Introduction
Randomized controlled trials (RCTs) for anorexia nervosa (AN) are limited, and this is especially true for severe and enduring anorexia nervosa (SE-AN) (Wonderlich et al. Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012). There is also limited evidence for the efficacy of any specific approaches from RCTs in adult AN and there have been no RCTs with a primary focus on SE-AN (Hay et al. Reference Hay, Touyz and Sud2012; Wonderlich et al. Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012). Such individuals merit research attention as they have the highest mortality rate of any mental illness (Steinhausen, Reference Steinhausen2002; Harbottle et al. Reference Harbottle, Birmingham and Sayani2008), and they suffer significant medical co-morbidity (Arkell & Robinson, Reference Arkell and Robinson2008; Robinson, Reference Robinson2009; Birmingham & Treasure, Reference Birmingham and Treasure2010; Wonderlich et al. Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012). Patients with SE-AN also have high levels of disability, often being under- or unemployed, supported by health benefit plans, and they can become a significant burden to parents, carers and health-care funders (Treasure et al. Reference Treasure, Murphy, Szmukler, Todd, Gavan and Joyce2001; Strober, Reference Strober2004a; Striegel-Moore, 2008).
One of the challenges in tailoring treatment for individuals with SE-AN is that treatments that focus on both physical and psychological recovery run the risk of misalignment with patient aims and readiness for recovery, resulting in high drop-out (Wonderlich et al. Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012). Insurance companies often refuse to treat on the basis that these individuals do not respond to known treatments and in the UK, for instance, some National Health Service (NHS) funders insist that specialist eating disorder (ED) services discharge such patients to generic psychiatric services or no treatment on the same grounds. Globally, treatment programs are limited in their capacity to treat these patients and it has been reported that non-specific medical palliation may become the default care (Strober, Reference Strober, Grilo and Mitchell2009; Lopez et al. Reference Lopez, Yager and Feinstein2010; Kaplan & Buchanan, Reference Kaplan and Buchanan2012).
Taking account of these challenges and complexities of treatment, a different paradigm is needed (Goldner, Reference Goldner1989; Yager, Reference Yager, Yager, Gwirtsman and Edelstein1992; Vitousek et al. Reference Vitousek, Watson and Wilson1998; Strober, Reference Strober2004b; Robinson, Reference Robinson2009; Tierney & Fox, Reference Tierney and Fox2009; Williams et al. Reference Williams, Dobney and Geller2010). Such a paradigm must reflect the severe and enduring nature of this debilitating disorder. Rather than recovery being the basic premise, treatment should focus more upon retention, improved quality of life with harm minimization, and avoidance of further failure experiences (Strober, Reference Strober, Grilo and Mitchell2009; Williams et al. Reference Williams, Dobney and Geller2010). This approach needs to take into account the challenges in treating patients with long-standing low levels of motivation for change, neurocognitive deficits, and a self-view and lifestyle dominated by the illness (Strober, Reference Strober2004b; Schmidt & Treasure, Reference Schmidt and Treasure2006; Hatch et al. Reference Hatch, Madden, Kohn, Clarke, Touyz and Williams2010; Treasure & Russell, Reference Treasure and Russell2011). Thus, in the absence of scientific guidelines, clinicians who have the responsibility of treating patients with SE-AN resort to modifying existing treatment protocols, seeking out alternative strategies that make accommodations for patients' chronic status, paying close attention to co-morbidities and blending supportive, harm reduction and recovery-based strategies (Wonderlich et al. Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012). Wonderlich et al. (Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012, p. 476) state that in the attempt to meet this therapeutic challenge, ‘treatments may devolve into relatively unfocused, intermittent, supportive interventions, where goals become unclear and monitoring of clinical status becomes impressionistic and imprecise.’
Our present study was designed to ascertain whether it is possible to retain patients with SE-AN in treatment and whether clinically meaningful improvement is possible by adapting existing psychotherapy protocols that have demonstrated some efficacy, albeit limited, in treating adults with AN. Specifically, the present study was designed to test the relative efficacy of a modified cognitive behavioral therapy for AN (CBT-AN; Pike et al. Reference Pike, Walsh, Vitousek, Wilson and Bauer2003), which has documented efficacy particularly for relapse prevention for adult AN, and a modified specialist supportive clinical management (SSCM; McIntosh et al. Reference McIntosh, Jordan, Luty, Carter, McKenzie, Bulik and Joyce2006, Reference McIntosh, Jordan, Bulik, Grilo and Mitchell2010), a treatment that has demonstrated utility in two trials in adult AN (McIntosh et al. Reference McIntosh, Jordan, Bulik, Grilo and Mitchell2010; Schmidt et al. Reference Schmidt, Oldershaw, Jichi, Sternheim, Startup, McIntosh, Jordan, Tchanturia, Wolff, Rooney, Landau and Treasure2012). Although individuals with SE-AN participated in the initial studies evaluating both CBT-AN and SSCM for adult AN, neither of the previous studies focused exclusively on SE-AN.
The primary outcomes in the current study were quality of life, mood disorder symptoms and social adjustment. Body mass index (BMI), ED psychopathology, motivation for change and health-care burden were secondary outcomes.
Method
Design
Two intervention sites (University of Sydney and St George's, University of London) randomized 63 female participants to CBT-AN (Pike et al. Reference Pike, Walsh, Vitousek, Wilson and Bauer2003) or SSCM (McIntosh et al. Reference McIntosh, Jordan, Luty, Carter, McKenzie, Bulik and Joyce2006, Reference McIntosh, Jordan, Bulik, Grilo and Mitchell2010). All participants were aged ⩾18 years and met DSM-IV (APA, 2000) criteria for AN, excluding criterion D (amenorrhea), for more than 7 years. Previous studies have reported a mean duration of illness between 5 and 7 years. Our aim was to address SE-AN, and to be conservative we selected 7 years as the lower limit because this was the upper limit of several adult RCTs (Pike et al. Reference Pike, Walsh, Vitousek, Wilson and Bauer2003; Walsh et al. Reference Walsh, Kaplan, Attia, Olmsted, Parides, Carter, Pike, Devlin, Woodside, Roberto and Rockert2006; Carter et al. Reference Carter, MacFarlane, Bewell, Olmstead, Woodside, Kaplan and Crosby2009). Individuals whose ED endures for this period of time have not only a long-standing disorder but also a severe one, which is evident from patient baseline social adjustment, weight, mood and health status (Strober, Reference Strober, Grilo and Mitchell2009). Patients were also included if they met all DSM-IV criteria but presented with a BMI between 17.6 and 18.5 kg/m2. We included patients within this BMI range because recent studies comparing full AN versus subthreshold AN in adult females showed no differences between these groups (McIntosh et al. Reference McIntosh, Jordan, Carter, Luty, McKenzie, Bulik, Frampton and Joyce2005; Le Grange et al. Reference Le Grange, Crosby, Engel, Cao, Ndungu, Crow, Peterson, Mitchell and Wonderlich2013).
Randomization was performed by a biostatistician in the Data and Coordinating Centre (DCC, The University of Chicago), independent from either intervention site. Participants were individually randomized using Ephron's biased coin approach stratified within sites based on subtype of illness [AN restricting type (ANR) versus AN binge-purging type (ANBP)] and current use of psychiatric medication. Participants were assigned therapists who conducted both forms of treatment to control for non-specific therapist effects. The therapists were three clinical psychologists with extensive experience in treating EDs in adults. Two 2-day in-person workshops were held to train the therapists in manualized CBT-AN and SSCM. The first workshop was held prior to randomization and the second was held 1 year later. Experts in CBT-AN (K. M. Pike) and SSCM (V. V. McIntosh) were involved in training the therapists. Therapists treated pilot cases with each treatment before being assigned randomized cases, and treatment was conducted in clinics for adults with EDs at each of the two intervention sites. The Institutional Review Boards of each site approved the study protocol. Weekly multi-site supervision sessions were conducted to ensure that the therapies were provided in accordance with the treatment manuals, and that treatment centers maintained consistent approaches in terms of clinical and practical decisions. Audiotaped sessions were reviewed by one of the authors upon completion of the study (D.L.G.).
Participants
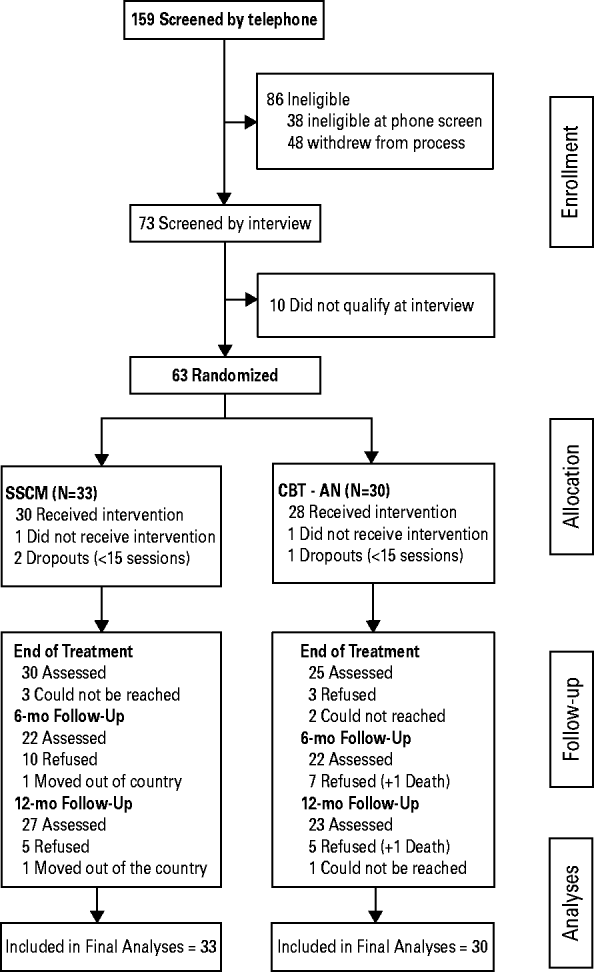
Participants were recruited from July 2007 to November 2010 by advertising to clinicians, clinics treating EDs, and on generic websites. After telephone screening (n = 159) to determine eligibility, 73 (46%) were invited for in-person assessment (see Fig. 1). Respective site study coordinators described the protocol in detail to participants before written informed consent was obtained and the assessments conducted. Participants were eligible if they were female (males were excluded as we estimated that the number of such cases would be negligible), aged ⩾18 years, met DSM-IV criteria for AN, excluding criterion D (amenorrhea), and had an illness duration of at least 7 years. Participants were excluded from the study if they presented with a current manic episode or psychosis, current alcohol or substance abuse or dependence, significant current medical or neurological illness (including seizure disorder), with the exception of nutrition-related alterations that impact on weight, were currently engaged in psychotherapy and not willing to suspend treatment for the duration of their participation in the study, had plans to move beyond commuting distance from the study site in the following 12 months, or did not live within commuting distance to the study site. Eighty-six percent (n = 63) of eligible participants agreed to randomization. The majority of those ineligible did not meet DSM-IV weight loss or illness duration criteria.

Fig. 1. Study flowchart.
Treatments
Both treatments involved 30 individual treatment sessions provided over 8 months in an out-patient setting. Participants were told that the focus of treatment would be on improving quality of life rather than weight gain per se, and that the specific treatment goals would be articulated collaboratively at the outset of therapy.
CBT-AN
The CBT-AN used in this trial was based on the CBT-AN protocol developed by Pike et al. (Reference Pike, Walsh, Vitousek, Wilson and Bauer2003), which focuses on the cognitive and behavioral disturbances linked to the core features of AN and also more global issues associated with AN, including motivational and schema-based work. As originally designed, CBT-AN includes four phases of treatment. Phase I provides specific strategies for initiating treatment, orienting patients to CBT and addressing issues of motivation. Phase II focuses on strategies for addressing weight gain, addresses cognitive distortions and behavioral disturbances associated with eating and weight. Phase III expands the focus of treatment to schema-based work that addresses relevant issues that extend beyond the specific domain of eating and weight. Phase IV focuses on reviewing the course of therapy, consolidating gains and preparing to continue the work of CBT-AN independently after therapy ends. Although the four phases of treatment are described sequentially, depending on the course of therapy for each individual, the treatment is flexible in terms of applying modules of the protocol as needed throughout the course of treatment. For the present study, CBT-AN was modified to reflect the shift in treatment goals. Specifically, weight gain and recovery from core features of the ED were not assumed to be treatment priorities. Instead, treatment goals were set collaboratively and weight gain was encouraged but not identified as the primary goal or focus of therapy (although medical safety was monitored and required to remain in the study). In this study CBT-AN allowed flexibility in approach, and the motivational enhancement section of the manual, for instance, was allowed to continue as long as clinically needed.
SSCM
SSCM (McIntosh et al. Reference McIntosh, Jordan, Luty, Carter, McKenzie, Bulik and Joyce2006, Reference McIntosh, Jordan, Bulik, Grilo and Mitchell2010) combines features of clinical management and supportive psychotherapy. Clinical management includes education, care and support, while fostering a therapeutic relationship that promotes adherence to treatment. Supportive psychotherapy aims to assist the patient through use of praise, reassurance and advice. As in the case of CBT-AN, SSCM was modified for this trial such that weight gain was not prioritized. Instead, SSCM encouraged patients to make changes to improve their quality of life and physical well-being. The rationale for this emphasis in treatment is that improvement in domains outside the core pathology can significantly affect patient well-being and disease burden, and research suggests that treatments that target psychosocial functioning are especially appropriate when there has been repeated relapse or a long duration of illness. Ultimately, SSCM aims to help individuals to improve their quality of life, which will further motivate and enable them to make progress on their core ED pathology.
CBT-AN and SSCM were both modified to prioritize quality of life and harm minimization associated with the ED, and they both made weight gain a secondary aim. The primary outcome measures were selected to assess at a macro-level the extent to which individuals were better able to find satisfaction in their lives and engage meaningfully with significant others as a result of treatment. The treatments were distinct in that CBT-AN made use of specific cognitive and behavioral strategies whereas SSCM made use of more general, supportive therapeutic strategies as outlined in the original manuals. Differences and similarities between these two study treatments are outlined in Table 1.
Table 1. Comparison of cognitive behavioral therapy (CBT-AN) and specialist supportive clinical management (SSCM) as modified in this study for treatment of severe and enduring anorexia nervosa (SE-AN)

Assessment and procedures
Assessment included diagnostic evaluation for weight and ED-related symptoms and psychopathology and also co-morbid psychiatric disorders. The patients were assessed at pretreatment, end of treatment (EOT), and at 6- and 12-month follow-ups. Independent assessors blind to treatment assignment conducted all assessments. With regard to treatment fidelity, all therapy sessions were digitally recorded, de-identified and then forwarded to the Data Management Site for fidelity checking.
Primary outcome measures
Eating Disorder Quality of Life Instrument (EDQOL; Engel et al. Reference Engel, Wittrock, Crosby, Wonderlich, Mitchell and Kolotkin2006)
The EDQOL is a standardized and validated 25-item instrument assessing quality of life in ED populations across four subscales: psychological, physical and cognitive, financial, and work or school. This is the first instrument designed to assess quality of life in ED patients. Because of an inherent bias in existing instruments towards assessing occupational attainment, it is often a challenge to accurately evaluate quality of life in this population. This instrument is uniquely designed to assess relevant components of quality of life for individuals with AN and has demonstrated reliability and validity.
Short Form-12 Health Status Questionnaire (SF-12; Ware et al. Reference Ware, Kosinski and Keller1996)
The SF-12 was used to assess quality of life. The well-validated SF-12 measures dimensions of health and role limitations due to physical and mental ill-health, for which Mental (MCS) and Physical Component Summary (PCS) scales can be derived.
Beck Depression Inventory (BDI; Beck et al. Reference Beck, Steer and Brown1996)
The BDI is a 21-question scale with each answer rated 0–3. This scale is widely used across the entire spectrum of studies of psychopathology and psychotherapy.
Weissman Social Adjustment Scale (WSAS; Weissman & Bothwell, Reference Weissman and Bothwell1976)
The WSAS assesses social adjustment in multiple areas of functioning, including marital, family, work, economic and leisure. The scale has well-established reliability and validity and has been used in a wide variety of populations.
Secondary outcome measures
BMI
Weight and height were assessed at baseline, and weight was assessed by the therapist before each therapy session. The participant was weighed in light indoor clothing, without shoes, on a balance beam scale that was recalibrated regularly. Weight change was calculated in BMI (kg/m2), which allowed us to track treatment course.
Eating Disorder Examination (EDE; Fairburn & Cooper, Reference Fairburn, Cooper, Fairburn and Wilson1993)
The EDE is a standardized investigator-based interview that measures the severity of the characteristic psychopathology of EDs. Studies consistently support its use, sensitivity, reliability and validity, making it the gold standard for assessing EDs.
Anorexia Nervosa Stages of Change Questionnaire (ANSOCQ; Rieger et al. Reference Rieger, Touyz and Beumont2002)
The ANSOCQ is a 20-item multiple choice questionnaire that assesses a patient's readiness to recover from AN. It has demonstrated good validity and has high levels of inter-rater and test–retest reliability.
Health-care utilization
A self-report questionnaire was designed specifically for the study with input from health economists, primary care physicians (PCPs), psychiatrists and medical specialists to assess the frequency and intensity of the patient's use of PCP services, ED treatment services, medical services and specialist appointments over the preceding 6-month period.
Participant safety
Participants were assessed prior to randomization to ensure medical stability for out-patient treatment. During treatment, patients could be referred to their general practitioner who was aware of this RCT and who had consented to follow the patient throughout the duration of this trial. In addition, patients could be hospitalized for a maximum of 21 days during the course of treatment and still remain in the study upon discharge. An in-patient stay was determined using well-established guidelines as provided by the American Psychiatric Association (APA, 2000) or the National Institute for Health and Clinical Excellence (NICE, 2004).
Statistical analyses
All analyses were conducted using SPSS version 19.0.0 (SPSS Inc., USA). A two-tailed α of 0.05 was used to evaluate all tests of significance. Participants were compared on sociodemographic and clinical characteristics and also primary and secondary outcome measures at baseline (treatment groups × site), using two-way analysis of variance for continuous measures and logistic regression for dichotomous measures.
All outcome analyses were based upon an intention-to-treat (ITT) approach. Missing data for continuous outcome measures at EOT and follow-ups were imputed using multiple imputation based upon fully conditional Markov chain Monte Carlo modeling (Schafer, Reference Schafer1997). Data for the final analysis model were based upon the averaged values of 100 separate imputations (Rubin, Reference Rubin2009). Treatment groups were then compared separately at the EOT, 6-month and 12-month follow-ups using a general linear model for continuous outcomes, a generalized linear model based upon a negative binomial distribution for count data (i.e. PCP visits, hospital visits), or a logistic regression for dichotomous outcomes (i.e. medication management, medical management). Covariates for all models included baseline observation (with the exception of medication management and medical management), site, AN subtype and psychotropic medication use. Between-group effect size was calculated using partial eta-squared (η 2p), which is interpreted as the unique proportion of variance in outcome attributed to the treatment group (small effect = 0.01, medium effect = 0.06, large effect = 0.14; Cohen, Reference Cohen1998). Sensitivity analyses were conducted to evaluate the impact of the multiple imputation procedures. The analyses were repeated based upon complete case analysis and imputation based upon last observation carried forward (LOCF) and the results were compared across the three methods. Within-group change from baseline to EOT and follow-up was compared using a repeated-measures general linear model. Within-group effect size was based upon the standardized effect size calculated as change from baseline to EOT or follow-up divided by the baseline standard deviation (small effect = 0.20, medium effect = 0.50, large effect = 0.80; Cohen, Reference Cohen1998).
The primary outcomes were change in measures assessing chronicity; that is, quality of life, mood disorder symptoms and social adjustment. Change in weight (BMI), core ED psychopathology, motivation for change and health-care utilization (use of medical services, for example number of hospital days, PCP and specialist visits) were all secondary outcome measures.
Results
Participant characteristics
All study participants were female with a mean age of 33.4 years (s.d. = 9.6, range 20–62) and a mean duration of illness of 16.6 years (s.d. = 8.5, range 7–49 years). The mean BMI for the sample was 16.2 kg/m2 (s.d. = 1.3, range 11.8–18.5). Nearly three-quarters of participants (n = 47, 74.6%) met criteria for ANR. In terms of co-morbid SCID-I (DSM-IV-TR; First et al. Reference First, Spitzer, Gibbo and Williams2002), Axis I diagnoses, 22 participants (35%) met criteria for a mood disorder or dysthymia, 20 participants (31.7%) met criteria for generalized anxiety disorder and 16 (25.4%) met criteria for social phobia. Six participants (9.5%) met criteria for obsessive–compulsive disorder and one participant met criteria for current substance dependence. Twelve participants in CBT-AN (38.7%) and 14 in SSCM (43.8%) were taking psychotropic medication.
Randomization and attrition
A total of 63 participants were randomized to CBT-AN (n = 31) or SSCM (n = 32). Table 2 presents baseline participant characteristics by group and site. No significant differences on any baseline characteristics were found between treatment groups, sites or group-by-site interactions.
Table 2. Baseline participant characteristics by treatment group and site

CBT, Cognitive behavioral therapy; SSCM, specialist supportive clinical management; BMI, body mass index; AN, anorexia nervosa; OR, odds ratio; s.d., standard deviation.
A total of 55 (87.3%) participants completed treatment, 26 of 31 (83.9%) in CBT-AN and 29 of 32 (90.6%) in SSCM (Fisher's exact p = 0.474). Forty-five (71.4%) participants completed the 6-month follow-up (22 CBT-AN, 23 SSCM) and 50 (79.4%) completed the 12-month follow-up (24 CBT-AN, 26 SSCM). In total, 54 (85.7%) participants completed at least one post-treatment assessment (27 CBT-AN, 27 SSCM). There were no significant differences between treatments in follow-up completion rates (p's = 0.763–1.00).
Seven patients were admitted to the hospital during out-patient treatment and six were discharged prior to the 21-day maximum stay allowed in this study. One patient required hospitalization for longer than 21 days, refused further treatment and subsequently died at home during the follow-up phase of this study. (This is the death reported in Fig. 1.) There was no significant difference between treatment groups in terms of hospital visits at EOT (p = 0.417), the 6-month (p = 0.154) or the 12-month follow-up (p = 0.059). Eleven patients received further out-patient treatment or partial hospitalization/hospitalization during the follow-up phase of this study.
Treatment outcome
The magnitude and significance of within-group changes on primary and secondary measures of outcome are summarized in Table 3. With the exception of the SF-12 PCS scale, both groups experienced significant changes on all primary and secondary measures of outcome at EOT, 6- and 12-month follow-ups. The magnitude of change ranged from moderate (e.g. 0.50 for BMI) to large (e.g. 1.52 for ANSOCQ). The magnitude of improvements for health-related quality of life, depression and social adjustment were somewhat larger for SSCM, whereas those for ED symptoms and readiness for change were generally larger for CBT.

CBT, Cognitive behavioral therapy; SSCM, specialist supportive clinical management; EDQOL, Eating Disorders Quality of Life; SF-12, Short Form-12 Health Status Questionnaire; MCS, Mental Component Summary scale; PCS, Physical Component Summary scale; BDI, Beck Depression Inventory; WSAS, Weissman Social Adjustment Scale; BMI, body mass index; EDE, Eating Disorder Examination; ANSOCQ, Anorexia Nervosa Stages of Change Questionnaire.
a Cell entries represent within-group standardized effect size (i.e. change from baseline in baseline standard deviation units).
b Positive effect size indicates improvement; negative effect size indicates worsening.
* p < 0.05, ** p < 0.01, *** p < 0.001.
Comparisons between treatment groups on outcome measures at baseline (Table 4) revealed that SSCM reported higher levels of depression on the BDI (F 1,59 = 5.99, p = 0.017, η 2p = 0.092) and poorer social adjustment on the WSAS (F 1,59 = 4.45, p = 0.039, η 2p = 0.070).
Table 4. Treatment outcome by treatment groupa

CBT, Cognitive behavioral therapy; SSCM, specialist supportive clinical management; EDQOL, Eating Disorders Quality of Life; SF-12, Short Form-12 Health Status Questionnaire; MCS, Mental Component Summary scale; PCS, Physical Component Summary scale; BDI, Beck Depression Inventory; WSAS, Weissman Social Adjustment Scale; BMI, body mass index; EDE, Eating Disorder Examination; ANSOCQ, Anorexia Nervosa Stages of Change Questionnaire; PCP, primary care physician.
a Cell entries represent unadjusted means (standard deviation) except as noted.
b Covariates include site.
c Covariates include baseline score, site, anorexia nervosa (AN) subtype and psychotropic medication status.
d Cell entries represent n (%). Missing data imputed by multiple imputation.
e SSCM > CBT: p < 0.05, WSAS η 2p = 0.073.
f CBT > SSCM: p < 0.05, EDE Global η 2 = 0.135, ANSOCQ η 2p = 0.104.
No significant differences were found between treatment groups at EOT on any measure of outcome or health-care utilization (Table 4). Analysis at the 6-month follow-up revealed that CBT-AN had significantly better social adjustment on the WSAS (F 1,57 = 4.51, p = 0.038, η 2p = 0.073); however, this finding was not confirmed by sensitivity analysis using complete case or LOCF. Comparisons at the 12-month follow-up revealed that CBT-AN was associated with lower ED symptoms on EDE Global (F 1,57 = 8.90, p = 0.004, η 2p = 0.135) and higher readiness for recovery on ANSOCQ (F 1,57 = 6.59, p = 0.013, η 2p = 0.104) compared to SSCM. Both findings were confirmed with complete case analysis but LOCF failed to confirm the difference for ANSOCQ.
Discussion
This study was designed to determine whether it is possible to treat patients with SE-AN and represents the first RCT to examine the relative efficacy of two manualized treatments specifically tailored for this patient population. The results of this study indicate that, with the exception of the SF-12 PCS scale, both treatment groups experienced significant improvements on all primary and secondary outcome measures at all assessment time points and in domains outside the traditional core psychopathology. The magnitude of change ranged from moderate (e.g. BMI) to large (e.g. ANSOCQ). In particular, the magnitude of improvements in health-related quality of life, depression and social adjustment were large for SSCM, in keeping with the basic tenets of this treatment.
It is important to note that there were no significant differences between the two treatment groups at EOT. However, some limited differences were seen at the 6- and 12-month follow-ups. CBT-AN showed better social adjustment (WSAS), lower ED symptoms (EDE Global) and improved readiness to change (ANSOCQ). We suggest that this is due to the active and structured nature of CBT-AN, which resulted in more clearly articulated increases in social functioning and eating pathology. Although speculative, the specific skills of CBT-AN may have had the effect of empowering participants to make significant gains in these areas.
Taken together, these findings challenge the established paradigm that individuals with an enduring course of AN have little or no motivation to change and are unlikely to respond to conventional psychosocial treatments (Strober, Reference Strober, Grilo and Mitchell2009; Wonderlich et al. Reference Wonderlich, Mitchell, Crosby, Myers, Kadlec, Lahaise, Swan-Kremeier, Dokken, Lange, Dinkel, Jorgensen and Schander2012). Moreover, low drop-out rates in this study may be attributed to the fact that therapists worked on areas that the patients deemed important, especially areas associated with quality of life, which improved engagement and motivation.
Based on our findings, we argue that individuals with SE-AN can make significant strides in terms of achieving a higher quality of life along with a reduction in ED pathology. By widening the treatment goals, focusing on quality of life and lessening the pressure to achieve weight gain, we were able to engage individuals with SE-AN in treatment, circumvent the ‘customary’ high drop-out rates, and bring about significant progress and achieve meaningful positive change in their lives. For individuals with SE-AN, we argue that it is more constructive to address eating and weight pathology with this patient group by setting minimum weight thresholds for treatment participation rather than setting weight gain or weight normalization as the treatment priority. Although nutritional improvement is encouraged, social activities and leisure pursuits with family members and supportive others are re-established, and appropriate medical follow-up is promoted.
The strengths of the study include a retention rate of 85% (which to our knowledge is the highest of any study of adult AN). Prior CBT studies have demonstrated strong retention rates (65–78%) in relapse prevention; however, drop-out is often higher in treatment studies for acutely ill patients (usually of the order of 30%) (Dare et al. Reference Dare, Eisler, Russell, Treasure and Dodge2001; Halmi et al. Reference Halmi, Agras, Crow, Mitchell, Wilson, Bryson and Kraemer2005; Bulik et al. Reference Bulik, Berkman, Brownley, Sedway and Lohr2007; Glasofer et al., Reference Glasofer, Attia, Pike, Castonguay and Oltmamsin press), so our ability to retain a strong majority of patients across treatments is especially important. Additional strengths are the 12-month follow-up, and an independent data center that monitored recruitment, eligibility and data quality closely. Outcome assessment consisted of standardized instruments with assessors blinded to treatment group conducting the interviews. Supervision was conducted on a weekly basis throughout the trial and sessions were recorded for quality control. Treatments were manualized with previously tested therapies (CBT-AN and SSCM), and the modified manuals were pilot tested by study therapists before their use. However, several limitations should also be considered, including a follow-up period of 12 months, which might be considered short for such a severe and enduring group of AN patients. Furthermore, as in most studies of adults with AN, the current study was hampered by a modest sample size. Nevertheless, the sample was sufficient to show differences in outcomes between groups for ED symptoms at follow-up but may have been underpowered to detect differences in other outcomes. Most of the outcome measures were self-report with their inherent shortcomings. In addition, this was an open follow-up and patients were able to seek additional treatment if desired. There were also differences in the primary outcome measures at randomization, although these were adjusted for in the analysis. As assignment to treatment group was based upon randomization, any differences between treatment groups at baseline are due by definition solely to chance. As such, using covariates in the analyses to control for these baseline differences controls for such confounds. Finally, it would have been desirable to have included a third treatment arm, such as ‘treatment as usual’ (TAU). Such a group would have controlled for non-specific therapy factors. Despite these limitations, this remains the only RCT to date to exclusively recruit AN patients with an illness duration of at least 7 years.
Conclusions
This study clearly shows that SE-AN patients do respond to, and benefit from, two specialized treatments when administered by clinicians with specialist knowledge of enduring EDs. Despite the prevailing pessimism in the published literature of retaining such patients in treatment and follow-up (Strober & Johnson, Reference Strober and Johnson2012; Waller, Reference Waller2012; Glasofer et al., in press), a retention rate of 85% at follow-up was achieved in our study.
The findings of the current study suggest that CBT-AN was superior in reducing core ED symptoms at follow-up, but that both CBT-AN and SSCM contributed to improvements over time in health-related quality of life, body weight, depression and motivation to change. The magnitude of these changes ranged from moderate to large. The findings of this study should provide hope for those suffering from severe and enduring AN and also stimulate interest in the development of new psychosocial treatment approaches.
Acknowledgments
Funding was received from the Australian National Health and Research Council (PG 457419; Drs Touyz, Le Grange, Lacey and Hay), South West London and St George's NHS Trust (Dr Lacey), the Butterfly Foundation (Dr Touyz), and the University of Western Sydney (Dr Hay). We thank E. Rieger, H. Henrichsen, C. Evans, M. Ward, J. Russell, C. Stiles-Shields, J. Roehrig, A. Brown, V. McIntosh, A. Heriseanu, V. Mountford, R. Ramsden and K. Petrie for their contributions to this study.
[Trial Registration: Australian New Zealand Clinical Trials Registry no. 12607000440426.]
Declaration of Interest
Drs Touyz and Hay receive royalties from Hogrefe and Huber, McGraw Hill Education and Blackwell Scientific Publications, Dr Le Grange receives royalties from Guilford Press and Honoraria from the Training Institute for Child and Adolescent Eating Disorders, LLC.