrenek et al., Reference Chrenek, Makarevich, Kubovicova, Bulla and Supuka2019). Most sheep in Slovakia are reared countrywide in local farms that do not have specialized laboratory equipment. Although a basic microscopic evaluation of semen quality (spermatozoa motility and concentration) could be provided in situ by an experienced technician, the transport of fresh semen samples to specialized laboratories is required to carry out flow-cytometric analysis. Unfortunately, ram spermatozoa are very sensitive to any cold shock (Mendoza et al., Reference Mendoza, Casao, Pérez-Pé, Cebrián-Pérez and Muiño-Blanco2013) or oxidative damage (Hamilton et al., Reference Hamilton, Mendes, Castro, Assis, Siqueira, Delgado, Goissis, Muiño-Blanco, Cebrián-Pérez, Nichi, Visintin and D’Ávila Assumpção2016), therefore the results of any quality assessment of transported fresh ram semen samples would not reflect the true physiological state of the spermatozoa at the time of collection as transport itself could last for several hours. To overcome this problem and maintain initial semen quality, fixation of the stained spermatozoa could be performed.
Fixation is a process that preserves the cell structure. Here, samples are chemically fixed to maintain them in a state very similar to that of their live counterparts by terminating all metabolic and enzymatic processes and therefore minimizing any possible change. Optimization of the fixation process is a crucial step, as underfixation could result in poor preservation of morphology or loss of fluorescent signal. Conversely, overfixation may also lead to signal loss or the generation of artefacts, or could increase nonspecific background signals (Fischer et al., Reference Fischer, Jacobson, Rose and Zeller2008). Generally, two basic types of fixatives have been used in immunochemistry: cross-linking agents and coagulants (Melan, Reference Melan1994).
Formaldehyde is a common choice of fixative for fluorescence microscopy, as well as for flow cytometry. This reagent forms chemical bonds between reactive groups, therefore cross-linking proteins; formaldehyde does not also contribute significantly to autofluorescence. In general, cells are fixed in phosphate-buffered saline (PBS) (pH 7.4) containing 2–4% formaldehyde for 15 min at 20°C (Fischer et al., Reference Fischer, Jacobson, Rose and Zeller2008). The second fixative type, coagulants (solvents such alcohols and acetone), have the tendency to form artefacts, but nevertheless are very useful in light microscopy and for antigens with large-molecular-weight or polymerized structural proteins (Melan, Reference Melan1994). Moreover, the rapid use of cold methanol solution or acetone to fix samples compares well with that of aldehydes, and these have been used for example to study cytoskeleton components. At the same time, however, they fix and permeabilize cells, which is not desirable in some cases. In addition, together with fixation, dehydration occurs that results in possible sample shrinkage (Fischer et al., Reference Fischer, Jacobson, Rose and Zeller2008).
The aim of this study was therefore to examine the stability of fluorescent signals from semen samples that had been fixed immediately after staining in a buffer containing formaldehyde and by repeated measurements for several hours after fixation.
Materials and methods
Animals
Three sexually mature and clinically health rams of the Slovak Dairy (SD) sheep breed, aged 2 years old, were used in this study. The rams were kept in external conditions in individual stalls at a breeding facility (NPPC, VUŽV Nitra, Lužianky, Slovak Republic), and fed with hay bales and oats; water and mineral salts were supplied ad libitum. Semen samples (n = 9) were collected once a week by electro-ejaculation and immediately transferred to the laboratory as described previously (Kulíková et al., Reference Kulíková, Baláži, Tóthová, Jurčík, Huba and Chrenek2018; Baláži et al., Reference Baláži, Vašíček, Svoradová, Macháč, Jurčík, Huba, Pavlík and Chrenek2020) for the duration of study (3 weeks).
Experimental design and flow cytometry
Aliquots of fresh semen samples from each ram were adjusted to 106 cells/ml in Ca- and Mg-free PBS; Biosera, France) and stained using selected chemicals for specific markers to identify different physiological cell attributes, as follows: (i) apoptosis-like changes using annexin V (annexin V-FITC Apoptosis Detection Kit, Canvax, Spain) and apoptosis using DNA fragmentation (YO-PRO-1) and detection of most caspases (FLICA; Vybrant FAM Poly Caspases Assay Kit); (ii) acrosomal status using PNA in Alexa Fluor 488 and antibody against intra-acrosomal protein in FITC (GAPDHS; SpermFlowEx Kit, EXBIO, Czech Republic); (iii) mitochondrial activity was analyzed via membrane mitochondrial potential using MitoTracker probes (MitoTracker Green FM and MitoTracker Red CMXRos); and (iv) generation of reactive oxygen species (ROS) using dihydroethidium (DHE) and CellROX Green Reagent, and cell death using LIVE/DEAD Fixable Dead Cell Stain kits (green and red fluorescent reactive dye). All chemicals were purchased from Thermo Fisher Scientific (USA) unless stated otherwise. Efficiency of the chemicals used to identify different physiological processes was confirmed following the induction of these processes in ram semen samples (see Supplementary Material Results S1). Briefly, ram semen samples were incubated with the mentioned reagents either in accordance with the producer’s manuals or as described previously (Koppers et al., Reference Koppers, De Iuliis, Finnie, McLaughlin and Aitken2008; Svoradová et al., Reference Svoradová, Kuželová, Vašíček and Chrenek2017). Reagents for staining were used at a final concentration in accordance with the producer’s manuals or as follows: YO-PRO-1 (100 μM), PNA (20 μM), MitoTracker Green FM (200 nM), MitoTracker Red CMXRos (100 nM), DHE (2 μM) and CellROX Green Reagent (2.5 μM). All samples were stained with a specific marker in combination with the LIVE/DEAD Fixable Dead Cell Stain kit (red or green depending on the fluorescence of the initial marker). Aliquots of stained samples were immediately analyzed by flow cytometry using a FACSCalibur instrument (BD Biosciences, USA) and the FL1 (green) and FL3 (red) channels. The remaining stained samples were washed and fixed with a formaldehyde-based fixation buffer (eBioscience™ IC Fixation Buffer; Thermo Fisher Scientific, USA) for 20 min at room temperature in accordance with the producer’s manual. After a final wash, fixed samples were immediately analyzed again by flow cytometry. Aliquots of fixed samples were re-analyzed after an additional 5 h and 20 h post-fixation. All fixed samples were stored in the dark at 2–8°C until the time of next measurement. At least 10,000 events were analyzed for each sample.
Statistical analysis
Experiments were repeated three times. Obtained results were evaluated using SigmaPlot software (Systat Software Inc., Germany) with one-way analysis of variance (Holm–Sidak method) and expressed as means ± SD. P-values at P < 0.05 were considered to be statistically significant.
Results
Two of the three measured apoptotic markers (annexin V and FLICA) maintained the proportion of positively stained cells after fixation and, moreover, also at several hours post-fixation (about 10 and 45%, respectively). However, the YO-PRO-1-stained samples loss their fluorescence intensity immediately after fixation (Fig. 1A) and the proportion of positive cells decreased significantly (P < 0.05) compared with the fresh sample (from 30 to 10%; Fig. 1B).

Figure 1. Expression of apoptotic markers in fresh and fixed ram semen samples. (A) Illustrative flow-cytometric histograms showing changes in fluorescence intensity. (B) Statistical evaluation of apoptotic cells using three different markers (means ± SD); *statistical significant at P < 0.05. Fresh, fresh samples analysed immediately after staining; Fixed, Fixed 5 h and Fixed 20 h, samples fixed after staining and analyzed either immediately after fixation, or at 5 h or 20 h post-fixation, respectively.
For acrosomal damage, the proportion of positively stained spermatozoa (PNA+ and GAPDHS+) was not affected by fixation when measured immediately or at several hours post-fixation. Both markers detected about 10% of cells as positive (Fig. 2A, B).

Figure 2. Proportion of spermatozoa in fresh and fixed ram semen samples with damaged acrosomes. (A) Illustrative flow-cytometric histograms showing changes in fluorescent intensity. (B) Statistical evaluation of acrosomal damage analyzed using two different markers (means ± SD). Fresh, fresh samples analysed immediately after staining; Fixed, Fixed 5 h and Fixed 20 h, samples fixed after staining and analyzed either immediately after fixation, or at 5 h or 20 h post-fixation, respectively.
Membrane mitochondrial potential (MMP) of fresh and fixed ram spermatozoa were analyzed using two different MitoTracker (MT) probes. Despite fixation, both probes (MT Green FM and MT Red CMXRos) showed similar values for MMP (about 60%) compared with the fresh sample (Fig. 3A, B).
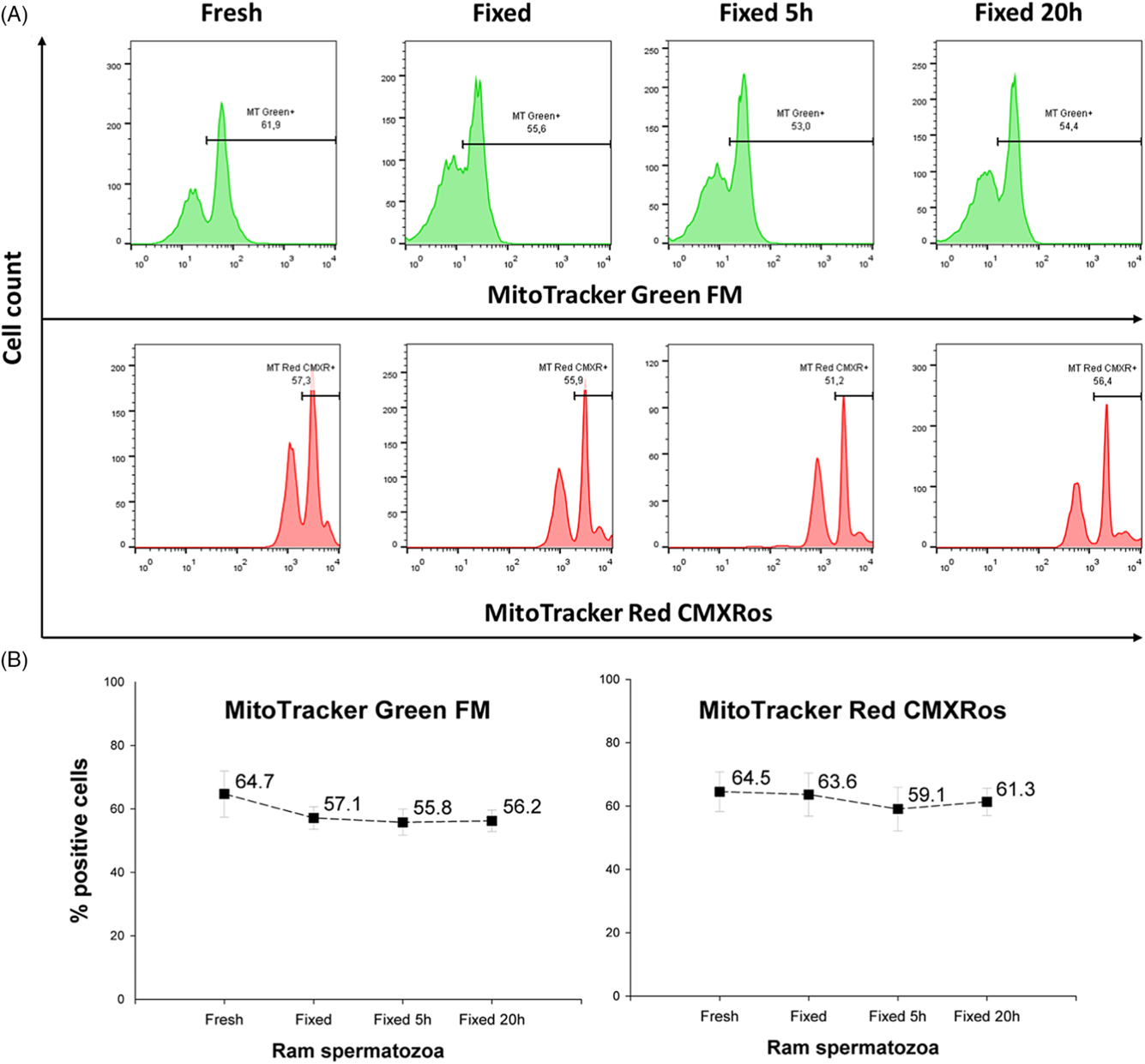
Figure 3. Membrane mitochondrial potential of spermatozoa measured in fresh and fixed ram semen samples. (A) Illustrative flow-cytometric histograms showing changes in fluorescent intensity. (B) Statistical evaluation of MMP analyzed using two different MT probes (means ± SD). Fresh, fresh samples analysed immediately after staining; Fixed, Fixed 5 h and Fixed 20 h, samples fixed after staining and analyzed either immediately after fixation, or at 5 h or 20 h post-fixation, respectively.
Similarly, no effect of fixation was observed between fresh and fixed samples when ROS-positive spermatozoa were measured using DHE or CellROX Green reagent (Fig. 4A, B). However, while DHE staining revealed about 60% ROS+ spermatozoa, the CellROX Green reagent detected only 20% ROS+ cells.

Figure 4. Generation of ROS analyzed in fresh and fixed ram semen samples. (A) Illustrative flow-cytometric histograms showing changes in fluorescent intensity. (B) Statistical evaluation of ROS-positive spermatozoa analyzed using two different markers (means ± SD). Fresh, fresh samples analysed immediately after staining; Fixed, Fixed 5 h and Fixed 20 h, samples fixed after staining and analyzed either immediately after fixation, or at 5 h or 20 h post-fixation, respectively.
Cell death was analyzed using LIVE/DEAD Fixable Dead Cell Stain kits with green and red fluorescence. Both reagents revealed similar numbers of positive cells (about 15%) in fixed samples compared with the fresh samples (Fig. 5A, B).
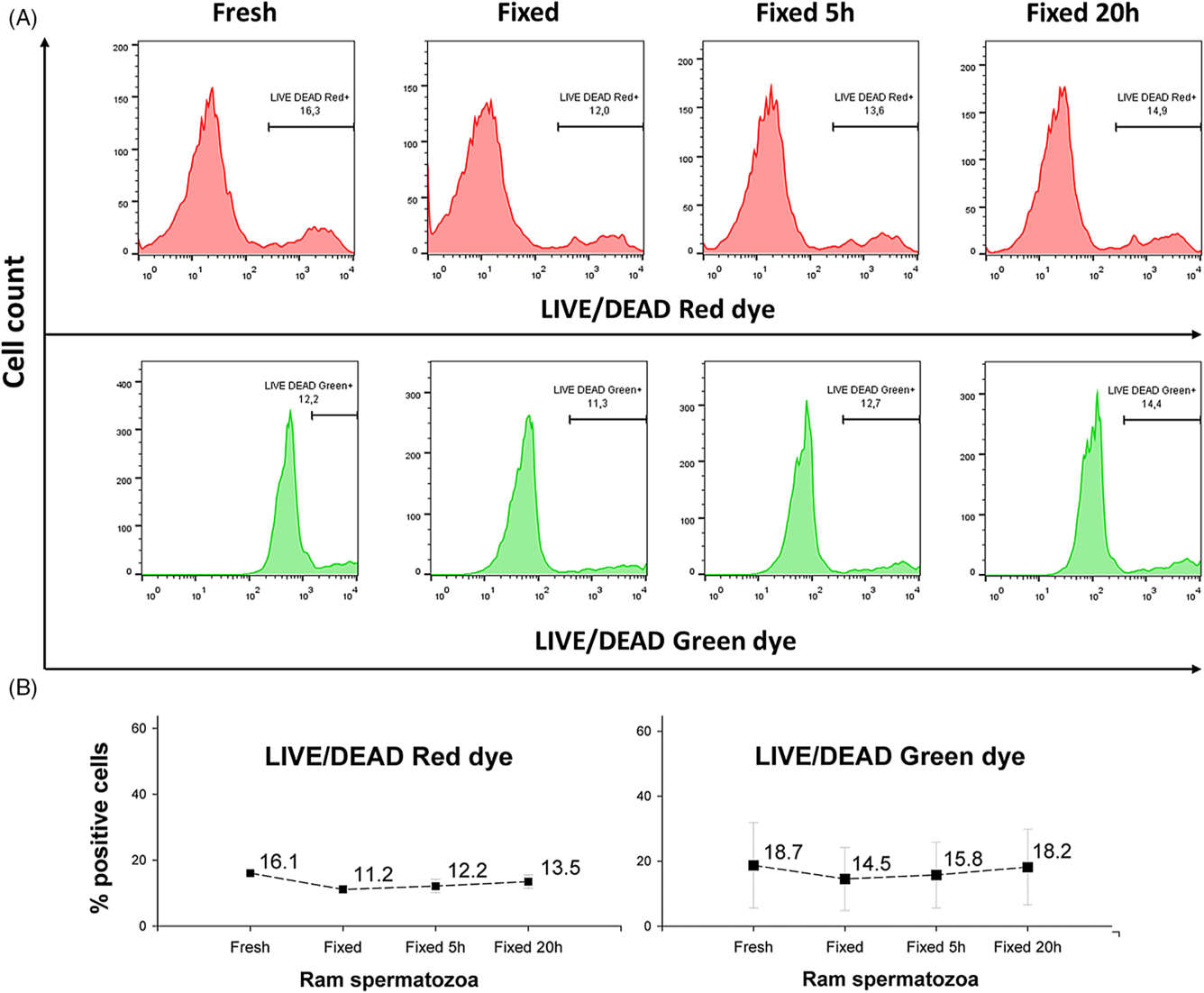
Figure 5. Comparison of the dead cell proportion in fresh and fixed ram semen samples. (A) Illustrative flow-cytometric histograms showing changes in fluorescence intensity. (B) Statistical evaluation of the cell death analyzed using the same marker in two different fluorescence spectra (means ± SD). Fresh, fresh samples analysed immediately after staining; Fixed, Fixed 5 h and Fixed 20 h, samples fixed after staining and analyzed either immediately after fixation, or at 5 h or 20 h post-fixation, respectively.
Flow-cytometric analysis for spermatozoa incubated with different inducers can be found in Supplementary Material Figs 1–5.
Discussion
In this study, we tested several sperm qualitative markers for their ability to retain fluorescence intensities after fixing stained semen samples in formaldehyde-containing buffer. First, apoptotic (YO-PRO-1 and FLICA) and apoptotic-like markers (annexin V) were examined. YO-PRO-1 is a common fluorescent marker for detection of early apoptosis (Idziorek et al., Reference Idziorek, Estaquier, De Bels and Ameisen1995). Based on the obtained data, YO-PRO-1 dye did not withstand formaldehyde fixation. A similar observation was seen in a human cell line stained with YO-PRO-1 and subsequently fixed with formaldehyde (Bradford and Buller, Reference Bradford and Buller2008). As also demonstrated in our study with stained and fixed ram semen, Bradford and Buller (Reference Bradford and Buller2008) stated that annexin V dyes maintained their fluorescence intensity even after 18 h post-fixation. Both YO-PRO-1 and annexin V belong to a group of supravital dyes (Wlodkowic et al., Reference Wlodkowic, Telford, Skommer and Darzynkiewicz2011), however YO-PRO-1 is a DNA intercalating dye. Its fluorescence properties may be changed due to cross-linking of DNA with DNA itself and/or nearby proteins during formaldehyde fixation (Jacobberger, Reference Jacobberger2000) and is probably a reason for the significant loss of YO-PRO-1 signal in post-fixed ram semen samples. Annexin V should be also used with caution in fixed cells, as its fluorescence may be quenched (Plenchette et al., Reference Plenchette, Filomenko, Logette, Solier, Buron, Cathelin and Solary2004) or reduced (Mariotti et al., Reference Mariotti, Pardini, Teloni, Gagliardi, Fraziano and Nisini2017) after sample fixation. FLICA, as a marker of active caspases, is relatively nontoxic and shows high permeability to plasma membranes. Moreover, intracellular binding of FLICA reagents to active caspases is covalent, hence it can withstand formaldehyde fixation of cells (Wlodkowic et al., Reference Wlodkowic, Telford, Skommer and Darzynkiewicz2011). Grunewald et al. (Reference Grunewald, Rasch, Reinhardt, Baumann, Paasch and Glander2008) reported stable fluorescence in semen samples that were stained with caspase-3 FLICA and fixed (4% formaldehyde) 10 days previously. These findings were confirmed by our results, as there were no significant changes in FLICA fluorescence intensity measured in fresh and post-fixed samples. Furthermore, the poly-caspase substrate FAM-VAD-FMK used in our experiments has very often been reported in other studies on flow-cytometric analysis of apoptosis in spermatozoa (Martínez-Pastor et al., Reference Martínez-Pastor, Mata-Campuzano, Álvarez-Rodríguez, Álvarez, Anel and De Paz2010).
Damage to the sperm acrosome is usually evaluated using Arachis hypogaea (peanut) agglutinin (PNA), a lectin that binds to glucosidic residues on the impaired acrosomal membrane (Martínez-Pastor et al., Reference Martínez-Pastor, Mata-Campuzano, Álvarez-Rodríguez, Álvarez, Anel and De Paz2010). However, recently, a new immunological approach has become available to characterize acrosomal status. GAPDHS, a sperm isoform of glyceraldehyde-3-phosphate dehydrogenase, is intra-acrosomal protein that was detected using mouse monoclonal antibody Hs-8 in human, boar and mouse spermatozoa. It is highly conserved among mammalian species (Margaryan et al., Reference Margaryan, Dorosh, Capkova, Manaskova-Postlerova, Philimonenko, Hozak and Peknicova2015), therefore cross-reactivity with ram sperm GAPDHS might be possible. In our study, no differences were found in the proportion of spermatozoa with acrosomal damage between fresh and fixed samples that were stained either with PNA coupled to Alexa Fluor 488 or with antibody against GAPDHS coupled to FITC. Fixation with formaldehyde is highly recommended for antibody-stained samples if storage is needed before analysis. Fixation increases cell stability, although staining fluorescence could decrease slightly (Givan, Reference Givan2000). In general, synthetic chemical dyes (such as FITC or Alexa Fluors) should be more stable to fixation compared with protein-based dyes such as PE and APC or their tandems (PE/Cy7, APC/Cy7, etc.). Nevertheless, they should withstand standard fixation with 1–4% formaldehyde in most cases (Shankey et al., Reference Shankey, Forman, Scibelli, Cobb, Smith, Mills, Holdaway, Bernal-Hoyos, Van Der Heiden, Popma and Keeney2006; Cossarizza et al., Reference Cossarizza, Chang, Radbruch, Acs, Adam and Adam-Klages2019). As with antibody-stained spermatozoa, lectins such PNA could be fixed subsequently and stored for later analysis (Martínez-Pastor et al., Reference Martínez-Pastor, Mata-Campuzano, Álvarez-Rodríguez, Álvarez, Anel and De Paz2010).
MitoTracker probes have been reported as MMP markers for more than 2 decades (Haughland, Reference Haughland2005). Some of them, including MitoTracker Red CMXRos (Poot et al., Reference Poot, Zhang, Krämer, Wells, Jones, Hanzel, Lugade, Singer and Haugland1996) or MitoTracker Deep Red (Peña et al., Reference Peña, Ball and Squires2018), are retained in somatic cells or spermatozoa mitochondria after formaldehyde fixation. Similar observations were made in our study, as MMP fluorescence was not significantly changed when comparing fresh and fixed samples incubated either with MitoTracker Red CMXRos or even with MitoTracker Green FM. Although, MitoTracker Green FM is commonly used for live cell staining and measurement, it might also be appropriate for analysis after formaldehyde fixation (Chazotte, Reference Chazotte2011).
DHE can detect intracellular superoxide radical anions (O2 •−), therefore identifying cells that generate intracellular ROS (Aitken et al., Reference Aitken, De Iuliis and Baker2012). In our study, fixed ram semen samples showed a similar proportion of ROS-positive spermatozoa compared with fresh samples when detected by both reagents (DHE and CellROX Green), although the numbers of DHE-positive cells increased more than two-fold compared with those positive for CellROX. However, despite DHE being reported to form red fluorescent 2-hydroxyethidium (2-OH-Et+) after oxidation by superoxide, another red fluorescent product named ethidium (Et+) was also formed by nonspecific oxidation. Et+ concentration was usually much higher than that of 2-OH-Et+ (Zielonka and Kalyanaraman, Reference Zielonka and Kalyanaraman2010; Dikalov and Harrison, Reference Dikalov and Harrison2014), possibly in our experiments caused the higher proportion of ROS-positive spermatozoa detected by DHE compared with CellROX Green reagent. Therefore, to detect superoxide objectively, both DHE products (2-OH-Et+ and Et+) should be identified using high-performance liquid chromatography or fluorescence spectroscopy rather than by flow cytometry, as suggested by Nazarewicz et al. (Reference Nazarewicz, Bikineyeva and Dikalov2013). CellROX reagent has been used successfully for flow-cytometric detection of superoxide anions in stallion (Gallardo Bolanos et al., Reference Gallardo Bolanos, Balao da Silva, Martin Muñoz, Morillo Rodriguez, Plaza Davila, Rodriguez-Martínez, Aparico, Tapia, Ferrusola and Peña2014; Davila et al., Reference Davila, Muñoz, Tapia, Ferrusola and Peña2015), ovine (Alves et al., Reference Alves, de Andrade, de Arruda, Batissaco, Florez-Rodriguez, Lançoni, de Oliveira, Tores, Ravagnani, de Almeida, Vellone and Celeghini2015) and bull (de Castro et al., Reference de Castro, de Assis, Siqueira, Hamilton, Mendes, Losano, Nichi, Visintin and Assumpção2016) spermatozoa using CellROX Deep Red or Green reagent. The latter two studies indicated that CellROX reagents were able to detect nonspecific types of free radicals. Nevertheless, based on information from the reagent manufacturer, CellROX Green reagent retains its fluorescent signal after formaldehyde fixation (Grinberg et al., Reference Grinberg, Dibbern, Levasseur and Kraig2013) if analyzed within 24 h.
Amine reactive dyes are able to identify dead cells in samples fixed later. These dyes are retained in cells after fixation (Perfetto et al., Reference Perfetto, Chattopadhyay, Lamoreaux, Nguyen, Ambrozak, Koup and Roederer2010), and are therefore called ‘live/dead fixable viability dyes’. They can be used combined with permeabilization techniques, therefore allowing multiparametric analysis of samples to proceed. The usefulness of these dyes for fixed samples (somatic cells or spermatozoa) has already been demonstrated by several studies (Bradford and Buller, Reference Bradford and Buller2008; Martínez-Pastor et al., Reference Martínez-Pastor, Mata-Campuzano, Álvarez-Rodríguez, Álvarez, Anel and De Paz2010; Ortega Ferrusola et al., Reference Ortega Ferrusola, Anel-López, Ortiz-Rodriguez, Martin Muñoz, Álvarez, De Paz, Masot, Redondo, Balao da Silva, Morrell, Rodriguez Martinez, Tapai, Gil, Anel and Pena2017; Peña et al., Reference Peña, Ball and Squires2018). Our observed data were in agreement with these findings, as fluorescent signals from both reagents (LIVE/DEAD Green and Red dyes) were maintained after formaldehyde fixation.
In conclusion, we propose here an optimized methodology for multiparametric quality assessment of ram semen samples that required prolonged storage or transportation prior to the flow-cytometric analysis. For ram semen samples, several tested markers were able to withstand formaldehyde fixation, and therefore could be used to analyse apoptosis (annexin V simultaneously with FLICA), acrosomal status (PNA), mitochondrial activity (MitoTracker Red CMXRos) and oxidative stress (CellROX Green) and could be combined with a suitable live/dead fixable viability dye.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0967199420000581
Financial support
The study was supported financially by the Slovak Research and Development Agency (no. APVV-17–0124), by the Scientific Grant Agency (grant no. VEGA 1/0049/19), the Educational Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic, and the Slovak Academy of Sciences (grant no. KEGA 026SPU-4/2018).
Ethical standards
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional guidelines on the care and use of laboratory animals. The experiments were carried out in accordance with the Code of Ethics of the EU Directive 2010/63/EU for animal experiments. (https://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm).
Conflicts of interest
None.







