INTRODUCTION
Idiopathic normal pressure hydrocephalus (iNPH) is a disorder of abnormal cerebrospinal fluid (CSF) absorption not due to a known cause, such as trauma, that results in ventriculomegaly. The clinical syndrome of iNPH was first described in 1965 and is characterized by the triad of gait disturbance, urinary incontinence, and cognitive impairment (Adams, Fisher, Hakim, Ojemann, & Sweet, Reference Adams, Fisher, Hakim, Ojemann and Sweet1965). Subsequent research advances have revealed additional clinical symptoms, including behavioral changes and neuropsychiatric syndromes (e.g., Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009).
Cognitive impairment in iNPH is most commonly characterized by a frontal–subcortical profile with deficits in attention, processing and psychomotor speed, and executive functioning (EF; Ogino et al., Reference Ogino, Kazui, Miyoshi, Hashimoto, Ohkawa, Tokunaga and Takeda2006; Williams & Malm, Reference Williams and Malm2016). Specifically, neuropsychological tests sensitive to functions mediated by the dorsolateral prefrontal cortex and the anterior cingulate, such as Trail Making Test Part B, have demonstrated sensitivity to cognitive impairment in iNPH patients (e.g., Katzen et al., Reference Katzen, Ravdin, Assuras, Heros, Kaplitt, Schwartz and Relkin2011). Disruption involving these brain regions may be secondary to involvement of subcortical projections from the caudate nucleus and white matter pathways that run anterior and lateral to the frontal horns of the lateral ventricles (for a review, see Rigamonti, Reference Rigamonti2014, Chapter 7). Compared to patients with Alzheimer’s disease (AD), iNPH patients demonstrate fewer difficulties with orientation and memory, but more pronounced executive dysfunction and slower processing speed (Ogino et al., Reference Ogino, Kazui, Miyoshi, Hashimoto, Ohkawa, Tokunaga and Takeda2006). However, there is considerable variability in the cognitive domains affected and severity of impairment among iNPH patients (Picascia et al., Reference Picascia, Minafra, Zangaglia, Gracardi, Pozzi, Sinforiani and Pacchetti2016).
Frontal Behavior Syndromes in iNPH
The presence of cognitive impairment in iNPH is relatively well established; however, neuropsychiatric manifestations are not as well understood. Apathy, which refers to diminished motivation not attributable to decreased level of consciousness, cognitive impairment, or emotional distress (Marin, Reference Marin1990), is the most common neuropsychiatric symptom associated with iNPH. Estimates suggest that clinically significant levels of apathy are present in approximately 60%–70% of patients with suspected iNPH (Allali et al., Reference Allali, Laidet, Armand, Saj, Krack and Assal2018; Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009). Such findings have been documented using multiple self-report and informant measures including the Apathy Evaluation Scale (Peterson et al., Reference Peterson, Housden, Killikelly, Devito, Keong, Savulich and Sahakian2016), Starkstein Apathy Scale (Allali et al., Reference Allali, Laidet, Armand, Saj, Krack and Assal2017), and Neuropsychiatric Inventory (NPI; Kanemoto et al., Reference Kanemoto, Kazui, Suzuki, Sato, Kishima, Yoshimine and Yoshiyama2016; Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009).
Diversion of CSF from the brain to abdominal cavity (or shunting) is commonly used to reverse or mitigate symptoms of iNPH (Nassar & Lippa, Reference Nassar and Lippa2016). Following shunting, apathy generally declines, and the extent of reduction is associated with improvements in global cognitive functioning, as measured by the Mini-Mental State Exam (MMSE; Peterson et al., Reference Peterson, Housden, Killikelly, Devito, Keong, Savulich and Sahakian2016) as well as improvement on cognitive measures of EF (Kanemoto et al., Reference Kanemoto, Kazui, Suzuki, Sato, Kishima, Yoshimine and Yoshiyama2016). Post-shunt reductions in neuropsychiatric symptoms, as measured by the NPI (an informant measure of dementia-related behavioral symptoms, which includes apathy) have also been shown, and these changes are associated with decreased caregiver burden (Kanemoto et al., Reference Kanemoto, Kazui, Suzuki, Sato, Kishima, Yoshimine and Yoshiyama2016).
Research examining aspects of neuropsychiatric functioning in iNPH beyond apathy is relatively scant. Aside from apathy, other neuropsychiatric/behavioral changes that have been observed in iNPH include anxiety, agitation, and stereotyped motor behaviors (e.g., opening and closing the door without purpose; Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009). In addition, it should be noted that depression has been a topic of growing interest in iNPH. Clinically significant depressive symptoms have been shown to be overrepresented in individuals with iNPH compared to the general population (Israelsson, Allard, Eklund, & Malm, Reference Israelsson, Allard, Eklund and Malm2016). The overlap between primary symptoms of iNPH, including apathy, and primary symptoms of depression present somewhat of a diagnostic challenge; however, it has been argued that depression is distinct from apathy and that properly identifying and treating comorbid depression in iNPH is likely to improve patient outcomes (Israelsson et al., Reference Israelsson, Allard, Eklund and Malm2016).
The Role of AD Pathology
The presence of AD biomarkers such as amyloid-beta 42 (Aβ-42) and tau proteins in CSF in iNPH is an emerging area of interest. AD is regarded as one of the most common comorbid conditions with iNPH (Bech-Azeddine, Høgh, Juhler, Gjerris, & Waldemar, Reference Bech-Azeddine, Høgh, Juhler, Gjerris and Waldemar2007; Golomb et al., Reference Golomb, Wisoff, Miller, Boksay, Kluger, Weiner and Salton2000). Initial evidence indicates that the presence of AD pathology is relevant to neuropsychiatric symptoms and behavior changes in iNPH (Kazui et al., Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato and Tanaka2016). In addition, the presence or absence of AD biomarkers has demonstrated clinical significance in predicting response to neurosurgical interventions. For example, iNPH patients with negative Aβ positron emission tomography (PET) scans were more likely to demonstrate a reduction in gait disturbance following shunting (Jang et al., Reference Jang, Beom, Yeshin, Kim, Lee, Tae and Duk2018). Similar findings regarding shunt response have been reported for CSF biomarkers of AD pathology, such as the p-tau/Aβ-42 ratio (Hong et al., Reference Hong, Kim, Jeong, Kim, Hwang, Lee and Na2018).
Emerging evidence suggests AD pathology may be linked to neuropsychiatric symptoms in iNPH. Kazui et al. (Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato and Tanaka2016) found a larger reduction in neuropsychiatric symptoms (measured with the NPI) post-shunt for iNPH patients without the presence of AD biomarkers as compared to those with a high likelihood of comorbid AD. The iNPH patients without AD biomarkers also displayed a larger reduction in caregiver burden after shunting, which the authors posited might be related to the differing changes in neuropsychiatric symptoms in response to shunting between groups.
Our research aims to assess the presence of frontal behavior change in iNPH with and without AD biomarkers using the Frontal Systems Behavior Scale (FrSBe; Malloy & Grace, Reference Malloy and Grace2001).
The FrSBe
The FrSBe consists of 46 items comprising three subscales (apathy, disinhibition, and executive dysfunction), for which informants rate behaviors both before the patient’s illness or injury and currently (within the 2 weeks prior to assessment). The apathy, disinhibition, and executive dysfunction scales have been shown to accurately discriminate among behavioral syndromes present in a variety of neurodegenerative/neurological conditions. For example, elevations in the apathy and executive dysfunction scales, but not the disinhibition scale, have been observed in patients with mild cognitive impairment (MCI) and dementia due to suspected AD (Ready, Ott, Grace, & Cahn-Weiner, Reference Ready, Ott, Grace and Cahn-Weiner2003). The disinhibition scale has demonstrated discriminative utility in differentiating between frontotemporal dementia and AD (Malloy, Tremont, Grace, & Frakey, Reference Malloy, Tremont, Grace and Frakey2007). The FrSBe has also established clinical utility in detecting behavioral changes associated with Huntington’s disease (Paulsen et al., Reference Paulsen, Stout, DeLaPena, Zeena, Swenson and Malloy1996), vascular dementia (Zawacki et al., Reference Zawacki, Grace, Paul, Moser, Ott, Gordon and Cohen2002), and multiple sclerosis (Chiaravalloti & Deluca, Reference Chiaravalloti and Deluca2003).
FrSBe’s convergent validity is supported by modest correlations observed between its scales and cognitive measures of EF (e.g., Paulsen et al., Reference Paulsen, Stout, DeLaPena, Zeena, Swenson and Malloy1996). These modest correlations demonstrate that FrSBe’s behavioral ratings are not redundant with cognitive tests and offer incremental value in predicting real-world outcomes. For example, research has identified associations between the disinhibition scale and burden for AD caregivers independent of cognitive test scores and functional impairment measures (Rymer et al., Reference Rymer, Salloway, Norton, Malloy, Correia and Monast2002). FrSBe’s behavioral ratings have also evidenced incremental utility in the prediction of activities of daily living beyond the variability accounted for by cognitive test results (Norton, Malloy, & Salloway, Reference Norton, Malloy and Salloway2001). Of note, apathy is cited as an especially powerful predictor of functional capacity among patients with mild to moderate AD (Boyle et al., Reference Boyle, Malloy, Salloway, Cahn-Weiner, Cohen and Cummings2003).
Possible Mechanisms of Apathy and Other Neurobehavioral Changes in iNPH
Neuroanatomical work in animal and human patient populations has linked frontal behavior syndromes to specific frontostriatothalamic circuits, some of which run adjacent to the lateral ventricles. The anatomy of these circuits may offer insight into behavior changes that are most commonly observed in iNPH. An apathetic behavioral syndrome has been linked to dysfunction in the anterior cingulate circuit, which involves direct pathways among the ventral anterior aspect of the thalamus, the anterior cingulate, caudate, and substantia nigra (Tekin & Cummings, Reference Tekin and Cummings2002). In addition, dysexecutive behavioral syndromes have been associated with disruption in the dorsolateral prefrontal subcortical circuit that runs proximal to the lateral ventricles; in contrast, disinhibition is linked to orbitofrontal circuitry that is more distal to the lateral ventricles (Rigamonti, Reference Rigamonti2014; Tekin & Cummings, Reference Tekin and Cummings2002). Given the lateral ventricles’ proximity to these cortical and subcortical circuits and structures, in the context of ventriculomegaly stemming from abnormal CSF absorption, a thorough evaluation of the frontal systems behaviors in iNPH is warranted.
The Current Study
This study aimed to replicate and advance findings regarding the presence of apathy and additional neuropsychiatric/behavioral changes in patients with iNPH by measuring a broader range of frontal systems behaviors using the FrSBe (Aim 1). Based on the proximity of the dorsolateral prefrontal subcortical circuit to the lateral ventricles as compared to orbitofrontal circuitry, as outlined above, we hypothesized that there would be clinically elevated levels of executive dysfunction in addition to apathy, but not disinhibition, among individuals with iNPH. Consistent with prior research, we also anticipated modest correlations between FrSBe scores and cognitive measures of EF. As the secondary aim, the study evaluated whether neuropsychiatric/ behavioral changes are exacerbated by the presence of AD biomarkers (Aim 2). Given the limited research to date, it was unclear whether the presence of AD biomarkers would alter the type or severity of frontal behavior changes. As such, we conducted an exploratory analysis to further appraise potential group differences across individuals with and without comorbid AD pathology.
METHODS
Participants
This study uses a subset of the baseline data from a larger iNPH study with specific aims to assess the presence of frontal behavior change in iNPH with and without AD biomarkers using the FrSBe. Sixty-three consecutive patients who met the inclusion/exclusion criteria were recruited from the Butler Hospital’s Normal Pressure Hydrocephalus Clinic. Inclusion criteria were referral to the Normal Pressure Hydrocephalus Clinic with at least one of the three core clinical features of iNPH (i.e., gait impairment, urinary incontinence, and cognitive impairment) and radiographic evidence of dilated ventricles out of proportion to the amount of cortical and subcortical atrophy. The iNPH diagnosis was established in an interdisciplinary discussion between two iNPH experts, a neurologist and a neurosurgeon (S.S. and P.K.), including clinical symptomatology and presence of ventriculomegaly on neuroimaging.
Exclusion criteria included secondary hydrocephalus, previous shunt insertion, or inability to meaningfully participate in the schedule of formal assessments (e.g., severe dementia, suboptimally controlled psychiatric impairments or primary psychotic disorder, or extensive sensory or motor limitations). Patients were also excluded on the basis of substance abuse within the past year or history of other neurologic disorder such as large vessel stroke, brain tumor, etc. Patients suspected of having a neurodegenerative disorder, such as AD, in addition to iNPH were not excluded, as one of the study goals is to improve differential diagnosis in this mixed population.
For the current analysis, 13 participants were excluded due to missing data on the FrSBe informant scalesFootnote 1. Of the 50 patients included, 52% were female and they were on average 75.72 years old (SD = 7.45) and had 13.36 years of education (SD = 2.44). The Butler Hospital Institutional Review Board approved the study, and both patients and their caregivers consented to all procedures.
Assessments
All assessments used for this article were completed at a baseline visit prior to any surgical intervention.
AD Biomarkers
AD biomarker status was determined by AβPET imaging (florbetapir PET standard uptake value ratio > 1.1; Clark et al., Reference Clark, Schneider, Bedell, Beach, Bilker, Minimi and Skovronsky2011) or CSF assays (CSF total tau to Aβ-42 ratio > 1; Andreasen et al., Reference Andreasen, Minthon, Pia, Vanmechelen, Vanderstichele, Winblad and Blennow2001; Molinuevo et al., Reference Molinuevo, Blennow, Dubois, Engelborghs, Lewczuk, Perret-Liaudet and Parnetti2014). CSF assay was run in Sweden by Dr. Kai Blennow’s lab. This CSF cut-off (i.e., CSF total tau to Aβ-42 ratio > 1) was determined by the standard used by his lab. Patients were classified as AD positive if they met biomarker status based either on AβPET imaging or CSF assays. Of the full sample, 44 patients had biomarker data that passed quality control (CSF only, n = 27; PET only, n = 4; both CSF and PET, n = 13). Four patients had AD-positive PET imaging but AD-negative CSF ratios. In total, 14 of the 44 (31.8%) patients had positive AD biomarkers. Moving forward, these groups will be referred to as iNPH (meaning those without AD pathology) and iNPH + AD (meaning those with AD pathology). Demographics of this subsample of patients as a function of AD biomarker status are displayed in Table 1. Groups did not significantly differ in terms of age, education, or gender (all p values > .068).
Table 1. Demographics as a function of AD biomarker status

Note. Age, age in years; SD, standard deviation; Education, educational attainment in years; iNPH, idiopathic normal pressure hydrocephalus; AD, Alzheimer’s disease; M, male; F, female.
Behavioral Ratings
Caregivers completed both “before” and “current” behavior ratings for the FrSBe. Informants were asked to estimate when they first noted symptoms that were likely consistent with iNPH. “Before” ratings assessed a patient’s behavior prior to the onset of these first suspected iNPH symptoms. “Current” behavior ratings assessed a patient’s behavior within the past 2 weeks preceding the assessment. FrSBe scores were adjusted for age, gender, and education and converted to standardized T-scores according to the test manual (Malloy & Grace, Reference Malloy and Grace2001). The rationale for focusing on informant ratings was due to concerns of limited insight from patients and to reduce the probability of fatigue effects on cognitive testing. As suggested in the FrSBe manual (Malloy & Grace, Reference Malloy and Grace2001), T-scores equal to or greater than one and a half SDs above the mean (i.e., T ≥ 65) were deemed clinically significant.
To better characterize the sample, informants also estimated the date when the first iNPH symptom was observed in the patient (which was then converted to the number of months prior to the clinic visit) and completed a brief screen for depression using the following question from the Neuropsychiatric Inventory–Questionnaire (NPI-Q; Cummings et al., Reference Cummings, Ketchel, Ed, Shelley, Lopez and Dekosky2000): “Does the patient seem sad or say that he /she is depressed?” When time allowed, caregivers also completed the Zarit Burden Interview (ZBI; Zarit, Orr, & Zarit, Reference Zarit, Orr and Zarit1985) to assess caregiver burden.
Cognitive Tests
Patients also completed a series of cognitive tasks. Global cognition was evaluated using the MMSE (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975). Psychomotor speed was evaluated using the Trail Making Test Part A completion time. EF was evaluated using the Trail Making Test Part B completion time. Completion time scores for Trail Making Test Parts A and B were adjusted for age and education and converted to standardized z-scores using meta-analytic norms (Mitrushina, Boone, Razani, & D’Elia, Reference Mitrushina, Boone, Razani and D’Elia2005). Patients who discontinued Trails B (n = 21) were given a z-score of −3 to indicate severe impairment at the normative floor. Of the total sample of 50 patients, 4 either refused or were unable to complete all cognitive tasks due to physical limitation, resulting in a subsample of 46 in analyses involving cognitive measures.
Statistical Design
For Aim 1, chi-square McNemar tests were performed to examine differences in the number of clinically significant “before” and “current” FrSBe scores for apathy, executive dysfunction, and disinhibition scales. Similarly, paired samples t-tests were used to compare mean differences across the three scales for “before” and “current” informant ratings. Zero-order correlations were performed to examine the relationship between FrSBe scores for apathy, executive dysfunction, and disinhibition scales and cognitive measures. For Aim 2, to examine differences between iNPH and iNPH + AD groups on FrSBe scores, an analysis of variance (ANOVA) was conducted by examining the interaction between the factors of time (“before” vs. “current” informant rating) by group (AD biomarker status). A separate ANOVA was conducted for each of the three FrSBe scales. Given that the second aim of our study was exploratory, we used the Benjamini–Hochberg method (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) with the false discovery rate (i.e., Q) set to 0.10 to correct for multiple comparisons for these three analyses.
RESULTS
Characterizing the Sample
The estimated time of onset of the first iNPH symptom in months prior to the study visit was available for 47 patients (M = 52.23, SD = 50.80). Informant ratings of the presence of depression using the NPI-Q were available for 45 patients and indicated concern for depression in 46.66% of those individuals. A chi-square test of independence showed that clinically significant ratings of post-illness apathy on the FrSBe (i.e., T ≥ 65) were not significantly associated with ratings of the presence of depression on this screening measure (p = .356), suggesting that informants were able to distinguish between these two constructs.
Presence of the Three Frontal Behavior Syndromes in the Complete iNPH Sample
“Before” Behavior Ratings
Mean informant ratings of patient behaviors prior to the onset of iNPH symptoms were all within normal limits (apathy: M = 52.12, SD = 18.54; executive dysfunction: M = 52.56, SD = 13.50; disinhibition: M = 46.14, SD = 9.34). Eight patients (16%) had a clinically significant rating on the apathy scale, seven patients (14%) had a clinically significant rating on the executive dysfunction scale, and only one participant (2%) was above the cut-off on the disinhibition scale.
“Current” Behavior Ratings
Mean informant ratings of patient behavior post-onset of iNPH were clinically elevated for the apathy (M = 72.44, SD = 20.87) and the executive dysfunction scale (M = 69.90, SD = 23.41). In contrast, the disinhibition scale mean remained within normal limits (M = 55.28, SD = 15.51). Thirty-one patients (62%) had a clinically significant rating on the apathy scale, and 28 patients (56%) had a clinically significant rating on the executive dysfunction scale. Fifteen patients (30%) were above the cut-off on the disinhibition scale.
Chi-square McNemar tests revealed that the number of patients that converted to clinically significant T-scores (T-score ≥ 65) when comparing current informant ratings to pre-illness informant ratings was statistically significant for all three of the behavior ratings scales (p values < .001). Similarly, paired samples t-tests comparing average “before” to “current” ratings were also statistically significant across all three behavior scales (t > 5.56, p values < .001). Large effect sizes were detected for changes in apathy (d = 0.94) and executive dysfunction (d = 0.91) ratings, and a medium effect size was detected for changes in disinhibition ratings (d = 0.78)Footnote 2. Pre- and post-illness informant ratings across the three scales are illustrated in Figure 1.

Fig. 1. The mean T-scores for the apathy, executive dysfunction, and disinhibition subscales across all iNPH patients (N = 50). Error bars represent the standard error of the mean. Higher scores indicate more severe frontal behavior symptoms. A statistically significant increase was seen across the three scales when comparing pre-illness (“before”) to post-illness (“current”) ratings. As can be seen, scores were highest for the apathy subscale, followed by executive dysfunction then disinhibited behaviors. * p < .05.
Relationships with Caregiver Burden
Self-reported caregiver burden using the ZBI was available from a subset of patient’s caregivers (n = 36). In this subset of the sample, greater burden was significantly correlated with more severe ratings of frontal behavior symptoms across all three post-illness FrSBe scales (all r values > .534, all p values < .002). Greater caregiver burden was also associated with more severe pre-illness executive dysfunction ratings on the FrSBe (r = .438, p = .007). The other two pre-illness FrSBe scales were not significantly associated with the ZBI (both r values < .213, both p values > .214).
Relationships with Objective Cognitive Test Performance
In addition to the behavioral measure (FrSBe), objective cognitive test data were available for a subset of patients (n = 46). Zero-order correlations were examined between scores on each of the three “current” FrSBe subscales and measures of global cognition (MMSE), psychomotor speed (age- and education-corrected Trails A completion times), and EF (age- and education-corrected Trails B completion times). Relationships between cognitive test scores and the three “current” FrSBe scales are presented in Table 2. As shown, no statistically significant associations were present among MMSE scores and any of the three frontal systems scales; similarly, no statistically significant relationships were observed between the disinhibition scale and any of the cognitive measures. Associations between Trails A and B scores and the apathy ratings as well as Trails A and executive dysfunction ratings reached statistical significance in the expected direction. The association between Trails B and executive dysfunction approached, but did not reach, statistical significance (p = .051). Partial correlations between Trails B and the apathy and executive dysfunction ratings controlling for Trails A performance were examined to isolate the executive aspect of this task from lower-order component processes that are necessary, but not sufficient, for performance on cognitive tests of EF. Neither of these partial correlations were significant (absolute value of both r values < −.210, both p values > .167).
Table 2. Zero-order correlations between cognitive test scores and “current” informant ratings of frontal behavior syndromes

Note. n = 46. Higher scores indicate better cognitive performance and more severe frontal behavior symptoms.
MMSE, Mini-Mental State Examination raw scores; AD, Alzheimer’s disease; Trails A, age- and education-corrected z-scores on Trails A; Trails B, age- and education-corrected z-scores on Trails B.
* p < .05. ** p < .001
Rates of Behavior Ratings across AD Biomarker Status
AD biomarker data were available for a subset of patients (n = 44). Mixed-model ANOVAs (Diagnostic group × Pre/post-behavior ratings) were conducted to examine whether there were significant interactions between group status (iNPH vs. iNPH + AD) and informant rated changes (“before” vs. “current” informant ratings) on the three FrSBe subscales. The results showed a statistically significant interaction only for executive dysfunction rating [F(1, 42) =6.07, p = .018], which remained statistically significant when controlling for multiple comparisons. Changes in apathy and disinhibition ratings from “before” to “current” did not significantly differ as a function of AD biomarker status (both interaction term p values > .202). For the interaction terms, small effect sizes were observed for the apathy (η p2 = .004) and disinhibition (η p2 = .038) scales, whereas a medium effect size was observed for the executive dysfunction scale (η p2 = .126)Footnote 3. Figure 2 illustrates the results of these analyses.

Fig. 2. The change in informant ratings from pre-illness (“before”) to post-illness (“current”) time points as a function of iNPH and iNPH + AD groups for each of the three FrSBe scales (n = 44). Error bars represent the standard error of the mean; higher scores indicate more severe frontal behavior symptoms. * p < .05.
The estimated time of onset of iNPH symptoms (number of months prior to the clinic visit) did not significantly vary between the iNPH and iNPH + AD groups [n = 43 (iNPH: n = 29, M = 49.59; iNPH + AD: n = 14, M = 53.79), t (41) = −0.246, p = .807]. Cognitive data were available for 40 patients. Table 3 shows the cognitive test data (MMSE and Trails A and B) as function of AD biomarker status. As can be seen, groups did not significantly differ across any of these three measures. Table 3 also shows the means for each of the three “current” frontal behavior syndrome ratings to facilitate comparison of the cognitive and behavioral data.
Table 3. Cognitive and FrSBe behavioral data as a function of AD biomarker status
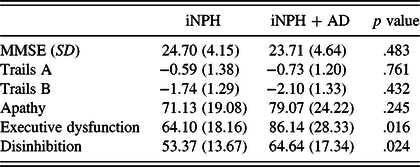
Note. n = 44 for MMSE and the three Frontal Systems Behavior Scales (apathy, executive dysfunction, and disinhibition); n = 40 for Trails A and B. SD, standard deviation; MMSE, Mini-Mental State Examination raw scores; Trails A, age- and education-corrected z-scores on Trails A; Trails B, age- and education-corrected z-scores on Trails B; Apathy, age-, education-, and gender-corrected T-scores for the “current” apathy informant ratings on the Frontal Systems Behavior Scale; Executive dysfunction, age-, education-, and gender-corrected T-scores for the “current” executive dysfunction informant ratings on the Frontal Systems Behavior Scale; Disinhibition, age-, education-, and gender-corrected T-scores for the “current” disinhibition informant ratings on the Frontal Systems Behavior Scale; higher scores indicate better cognitive performance and more severe frontal behavior symptoms.
For the subjects where both AD biomarker data and ZBI caregiver burden ratings were available (iNPH: n = 20, iNPH + AD: n = 11), the mean burden rating was higher for the iNPH + AD group but the difference in means was not statistically significant across the groups (iNPH: M = 19.10, iNPH + AD: M = 29.45; t(29) = −1.808, p = .081).
DISCUSSION
This study aimed to replicate and advance findings involving the presence neuropsychiatric/behavioral changes in patients with iNPH through novel use of the FrSBe. As a secondary aim, the study examined whether neuropsychiatric/behavioral changes are exacerbated by the presence of AD biomarkers. The results were largely supportive of our a priori hypotheses. While informant ratings on all three frontal behavior scales increased from pre- to post-illness, post-illness ratings were in the clinically significant range on average for apathy and executive dysfunction, but not disinhibition. Apathy scores were highest, on average, among the three scales (see Figure 1). FrSBe subscales showed modest correlations with the cognitive measures examined in this study, indicating that the behaviors captured by the FrSBe may be distinct from deficits detected using these commonly used cognitive measures (MMSE and the Trail Making Test). Finally, the iNPH + AD group evidenced elevated informant ratings of executive dysfunction compared to the iNPH group, but no differences were observed for apathy or disinhibition behavior ratings.
When interpreting the current results, it is important to highlight that the current project relied on informant ratings of frontal behavior change. Caregiver ratings have been shown to be more discrepant from patient and clinician ratings of neuropsychiatric symptoms, including apathy, for caregivers who themselves have depression and/or elevated caregiver burden (Gomez-Gallego, Gomez-Garcia, & Ato-Lozano, Reference Gomez-Gallego, Gomez-Garcia and Ato-Lozano2015; Pfeifer et al., Reference Pfeifer, Drobetz, Fankhauser, Mortby, Maercker and Forstmeier2015; Schulz et al., Reference Schulz, Cook, Beach and Lingler2013). Indeed, in the current sample, greater burden was significantly correlated with more severe ratings of frontal behavior symptoms across all three post-illness FrSBe scales as well as with more severe pre-illness executive dysfunction ratings. However, caregiver burden did not significantly differ across the iNPH and iNPH + AD groups, while post-illness dysexecutive FrSBe ratings were higher in the iNPH + AD group. Taken together, this pattern of findings indicates that the increase in informant ratings may be capturing both true changes in patients’ behavior as well as the perception of behavior changes attributable to caregiver characteristics, such as increased caregiver burden due to other patient symptoms (e.g., gait disturbance). It is important to highlight that these two possibilities (i.e., true behavior change and caregiver perception) are not mutually exclusive and are likely both contributing to the FrSBe informant ratings in this study. Therefore, the discussion of the findings in the following should only be interpreted as the caregiver experience of frontal behavior change in the context of iNPH.
Frontal Behavior Systems and iNPH
Regarding iNPH and neuropsychiatric function, these results replicate and advance two specific aspects of the current literature. First, increased apathy has been well documented in research examining neuropsychiatric changes associated with iNPH (Allali et al., Reference Allali, Laidet, Armand, Saj, Krack and Assal2018; Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009; Peterson et al., Reference Peterson, Housden, Killikelly, Devito, Keong, Savulich and Sahakian2016). The current results provide additional evidence for apathy as a primary and prominent neuropsychiatric symptom in iNPH by comparing informant ratings of apathy to other frontal behavior syndromes. In addition, our findings support the hypothesis that elevated dysexecutive behaviors, but not disinhibition, would also be reported on the FrSBe, given the proximity of the dorsolateral prefrontal subcortical circuit to the lateral ventricles relative to orbitofrontal circuitry (Rigamonti, Reference Rigamonti2014; Tekin & Cummings, Reference Tekin and Cummings2002).
The results were largely consistent with this hypothesis as evidenced by informants’ mean endorsement of clinically significant levels of apathy and dysexecutive behaviors, but not disinhibited behaviors (see Figure 1). Of note, analyses of pre- and post-illness informant ratings revealed statistically significant changes across all three scales both in terms of mean ratings and the proportion of patients converting to clinically significant levels. Although the increase in disinhibition was unexpected, the magnitude of the change aligned with expectations such that larger effect sizes were observed for the apathy and executive dysfunction scales relative to the disinhibition scale. Taken together, our results advance the current conceptualization of the iNPH neuropsychiatric profile to include not only apathy, but also executive dysfunction in behavior.
The current findings also align with past literature showing that executive dysfunction is often a prominent feature of the cognitive profile of iNPH (Katzen et al., Reference Katzen, Ravdin, Assuras, Heros, Kaplitt, Schwartz and Relkin2011; Ogino et al., Reference Ogino, Kazui, Miyoshi, Hashimoto, Ohkawa, Tokunaga and Takeda2006) in that the means for Trails B performance for both iNPH and iNPH + AD groups were both more than one and a half SDs below the normative mean (see Table 3). However, the current results also demonstrate the importance of controlling for lower-order component processes when examining relationships between cognitive performance on EF tests and outcomes of interest for individuals with iNPH. Specifically, in this sample, relationships between Trails B and informant ratings of frontal behavior changes were no longer statistically significant when controlling for Trails A indicating that informant ratings were associated with cognitive deficits in processing speed and motor difficulties rather than a pure EF deficit. Future research will benefit from careful consideration of lower-order component processes when examining performance on tests of EF in iNPH.
Specific mechanisms underlying disruptions in frontal systems behaviors in iNPH may include hypoperfusion, white matter tract pathology, and neurotransmitter disruption. Hypoperfusion has been observed in the frontal lobes (for reviews, see Klinge et al., Reference Klinge, Brooks, Samii, Weckesser, van den Hoff, Fricke and Berding2008; Owler & Pickard, Reference Owler and Pickard2001). Regarding white matter tracts, increased mean diffusivity has been documented in the anterior thalamic radiation and inferior fronto-occipital/uncinate tracts (Keong et al., Reference Keong, Pena, Price, Czosnyka, Czosnyka, Devito and Pickard2017). Dopaminergic abnormalities have also been evidenced in asymmetric changes in binding potential of pre-synaptic and post-synaptic radiotracers in the dorsal putamen (Ouchi et al., Reference Ouchi, Nakayama, Kanno, Yoshikawa, Shinke and Torizuka2007). Moreover, neuroanatomical changes in the caudate have been implicated (DeVito, Salmond, Owler, Sahakian, & Pickard, Reference DeVito, Salmond, Owler, Sahakian and Pickard2007; Peterson et al., Reference Peterson, Housden, Killikelly, Devito, Keong, Savulich and Sahakian2016), and reduced caudate volume has been associated with higher levels of reported apathy (Peterson et al., Reference Peterson, Housden, Killikelly, Devito, Keong, Savulich and Sahakian2016).
To date, measures such as the Apathy Evaluation Scale (Peterson et al., Reference Peterson, Housden, Killikelly, Devito, Keong, Savulich and Sahakian2016), Starkstein Apathy Scale (Allali et al., Reference Allali, Laidet, Armand, Saj, Krack and Assal2017), and NPI (Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009) have been used to measure neuropsychiatric symptoms in iNPH. The use of the FrSBe in this study allowed for a more extensive survey of neuropsychiatric symptoms affected in iNPH that included, but was not limited to, apathy. Moreover, the modest and often non-significant correlations with commonly used measures of processing speed and executive abilities (Trail Making Test Parts A and B) could indicate that frontal behavior changes are not fully characterized by this cognitive measure. EF is a highly complex construct that cannot be captured by performance on a single cognitive measure, such as the Trail Making Test (Stuss, Reference Stuss2011; Suchy, Reference Suchy2016). Trail Making Test is commonly considered to be a measure of set-shifting/mental flexibility and does not capture the same aspects of the EF construct as other measures, such as Color–Word Interference Tests or card-sorting measures (Suchy, Reference Suchy2016). It is possible that a more comprehensive cognitive assessment of the various aspects of EF would have revealed stronger associations between FrSBe subscales and cognitive test performance. Nonetheless, past literature has shown that behavioral ratings of frontal behavior symptoms have shown incremental utility beyond cognitive measures in their ability to predict functional outcomes in MCI and dementia populations (Norton et al., Reference Norton, Malloy and Salloway2001; Puente et al., Reference Puente, Cohen, Aita and Brandt2016). Taken together with that past work, the current findings indicate that future research aimed at understanding both cognitive and behavioral manifestations of frontal pathology in iNPH patients is warranted.
Notably, informants endorsed sadness and/or depression in almost half of the current patient sample, and endorsement of concern for depression was unrelated to the presence of clinically significant post-illness apathy ratings. While this finding is limited by the use of a single item screener, it nonetheless aligns with recent acknowledgements suggesting that depression is not only distinct from apathy, but also commonly comorbid in iNPH (Israelsson et al., Reference Israelsson, Allard, Eklund and Malm2016).
The Role of AD Pathology in Neuropsychiatric Symptoms
The exploratory analysis revealed that the presence of AD biomarkers was not associated with group differences in the reported increase in post-illness apathy; however, the iNPH + AD group had a greater increase in post-illness executive dysfunction informant ratings relative to the iNPH group. This finding aligns with results from a prior study that found that patients with suspected AD demonstrate more behavioral disturbance than individuals with suspected iNPH (Kito et al., Reference Kito, Kazui, Kubo, Yoshida, Takaya, Wada and Takeda2009).
The presence of more severe behavioral executive dysfunction among iNPH patients with positive AD biomarkers is likely due to complex or multiple pathologies affecting executive dysfunction among this patient group. Indeed, the presence of executive dysfunction in all stages of AD has been increasingly appreciated in the past 20 years (Allain, Etcharry-Bouyx, & Verny Reference Allain, Etcharry-Bouyx and Verny2013), including the presence of increased dysexecutive syndrome behaviors early in the disease course (Stout, Wyman, Johnson, Peavy, & Salmon, Reference Stout, Wyman, Johnson, Peavy and Salmon2003). Patients with positive AD biomarkers likely have more types of neuropathology affecting their dorsolateral prefrontal subcortical circuits compared to patients with only iNPH in isolation, which in turn may result in more severe behavioral manifestations of EF deficits. Importantly, the iNPH and iNPH + AD groups did not significantly differ in terms of time of onset of initial iNPH symptoms or MMSE scores during the study visit. This suggests that disease duration or overall dementia severity is unlikely to account for the current findings regarding more pronounced post-illness behavioral executive dysfunction in the iNPH + AD group.
The observed difference in the behavioral presentation across iNPH patients with and without comorbid AD likely has clinical relevance. While future research is needed, the current findings suggest that obtaining AD biomarker status at baseline may be useful to neurologists and neurosurgeons in counseling patients about how frontal behavior symptoms fit into the diagnostic picture. AD biomarker information may also be useful in assisting patients and families in decision-making about treatment options and expectations about treatment response. Research has suggested that the absence of AD biomarkers is associated with a larger reduction in neuropsychiatric symptoms and decreased caregiver burden following shunt intervention (Kazui et al., Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato and Tanaka2016). In addition, increased behavioral disturbance as measured by total scores on the FrSBe is associated with increased caregiver burden beyond dementia severity for patients with dementia due to suspected AD (Rymer et al., Reference Rymer, Salloway, Norton, Malloy, Correia and Monast2002). Taken together, the current results suggest that caregiver burden post-intervention may depend on the degree of frontal behavior changes not only for apathy, but also for executive dysfunction. Further research is warranted to investigate whether the presence of AD pathology alters the response of frontal behavioral syndromes in iNPH to surgical intervention and resultant caregiver burden.
Limitations and Future Directions
This study was subject to limitations, including a modest sample size of 50 which resulted in reduced statistical power restricting the range of potential analyses. In addition, replication in new samples is warranted, especially given the fact that the analyses examining pre- to post-illness changes among the FrSBe scales as a function of iNPH versus iNPH + AD groups were exploratory. In addition, the absence of a demographically matched healthy control group also represents a limitation inherent to the analysis of this dataset. This is somewhat offset by the transformation of raw scores to standardized scores based on normative data derived from samples of healthy individuals. However, given the large number of patients (46%) that obtained scores at the normative floor on the cognitive measure of EF (Trails B), the strength of the associations with FrSBe subscales was likely reduced due to the restriction of range.
In addition, CSF biomarkers of AD pathology (e.g., Aβ-42 and tau levels) may be misleading in iNPH due to alterations in amyloid precursor protein fragment drainage into the CSF resulting in reduced levels of all proteins (Graff-Radford, Reference Graff-Radford2014). In the current sample, 61% of patients only had CSF biomarker data available, which may have obscured the determination of comorbid AD in this sample. However, the use of total tau to Aβ-42 ratio versus just Aβ-42 or tau levels in isolation guarded against this possibility to an extent.
As discussed earlier, the sole reliance on informant ratings of frontal behavior change also represents a limitation for this study. The use of clinician ratings to characterize the presence of frontal behavior changes and inclusion of more diverse cognitive measures of EF will be important in future designs to help separate out to what extent increased caregiver burden or depression may have influenced the frontal behavior ratings in this study. In addition, the retrospective nature of the current design also renders the pre-illness ratings subject to variability in caregiver memory and influence of expectations for disease progression. The use of informant ratings to characterize time of initial iNPH symptom onset is also vulnerable to similar biases. Future studies will benefit from the use of prospective designs. This study also did not characterize the current sample in terms of ethnicity or the presence of neurodevelopmental conditions, such as learning disabilities. This represents a limitation given these factors potential influence on cognitive test data and behavioral ratings. Future research will benefit from better characterization of additional factors that may affect the cognitive and behavioral presentation of iNPH.
The prominent neuropsychiatric symptoms in iNPH can be quantified by behavioral ratings of apathy and executive dysfunction, which help clinicians to counsel patients and their family and to track disease progression, complementing other neurodiagnostic and cognitive tests. Moreover, given the FrSBe’s inclusion of pre- and post-scales measuring frontal systems behavior, it offers a useful method to assess the response of neuropsychiatric symptoms to neurosurgical intervention.
Future research should include a healthy control group, a larger sample size, and a variety of cognitive and behavioral measures (e.g., clinician ratings and additional objective cognitive measures of EF) to enhance the generalizability of the present results. Furthermore, given the relatively nascent state of research surrounding the role of comorbid AD pathology regarding how neuropsychiatric features respond to iNPH intervention, additional research is warranted to better understand this interaction. There is considerable variability in the type and severity of cognitive domains affected in iNPH, which is thought to be explained in part by differences in age and disease duration (Picascia et al., Reference Picascia, Minafra, Zangaglia, Gracardi, Pozzi, Sinforiani and Pacchetti2016). The current results suggest that comorbid AD may also contribute to this variability, specifically in the behavioral manifestations of cognitive dysfunction. Uncertainty remains regarding whether comorbid AD or other comorbid conditions could help to explain additional variability in the severity and diversity of other cognitive impairment seen in iNPH and any differences in response to intervention. Future studies would benefit from having PET imaging in addition to CSF assays to examine the predictive value of comorbid AD pathology to baseline behavioral presentation and shunt response in this CSF disorder.
ACKNOWLEDGMENTS
The authors would like to thank Kaj Blennow M.D., Ph.D, University of Gothenburg, for providing the CSF analysis and also thank all patients and their families for participating in this study. This work was supported by internal funding from the Butler Hospital.
Athene Lee is partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors have nothing to disclose.







