Atrial septal defect is a central issue in the pathophysiology of hypoplastic left heart syndrome. At the worst end of the spectrum, with virtual or absent left ventricle, an unrestrictive atrial septal defect is crucial to allow pulmonary venous blood flow to reach the systemic right ventricle.Reference Feinstein, Benson and Dubin 1 Otherwise, when the left ventricle is borderline, yet with preserved inflow and outflow, a restrictive defect has been claimed as a trigger for left ventricular development, by virtue of its augmented preload.Reference Emani and del Nido 2 , Reference Hickey, Caldarone and McCrindle 3
A total of five different morphologies of atrial septal defect have been described in hypoplastic left heart syndrome. The most common type is the ostium secundum (1), accounting for about 50% of cases;Reference Chin, Weinberg and Barber 4 however, the variant with leftward anterior deviation of the septum primum (2) seems to be equally common (Fig 1), at least at the severe end of the spectrum.Reference Park, Fedderly and Frommelt 5 The ostium primum (3) is associated with unbalanced right-dominant atrio-ventricular septal defect. Less frequently, congenitally small or absent atrial septal defect (4) and aneurysm of septum primum (5) have been reported.

Figure 1 Malalignement of the septum primum. The septum primum is indcated using red arrowheads in ( a ) and its curved shape is determined by the leftward deviation of its superior attachment. A subcostal view of the heart is represented in ( b ), the leftward deviation of septum primum is present along with mitral atresia and severely hypoplastic left ventricle. The atrial septal defect appears to be “non-restrictive”, either on two-dimensional or on color-flow Doppler view.
A restrictive atrial septal defect in hypoplastic left heart syndrome may lead to severe hypoxaemia along with elevated pulmonary venous pressure, unless a decompression pathway from the left atrium is present.Reference Hoque, Richmond, Vincent, Bacha and Torres 6 For these reasons, restrictive defect has been identified as a significant risk factor affecting survival, despite aggressive treatment.Reference Rychik, Rome, Collins, DeCampli and Spray 7 Restrictive atrial septal defect physiology occurs when the defect is critically small or absent, and sometimes in the case of malaligned, deviated septum primum, as pulmonary venous return lacks a straightforward pathway to the right atrium;Reference Chin, Weinberg and Barber 4 however, even when ostium secundum defect seems to be adequate at birth, it may become restrictive over time.
Hybrid stage-I palliation with pulmonary artery branch banding and transcatheter ductal stenting has modified the approach to atrial communication, compared with classical Norwood stage-I operation, either in terms of timing of the intervention or of procedural aspects. In fact, although atrioseptectomy is always part of the Norwood operation, the atrial septum is not necessarily addressed at the time of hybrid palliation, unless restriction occurs at birth.
The aims of our study were to describe atrial septal defect morphology in our patients with hypoplastic left heart syndrome, to report the incidence of restrictiveness and its relationship with morphology, and to correlate restriction to midterm survival. In addition, we focused on the interventional approach we performed to deal with restrictive defect.
Methods
We retrospectively reviewed our database at Pediatric Cardiology Center, Bambino Gesù Children’s Hospital in Taormina, searching for clinical and echocardiographic characteristics of the atrial septal defect in patients with hypoplastic left heart syndrome. Research was carried out in compliance with the Helsinki Declaration and the Ethical Committee of Messina University gave formal approval (Protocol No. 0008421). Between October 2011 and February 2015, 31 consecutive neonates of whom 19 were male, with a mean weight of 2.99 kg, were diagnosed with hypoplastic left heart syndrome. Thirty patients underwent hybrid stage-I palliation, consisting of surgical pulmonary artery branch banding and transcatheter stenting of the arterial duct, without cardiopulmonary bypass. Only one patient was designated for Norwood operation because of the diameter of the arterial duct that was unsuitable for stenting. Patients’ mean age at the time of operation was 3.6 days (range 1–7 days).
All patients underwent a thorough echocardiographic study immediately after birth, aimed to define cardiac anatomy and flow dynamics. Informed consent was obtained for each invasive procedure and for data collection, according to our Institution’s policy.
Overall, four variants of hypoplastic left heart syndrome were present in our population: mitral atresia/aortic atresia, mitral atresia/aortic stenosis, mitral stenosis/aortic atresia, and mitral stenosis/aortic stenosis.Reference Tchervenkov, Jacobs and Weinberg 8 Moreover, we considered right-dominant atrio-ventricular septal defect as a part of the spectrum of hypoplastic left heart syndrome-like forms.
We classified defect morphology in “standard” – that is, ostium secundum – and “complex”, which included malalignement defect, critically small foramen ovale, and aneurysmal defect. Ostium primum was observed exclusively in atrio-ventricular septal defects. Restrictive atrial septal defect physiology was diagnosed on the basis of echocardiography (Fig 2), when continuous Doppler interrogation through the defect recorded a mean gradient higher than 6 mmHg over three cardiac cycles, and on clinical signs of increased pulmonary artery pressure, such as transcutaneous oxygen saturation lower than 80% or failure to thrive.Reference Holzer, Wood and Chisolm 9 Subsequently, the diagnosis was confirmed by invasive measurements of left and right atrial pressure. Moreover, we distinguished between patients presenting early with restrictive defect – that is, within 1 week – patients with late occurrence, and patients without restrictive defect. We reported the prevalence of each morphological variant of the atrial septum in patients with and those without restriction, aiming to investigate whether standard or complex morphologies can be associated with restrictiveness.

Figure 2 Restrictive atrial septal defect (ASD). A very small ASD, indicated using a blue arrow, is present in this subcostal view of the heart ( a ). Color-flow Doppler in ( b ) shows high flow velocity of the left-to-right shunt. Continuous-wave Doppler through the ASD is showed in ( c ); the mean gradient is ~12 mmHg.
All patients diagnosed with restrictive defect underwent an interventional procedure to relieve obstruction. Surgery was performed in case of unsuccessful interventional treatment. Timing of the interventional procedure was distinguished as “early”, when required along with hybrid stage-I palliation within the 1st week of life, or “late”, if performed thereafter.
The interventional technique and materials have been described. The procedure was performed under general anaesthesia and through the femoral vein.
After access to the left atrium has been obtained, a preformed wire was positioned in the left upper pulmonary vein or looping in the left atrial cavity, drawing the atrial septum. A 6- or 7-Fr long sheath (Cook Inc., Bloomington, Indiana, United States of America) was then advanced over the wire with the tip across the atrial septum. Palmaz-Genesis premounted stents (Cordis, Cashel, Co. Tipperary, Ireland) were utilized (Table 1). The length and diameter of the stent was selected based on the patient age and size and on the left atrial size, with the aim to provide an unrestrictive and potentially durable flow through the atrial septum for some months. The stent was then advanced through the long sheath into the left atrium. Half of the stent was exposed by pulling back the sheath and the balloon was inflated in the left atrium, expanding the distal half of the stent. Subsequently, the long sheath and the partially expanded stent were pulled back against the left face of atrial septum. The right atrial portion of the stent was unsheathed and the balloon was gently inflated, expanding the proximal portion of the stent until a “diabolo shape” or “hourglass shape” was obtained (Fig 3 and Supplementary Fig 1). The thick atrial septum of mitral atresia or hypoplasia contributed to constrict the central part of the stent with respect to its extremities. The balloon was deflated and carefully removed out of the stent and into the long sheath, avoiding stent dislodgment. The guidewire was withdrawn using a catheter.

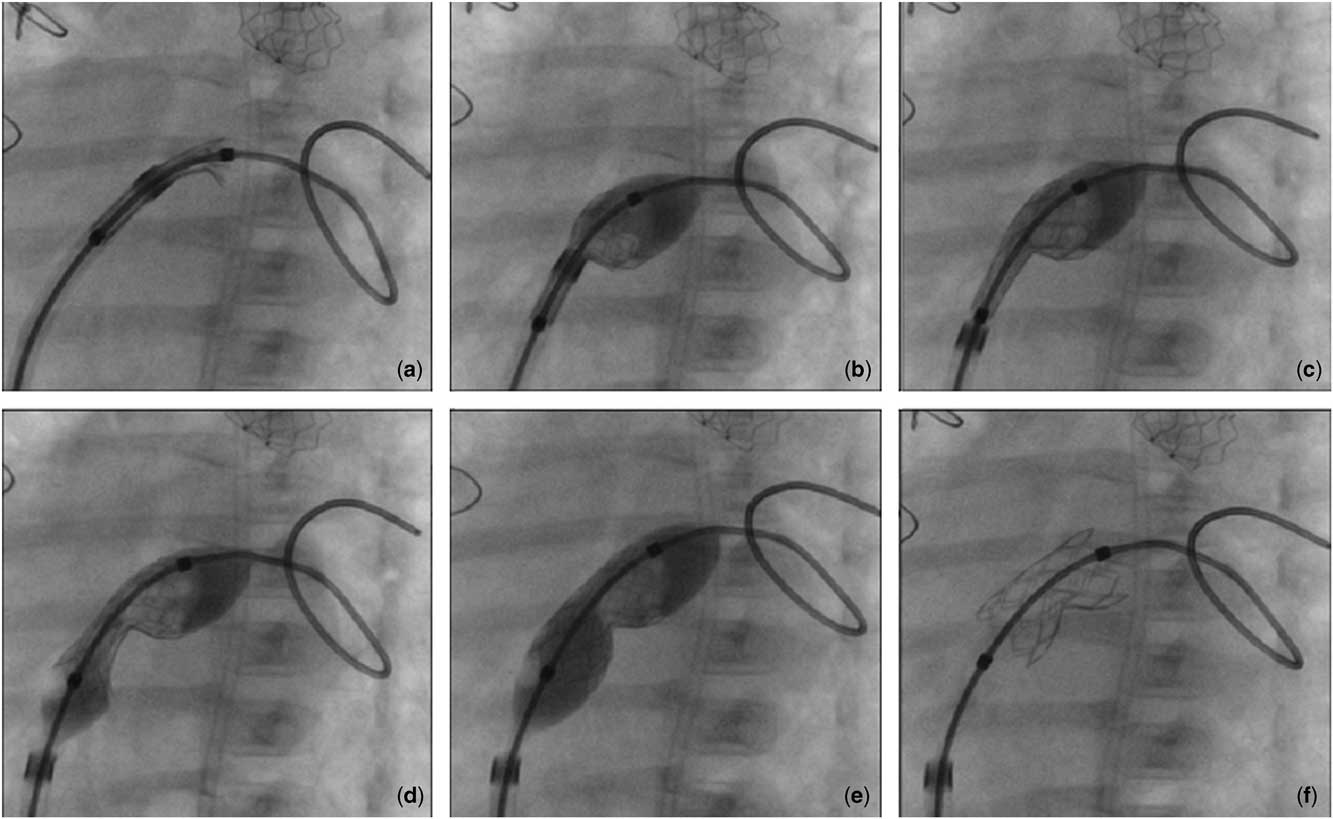
Figure 3 Transcatheter stenting of atrial septal defect (ASD). Left oblique fluoroscopic view. In ( a ) only the distal edge of the stent protrudes outside the long sheath; first, the distal half of the stent is exposed and inflated on the left side of ASD ( b ), then the long sheath is withdrawn ( c ), and the stent is delivered completely on the right side of the ASD ( d ). The whole balloon is inflated and the stent expanded, assuming a pronounced central waist with a peculiar hourglass-shape ( e ); the “shoulders” of the stent can be observed in ( f ).
Table 1 Characteristics of patients with restrictive atrial septal defect (ASD).

AA=aortic atresia; AS=aortic stenosis; MA=mitral atresia; MS=mitral stenosis; M – ASD=malalignement atrial septal defect; OS – ASD=ostium secundum atrial septal defect
Anti-aggregation treatment with acetylsalicylic acid at a dose of 2–5 mg/kg/day was started to prevent thrombus formation.
Interventional procedure was considered successful if transatrial gradient decreased below 6 mmHg and if no major adverse event occurred. Periprocedural mortality was defined as all-cause death occurring within 30 days after the interventional procedure. Midterm outcome was evaluated at three time points: survival at 2 months after the hybrid procedure, survival to surgical stage II and 12-month survival. The reason to consider a 2-month interval is that signs and symptoms of atrial septal defect restriction have been usually observed after such a mean time interval. We also aimed to test whether any association between restrictive defect and survival was present in our population.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 17.0 (Chicago, Illinois, United States of America). Comparison between two groups, with reference to continuous variables, were performed using the Mann–Whitney test conditioned on “restriction” event; categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. To assess the contribution of each variable on the study variables, we performed a multivariate stepwise linear regression to consider together continuous and categorical variables; Binary Logistic Regression model was estimated to assess the dependence of the binary variable “restriction” from defect morphology: standard versus complex. A two-tailed α of 0.05 was used to denote statistical significance.
Results
The most common variants of hypoplastic left heart syndrome were mitral atresia/aortic atresia (10 patients) and mitral stenosis/aortic stenosis (13 patients), whereas mitral atresia/aortic stenosis (one patient) and mitral stenosis/aortic atresia (four patients) were less frequent. In all, three patients had right-dominant atrio-ventricular septal defect, but, as ostium primum is not subjected to restriction, they were excluded from our analysis. For the same reason the patient who underwent Norwood operation was excluded.
Out of 27 patients, 11 (40%) fulfilled echocardiographic and/or clinical criteria for restrictive atrial septal defect, of whom three had mitral atresia/aortic atresia, three had mitral stenosis/aortic atresia, one had mitral atresia/aortic stenosis, and four had mitral stenosis/aortic stenosis – and their characteristics are listed in Table 1.
Defect morphology was classified as follows: the restrictive group showed three “standard” ostium secundum (27%) and eight “complex” morphologies, of which five were malalignement defect cases (46%), two were critically small foramen ovale cases (18%), and one was a case of aneurysmal defect (9%), whereas in the non-restrictive group (16 patients) we observed 11 ostium secundum (69%) and five complex morphologies, of which four were cases of malalignement (25%) and one was a case of aneurysmal defect (6%). A significant association was noted between complex morphologies and restriction (odds ratio 5.87, confidence interval (CI) 1.075–32.002; p=0.034).
We observed a tendency for a higher prevalence of restrictive defect in variants with mitral atresia, aortic atresia, or both (Fig 3), although the association did not reach statistical significance (p=0.44). We, however, could find an odds ratio of 2.250 (95% CI 0.465–10.883) for restriction in patients with mitral atresia or aortic atresia, or both compared with patients with patency of both valves.
A total of 11 patients underwent 14 interventional procedures to relieve restriction.
In all, three patients, of whom two had mitral stenosis/aortic stenosis and one had mitral stenosis/aortic atresia, presented with early restriction, manifested as failure to thrive and transcutaneous O2 saturation around 80%; they underwent balloon atrioseptostomy during the 1st week of life (mean age 5.6 days, mean weight 3.5 kg), during the same procedure as hybrid stage I, just before pulmonary artery banding. A 6-Fr sheath has been used through a transfemoral vein approach; in all three cases predilatation of the atrial septal communication was required (Table 1). One of them, with mitral stenosis/aortic stenosis, had an aneurysmal defect and the atrioseptostomy was unsuccessful, with the patient requiring urgent surgery. The patient with mitral stenosis/aortic atresia, developed recurrent restriction over time and needed further intervention with stenting of the defect after 60 days.
Late occurrence of restriction occurred in eight patients, at a mean age of 66 days. In all, three patients underwent transcatheter balloon dilatation of the defect, whereas five were elected for stenting, depending on the preference of the attending physician. Only one patient from this latter group was then switched to surgical atrioseptectomy, because it was impossible to cross the defect. After balloon dilatation, two patients required stenting as well, which was performed later in a separate procedure.
A total of seven patients of whom three had mitral stenosis or aortic atresia, two had mitral stenosis/aortic atresia, two had mitral atresia/aortic atresia, one had mitral atresia/aortic stenosis, and one had mitral stenosis/aortic stenosis underwent transcatheter stenting, at a mean of 64 days after the hybrid procedure. The devices used are listed in Table 1 and include balloon-expandable Palmaz Genesis 19×10 mm and 25×10 mm premounted stents (Cordis, Cashel, Co. Tipperary, Ireland), along with NuMED Tyshak II 20×8 mm, NuMED Tyshak Mini 20×6 mm or 20×8 mm (NuMED Canada Inc, Cornwall, ON, Canada) and Abbott Vascular Fox Plus 20×8 mm (Abbott Vascular Knoll-Ravizza, Milan, Italy) and Advance Low Profile 20×8 mm (Cook Inc, Bloomington, IN, United States of America) balloons.
All stenting procedures were successful except for one case, which was a patient with malalignement of septum primum. This case was complicated by sudden migration of the stent into the right atrium immediately after stent deployment, because of the leftward deviation of the septum primum that made the stent unsupported by the antero-inferior septal tissue, which was not stable enough. This required prompt surgical intervention for either retrieval of the stent AND for atrioseptectomy.
It is worth considering that the small size of the left atrium is another factor that contributes to raise the risk for stenting of the atrial septal defect. We measured the left atrial diameter in the subcostal view, from the atrial septum to the lateral wall. In our population the mean left atrial diameter was 9.8±1.9 mm (range 6.8–14.0). The smallest left atrial diameter of those patients who received a stent was 8.4 mm at birth, and, in this case, early balloon atrioseptostomy was required.
The mean invasive transatrial pressure gradient was 16.8 mmHg before and 2.7 mmHg after successful interventional treatment.
Need for reintervention occurred in 3/11 patients, 8 weeks after early balloon atrioseptostomy in one case, and 11 weeks after and 1 week after balloon dilatation in two cases. All three cases were elected for stenting, which was complicated in the last case by migration of the stent.
Surgery was required for 3/11 patients, after unsuccessful balloon atrioseptostomy in one case, after unattempted stenting of a non-crossable defect in the second case, and after stent migration in the last case.
Perioperative death occurred in 2/11 (18%) patients with restrictive atrial septal defect. Only one patient developed reverse coarctation along with restriction; after attempted balloon dilatation of the aortic arch she underwent Norwood operation but died after 1 week. The second patient experienced ductal stent migration after a non-successful balloon atrioseptostomy and did not survive urgent surgery.
We found no significant difference between restrictive and non-restrictive groups in terms of 2-month survival (p=0.35, Fisher’s exact test), of 7-month survival (p=0.44, Fisher’s exact test), or of 12-month survival (p=0.68) (Fig 4; Table 2). The only variable affecting 12-month survival was weight at birth (β=0.441; p=0.021). Moreover, standard or complex morphology of the defect did not show a different impact on survival (B=−0.182; CI −0.179, 3.884; ExpB 0.833; p=0.816).

Figure 4 Kaplan–Meier survival function for restrictive and non-restrictive atrial septal defect groups.
Table 2 Number and rate of survivors at 2, 7, and 12 months; χ2 and Fisher’s exact tests for comparison; p: two-sided difference test significance level.

Discussion
The reported incidence of restrictive atrial septal defect in hypoplastic left heart syndrome ranges from 5.7 to 28%.Reference Hoque, Richmond, Vincent, Bacha and Torres 6 , Reference Vida, Bacha and Larrazabal 10 , Reference Schranz 11 Although the prevalence in our population appears to be higher (40%), it should be noted that it refers to the total of the perinatal and interstage period, which applies only to the hybrid palliation. In fact, restrictive defect is expected to be uncommon after classic Norwood stage-I operation, as atrioseptectomy is part of the intervention, whereas the hybrid procedure does not necessarily affect atrial septal communication; therefore, the total incidence of restrictive defect requiring any sort of intervention was 40% in our population, but 11% of cases presented immediately after birth, whereas 29% of cases experienced restriction later during interstage.
Other studiesReference Holzer, Wood and Chisolm 9 , Reference Galantowicz, Cheatham and Phillips 12 , Reference Schranz, Bauer and Reich 13 report a lower rate of atrial septal defect stenting during interstage, perhaps related to a higher rate of neonatal balloon atrioseptostomy, which, in some centres, is performed in all patients a few weeks after the hybrid stage-I palliation, just before discharge; however, with this strategy all patients are exposed to an additional invasive procedure, irrespectively of the advent of restriction, and, still, the need for reintervention for recurrent restriction during interstage is reported to be around 18–21%.Reference Holzer, Wood and Chisolm 9
There is a relationship between atrial septal morphology and restrictiveness in our population. Complex morphologies including malalignement defect, critically small foramen ovale, and aneurysmal defect were associated with restriction more frequently than was standard ostium secundum.
As regards relationships between atrial septal defect restriction and anatomical variant of hypoplastic left heart syndrome it can be observed that restriction occurred in 7 out of 14 cases of mitral atresia and/or aortic atresia (50%) and in 4 out of 13 cases of mitral stenosis or aortic stenosis (30%) (Fig 3). Although restrictive atrial septal defect physiology is a natural tendency in the whole spectrum of hypoplastic left heart syndrome, we observed that in the severe forms, namely those with aortic and/or mitral atresia, intervention was required about twofold (odds ratio 2.250; 95% CI 0.465–10.883). Conversely, in the presence of patent mitral and aortic valves, intervention was less frequently needed during interstage, maybe because a restrictive defect might be better tolerated in these variants, because of the ability of the hypoplastic left ventricle to provide some antegrade flow.
The fate of the atrial septal defect is critical in the setting of hybrid approach to hypoplastic left heart syndrome, as hybrid palliation crucially relies on atrial septal defect patency; therefore, interstage monitoring requires high-level awareness in a setting of such a high incidence (40%) of defect restriction, as we observed in our population. Despite the current knowledge reports that restriction carries a poor outcome, however, we could not find any difference between restrictive and non-restrictive groups in terms of midterm survival. Presentation of restriction occurred at a mean time interval of 2 months. For this reason special attention must be paid, at approximately 2 months after hybrid palliation, so as not to miss early clinical and echographic signs of restriction. Although restriction may recur after sole balloon dilatation, we did not observe any relapse after stenting. In our population we did not find any significant difference between restrictive and non-restrictive groups as regards survival either at 2 months, nor at 7 months (mean age at surgical stage II), nor at 12 months. Indeed, we observed these comparable survival rates in relatively small groups of patients, and we do not know if the same would apply to a larger population; however, we can hypothesise that timely and effective treatment of patients with restrictive defect may lead to a better outcome, at least similar to those for patients who do not experience restriction, as already reported in a larger cohort.Reference Hoque, Richmond, Vincent, Bacha and Torres 6
Nonetheless, with the purpose of avoiding the advent of atrial septal defect restriction and, at the same time, to reduce the damage to pulmonary branches, it may be reasonable to perform the hybrid stage II earlier, as soon as it is deemed possible. Actually, the mean age of 7 months in our population is altered by the fact that in the 1st year of our experience the surgical stage II was quite delayed, compared with the subsequent years in which the age for stage II moved towards 4 months. Some centres have also suggested a four-stage approach, with conversion to Norwood stage-I operation after neonatal hybrid palliation, beyond the neonatal period.Reference Trezzi, Cetrano and Carotti 14
On the other hand, a restrictive atrial septal defect during fetal life is responsible for pulmonary venous hypertension, which may not reverse even after successful postnatal decompression of the left atrium, because of the muscular remodelling of the vessel walls.Reference Rychik, Rome, Collins, DeCampli and Spray 7 , Reference Taketazu, Barrea, Smallhorn, Wilson and Hornberger 15 To prevent such a complication some centres have attempted fetal transcatheter atrioseptoplasty or even stenting. Fetal intervention to create an unrestrictive defect has been reported to be technically feasible with a non-negligible complication rate, and although results are encouraging, its overall impact on neonatal outcome needs further clarifications.Reference Marshall, van der Velde and Tworetzky 16 , Reference Kalish, Tworetzky and Benson 17
So far, we did not observe patients who developed pulmonary vein stenosis during follow-up investigation, nor did we have a loss of Fontan candidacy because of pulmonary hypertension in the group of patients treated for atrial septal defect restriction, except for one case of complete obstruction of one pulmonary artery due to the deleterious effect of neonatal banding.
In our experience, the technique of stent deployment is a crucial aspect. As for the conventional technique, the stent is usually expanded once the long sheath has been withdrawn, so that the stent is freely and uniformly expanded by the balloon inflated, assuming a nearly cylindrical shape. Special attention can be paid not to inflate the balloon to its maximum rate, so that the extremities would expand more than the waist, giving the stent more stability. We have recently experienced a different technique, which, to our knowledge, has never been reported in literature. In detail (Fig 5 and Supplementary Fig 1), we prefer to first expose and inflate the distal half of the stent, leaning on the left side of the defect, then withdraw the long sheath, and complete the stent deployment on the right side of the defect. This allows to obtain a pronounced central waist with a peculiar hourglass-shaped stent; therefore, the “shoulders” of the stent may ensure more stability across such a thin structure like the foramen ovale.

Figure 5 Incidence of restrictive atrial septal defect (ASD) in different hypoplastic left heart syndrome (HLHS) variants. The incidence of early and late ASD restriction requiring intervention in our population is represented in the central pie-diagram. The incidence of restrictive ASD is depicted for mitral stenosis/aortic stenosis (MS/AS) and for the association of mitral and/or aortic atresia (MA/AA, MS/AA, MA/AS). Restrictive ASD physiology appears to be more frequently associated with aortic and/or mitral atresia.
As concerns leftward deviation of septum primum, a particular comment is well deserved. It should be noted that not only the malalignement of the two components of the septum but also the curved shape of the septum primum determine the impossibility to safely embed a stent in. In fact, the antero-inferior aspect of the defect is not a definite rim, as usual, but, rather, a curved plane, unsuitable for the stable engagement of a device. The same complication has been reported by Kim et alReference Kim, Sobczyk, Yang, Mascio, Austin and Recto 18 who dealt with a stent embolization from what they called a “tunnel patent foramen ovale”, which was actually a leftward deviation of the septum primum, as clearly depicted. Our feeling, therefore, is that the safest technique for stenting the malalignement defect in this setting might be a transeptal puncture of the septum primum with subsequent stenting of the artificially obtained defect, without ever involving the malalignement defect.Reference Kim, Sobczyk, Yang, Mascio, Austin and Recto 18
Few data are available about atrial septal defect intervention in patients with hypoplastic left heart syndrome and larger experiences are needed, given that the hybrid approach is still evolving and, at the same time, is becoming increasingly widespread.
Our study is limited by the retrospective nature of the analysis. Indications and timing for intervention were at the discretion of the attending physician. In addition, the small number of patients limits the predictive power of the analysis; however, the incidence of hypoplastic left heart syndrome is low even in a tertiary care Institution and, therefore, it is difficult to obtain a larger sample size. Finally, these data pertain to the early phase of our Institution’s learning curve with the hybrid procedure and this may also have influenced the results.
Conclusions
Restrictive atrial septal defect has a negative impact on the outcome of patients with hypoplastic left heart syndrome and may occur in up to 40% of patients in case of hybrid palliation. Sometimes restrictive atrial septal defect physiology manifests immediately after birth, although a significant proportion of patients after hybrid palliation will probably need intervention during interstage. Complex morphology of the defect is more often associated with restriction than ostium secundum; therefore, higher level of attention should be reserved to patients with complex morphology of the defect, approximating to 2 months of age. Transcatheter dilatation and/or stenting are nearly always feasible, unless atrial septal communication is extremely tight. The technique of stent deployment has a crucial role as the procedure itself carries a significant risk, particularly for stent migration. Timely and effective treatment of patients with restrictive defect is related to a better outcome.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
Research was carried out in compliance with the Helsinki Declaration and the Ethical Committee of Messina University gave formal approval (Protocol No. 0008421).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951117001792