Lignin is the second most abundant polymer in nature (15–30 wt.% of lignocellulosic biomass) and is obtained as a byproduct of the kraft process during the production of pulp and paper. The process allows extraction of >90% of the lignin from wood. The amount of lignin extracted from pulp-manufacturing operations worldwide is estimated to exceed 70 million tonnes per year. However, <2% is recovered for use as a renewable chemical (Chakar & Ragauskas, Reference Chakar and Ragauskas2004; Lora, Reference Lora2008).
Kraft lignin chemistry has been a subject of great interest because of the interesting properties of lignin derivatives. In fact, it is expected that lignin will play an important role as an alternative in petroleum-based chemistry. One of the potential applications of lignin is as a natural surfactant. Polar groups from the aromatic moieties confer on lignin the dual affinity typical in surfactants (Selyanina & Selivanova, Reference Selyanina and Selivanova2007; Rojas et al., Reference Rojas, Bullón, Ysambertt, Forgiarini and Argyropoulos2007; Selyanina et al., Reference Selyanina, Trufanova, Afanas′ev and Selivanova2007). However, the surface activity of lignin must be modified for the applications envisaged (Laurichesse & Avérous, Reference Laurichesse and Avérous2014). The grafting of polar or non-polar functional groups may vary the hydrophilic-lipophilic balance (HLB) for the design of lignin-based surfactants (Homma et al., Reference Homma, Kubo, Yamada, Matsushita and Uraki2008, Reference Homma, Kubo, Yamada, Koda, Matsushita and Uraki2010; Cerrutti et al., Reference Cerrutti, De Souza, Castellan, Ruggiero and Frollini2012; Gan et al., Reference Gan, Zhou, Yang and Qiu2013; Chen et al., Reference Chen, Li, Wu and Sun2014; Li et al., Reference Li, Zhu, Yang, Zhang, Xu and Lu2014; Lin et al., Reference Lin, Zhou, Wang, Lou, Yang and Qiu2014; Zhou et al., Reference Zhou, Wang, Yang and Qiu2015; Konduri et al., Reference Konduri, Kong and Fatehi2015; Chen at al., Reference Chen, Shen, Yan, Mi, Wang, Zhang and Zhou2016).
On the other hand, cyclic anhydrides are commonly used for lignin derivatization, as the bifunctional ester-carboxylic acid moieties confer significant change on physicochemical properties (Xiao et al., Reference Xiao, Sun and Sun2001; Thielemans & Wool, Reference Thielemans and Wool2005).
The best known commercial lignin-based surfactants are lignosulfonates. Sulfonated lignin has been evaluated as a dispersing agent for gypsum particles (Matsushita & Yasuda, Reference Matsushita and Yasuda2005; Matsushita et al., Reference Matsushita, Inomata, Hasegawa and Fukushima2009), coal (Zhou et al., Reference Zhou, Qiu, Yang, Lou and Ouyang2007) and cement (Ouyang et al., Reference Ouyang, Ke, Qiu, Guo and Pang2009). Previous works (Zhou et al., Reference Zhou, Qiu, Yang, Lou and Ouyang2007; Yang et al., Reference Yang, Qiu, Zhou and Lou2007) indicated that sulfonated lignin fractions of high molecular weight showed greater dispersing capacity and lower viscosity.
The main objective of this work was to evaluate the bentonite dispersant properties of kraft lignin derivatives esterified with cyclic anhydrides (succinic, maleic and glutaric), by using microwave radiation (Delgado et al., Reference Delgado, Ysambertt, Chávez, Bravo, Márquez and Bullón2012, Reference Delgado, Ysambertt, Bravo, Chávez and Márquez2015). The dispersing properties of lignin and lignin derivatives were compared with those of a high molecular weight (MW) fraction of lignin and the corresponding derivatives. In addition, two commercial lignosulfonate dispersants commonly used in the formulation of drilling sludge were used in order to compare the results.
MATERIALS AND METHODS
Pine (Pinus caribaea) kraft lignin (KL) was used. The KL was isolated from the black liquor of Smurfit Mocarpel Carton de Venezuela Company by acid precipitation (pH = 3). The high molecular weight fraction (HKL) was obtained by ultrafiltration using a 15 KDa ceramic membrane according to a method previously reported (Delgado et al., Reference Delgado, Ysambertt, Chávez, Bravo, Márquez and Bullón2012). Both lignins were esterified with succinic (SA, Riedel-de-Haën, 99%), maleic (MA, Riedel-de-Haën, 99.9%) and glutaric (GA, TCI America, 99.9%) anhydride. Acetonitrile (Burdick & Jackson, 99.9%), ethanol (Merck, 99.9%), hydrochloric acid (Sigma Aldrich, 37%), sodium hydroxide (Merck, 99.9%) and potassium bromide (Sigma Aldrich, ≥ 99.0%) were used. Bentonite was provided by Baker Hughes Company (USA). Two commercial dispersants were used (ammonium lignosulfonate (DP875-LS) provided by M&P Supply & Services, C.A., Italy, and sodium lignosulfonate (CF-LS) provided by Borregard Lignotech, Norway).
Lignin esterification
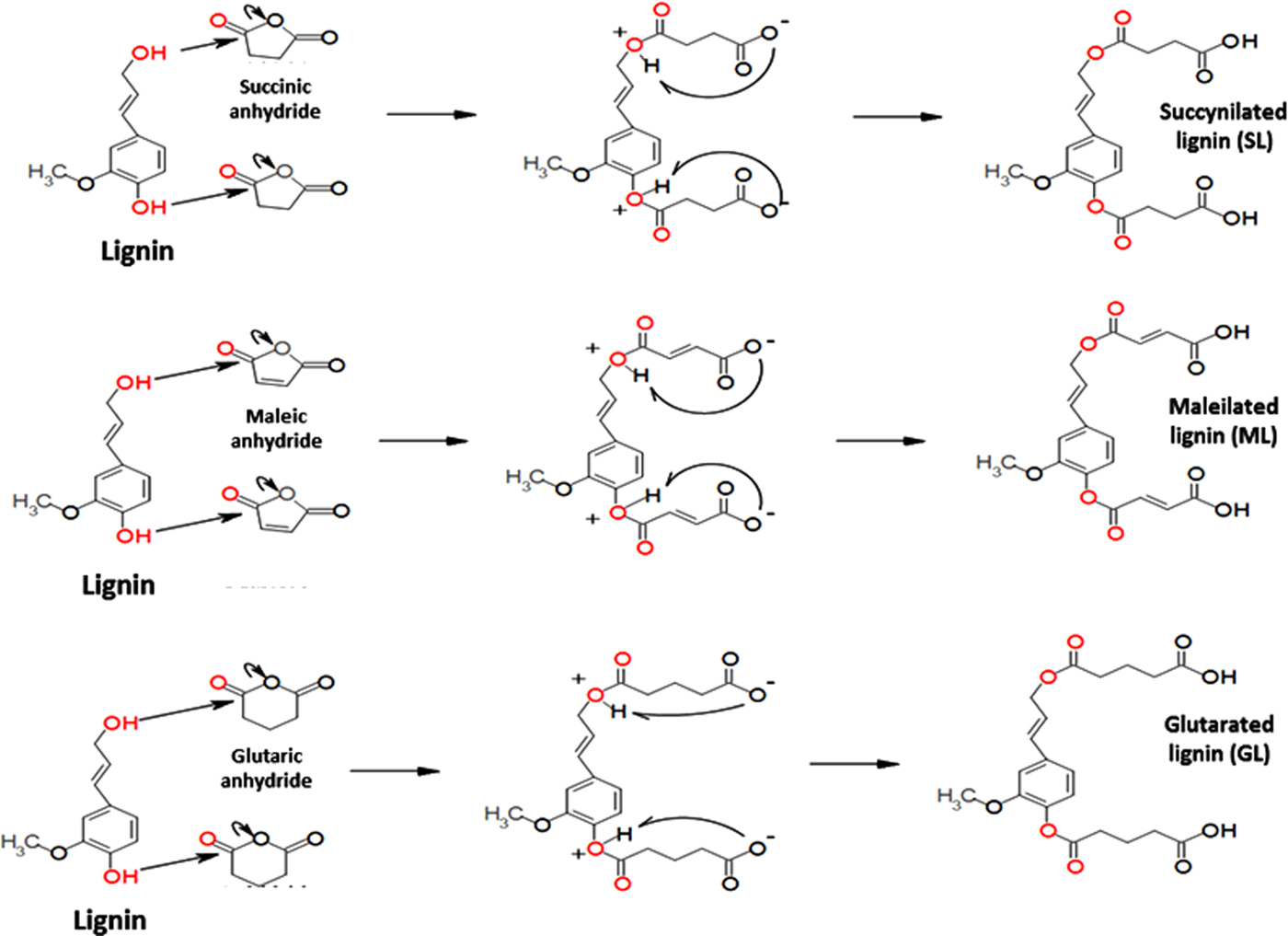
The proposed mechanism for lignin esterification is shown in Fig. 1. Two fundamental steps occur: (1) a nucleophilic attack by the hydroxyl groups (both phenolic and aliphatic), causing ring opening of the cyclic anhydride, and (2) the deprotonation of the hydroxyl groups for the formation of the novel carboxylic acid functional groups.
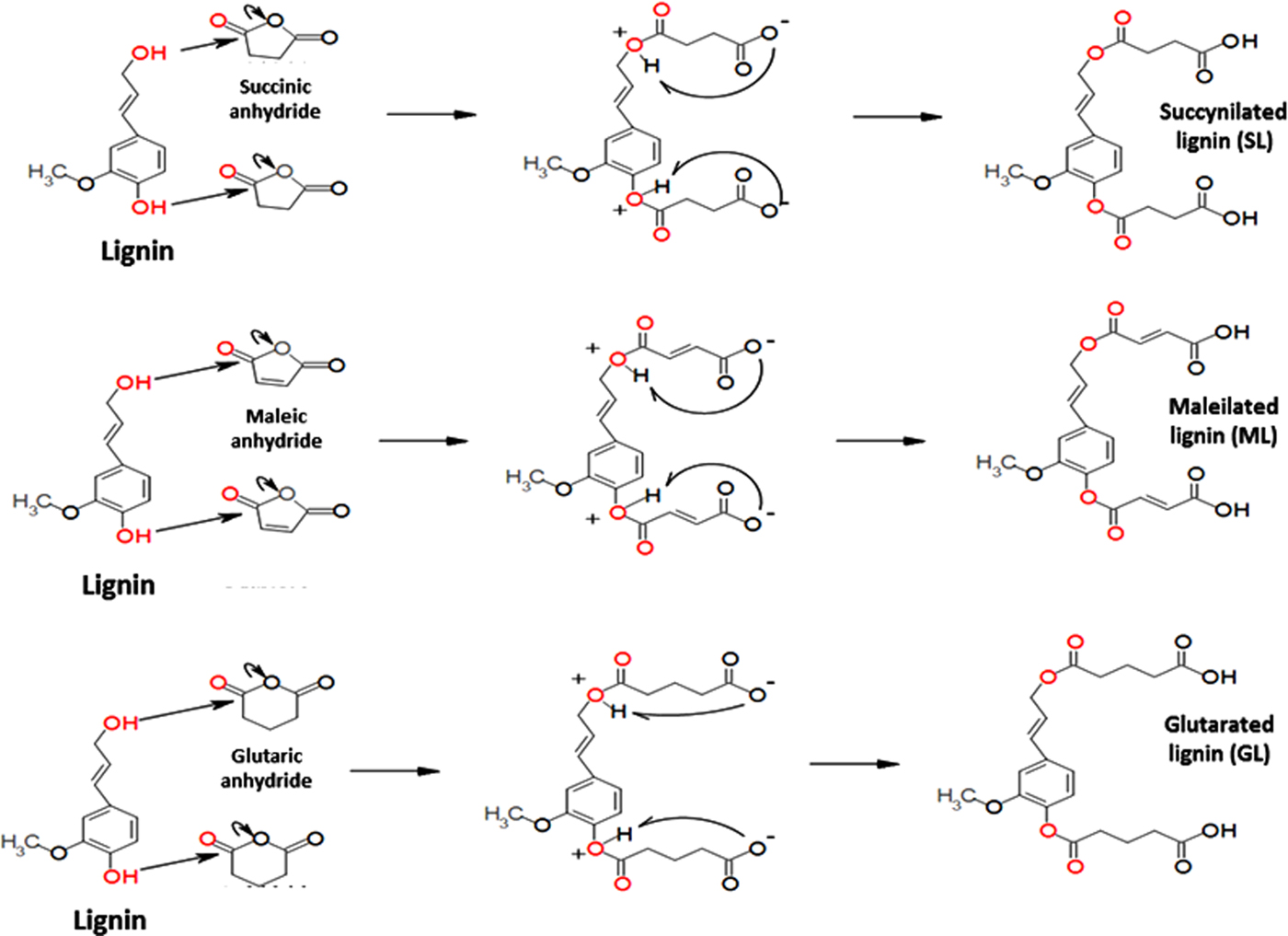
Fig. 1. Mechanism of lignin esterification with cyclic anhydrides.
The esterification reactions were carried out in a microwave oven (Panasonic, 1200 W). The reaction conditions are summarized in Table 1. Succinylated lignin (SL), maleilated lignin (ML), glutarated lignin (GL), high MW succinylated lignin (HSL), high MW maleilated lignin (HML) and high MW glutarated lignin (HGL) were obtained. In order to synthesize SL and HSL, the lignin and succinic anhydride were mixed in a glass reactor with 5 mL of an acetonitrile/ethanol (ACN/EtOH ratio, 4:1). The mixture was placed in an ultrasonic bath (Branson, CPX) for 5 min and then the reactants were subjected to microwave irradiation (60–80 s); 5 mL of 5% HCl solution (wt./v) was then added to precipitate the products, which were centrifuged (IEC, Centra CL2) for 15 min at 5000 rpm. The product was washed twice with distilled water to remove excess anhydride, centrifuged and finally dried in a vacuum oven (Cole-Parmer, EW-05053-10) at 50°C for 12 h (Delgado et al., Reference Delgado, Ysambertt, Bravo, Chávez and Márquez2015). In order to synthesize ML and HML as well as GL and HGL products the same procedure was followed, except for the fact that both reactions were carried out in solid phase (Delgado et al., Reference Delgado, Ysambertt, Chávez, Bravo, Márquez and Bullón2012).
Table 1. Reaction conditions for lignin esterification using microwave irradiation.
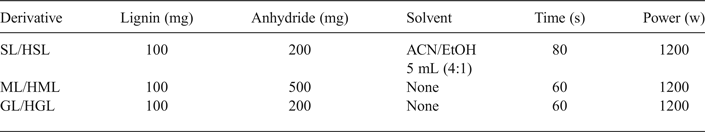
SL: succynilated lignin; HSL: High MW succynilated lignin; ML: maleilated lignin; HML: high MW maleilated lignin; GL: glutarated lignin; HGL: high MW glutarated lignin; ACN: acetonitrile; EtOH: ethanol.
After the chemical modification the lignin products were weighed to determine the increase in weight (WI %) according to the following equation (Gordobil et al., Reference Gordobil, Egüés and Labidi2016):
where m o is the initial weight of dried lignin sample (g) and m 1 is the weight of lignin after the esterification reaction (g).
Characterization of lignin and esterified lignin
Lignin and esterified derivatives were characterized by Fourier Transform Infrared Spectroscopy (FTIR, Shimadzu; 8400S) with IR Solution software. The FTIR spectra were collected between 4000 and 400 cm−1. A resolution of 4 cm−1 and 25 scans were used during spectra collection.
Preparation of bentonite suspensions
Aqueous bentonite suspensions were prepared by mixing either 50 mL of (1) a solution of lignin or (2) a solution of the corresponding derivatives (0–5000 mg/L) in 0.1 M NaOH with bentonite (2% wt/v). Bentonite was added gradually under constant stirring using a blender mixer (IKA-WERKE; Eurostar) at 150 rpm.
After bentonite addition, the suspensions were stirred at 500 rpm (10 min) for homogenization. In order to compare the dispersant properties of the synthesized derivatives, the commercial dispersants, as well as the high molecular weight lignin derivative solutions, were prepared. A dispersant concentration of 5000 mg/L, and a bentonite content of 7% (wt/v) were used. These conditions were defined by considering the concentration of components commonly used for drilling mud (Caenn et al., Reference Caenn, Darley and Gray2011).
Stability of bentonite suspensions
The stability of the suspensions was determined under static conditions by the volumetric separation method. The suspensions were taken to graduated tubes, which were maintained at 25°C. The volume of separated water was monitored every 24 hours over a period of 30 days. The experiments were carried out in triplicate and the average value was reported.
Viscosity and rheological behaviour of bentonite suspensions
A rheometer (Brookfield; LVDV-III+) was used. The relationship between the shear stress and the shear rate was evaluated. Viscosity was estimated using the consistency index described in the equation of the Power Law model (2), determined with the Rheocalc32 software.
where τ = shear stress, γ = shear rate, K = consistency index and n = flow index.
The experiments were carried out in triplicate and the average value was reported.
Dispersibility of bentonite suspensions
Dispersibility of lignin and lignin derivatives for bentonite suspensions was measured with modifications according to previous reports (Homma et al., Reference Homma, Kubo, Yamada, Koda, Matsushita and Uraki2010). The bentonite suspension (10 mL) was poured into a plastic cylinder (25 mm diameter, 25 mm height), the bottom of which was placed on a glass plate. After 60 s, the cylinder was removed to give the bentonite suspension spread on the glass. The final diameter of the bentonite suspension was measured. The experiment was repeated at least three times. The average value was used to calculate the flow value (3) (Nadif et al., Reference Nadif, Hunkeler and Käuper2002; Homma et al., Reference Homma, Kubo, Yamada, Koda, Matsushita and Uraki2010):
where Φfinal is the final diameter of bentonite suspension and Φinitial is the initial diameter (25 mm).
RESULTS AND DISCUSSION
Lignin esterification evidence
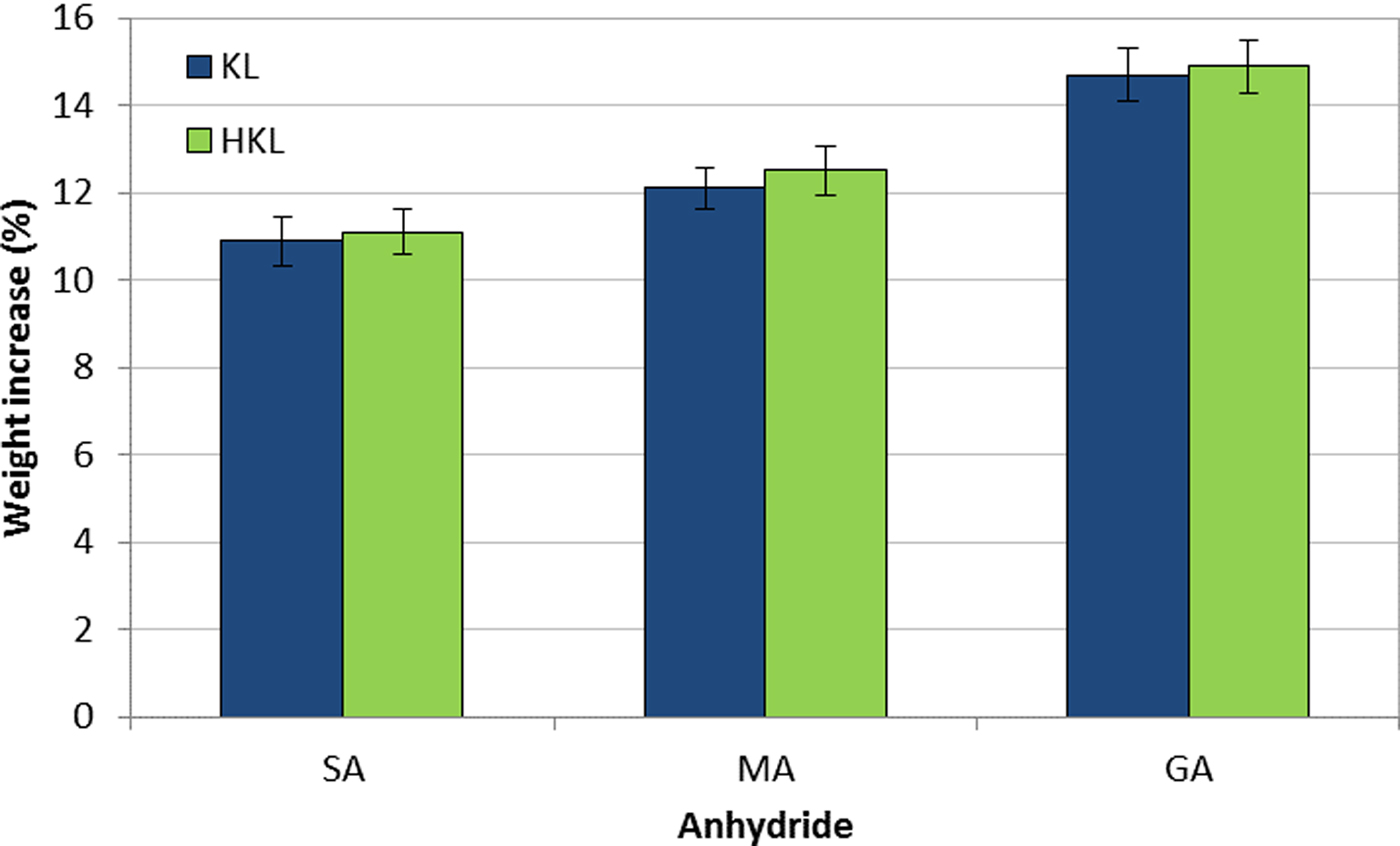
The yield of the lignin esterification reaction was evaluated using the weight increase (WI%) value, as an indication of the degree of conversion to esters of several macromolecules (Liu et al., Reference Liu, Sun, Qin, Zhang, Ren, Ye, Luo and Cao2008; Maldhure et al., Reference Maldhure, Chaudhari and Ekhe2011; Ratanakamnuana et al., Reference Ratanakamnuana, Atong and Aht-Ong2012; Gordobil et al., Reference Gordobil, Egüés and Labidi2016). The WI values which are shown in Fig. 2 are in accord with those reported in previous works (Delgado et al., Reference Delgado, Ysambertt, Chávez, Bravo, Márquez and Bullón2012, Reference Delgado, Ysambertt, Padilla, Chávez, Bravo and Márquez2014, Reference Delgado, Ysambertt, Bravo, Chávez and Márquez2015).
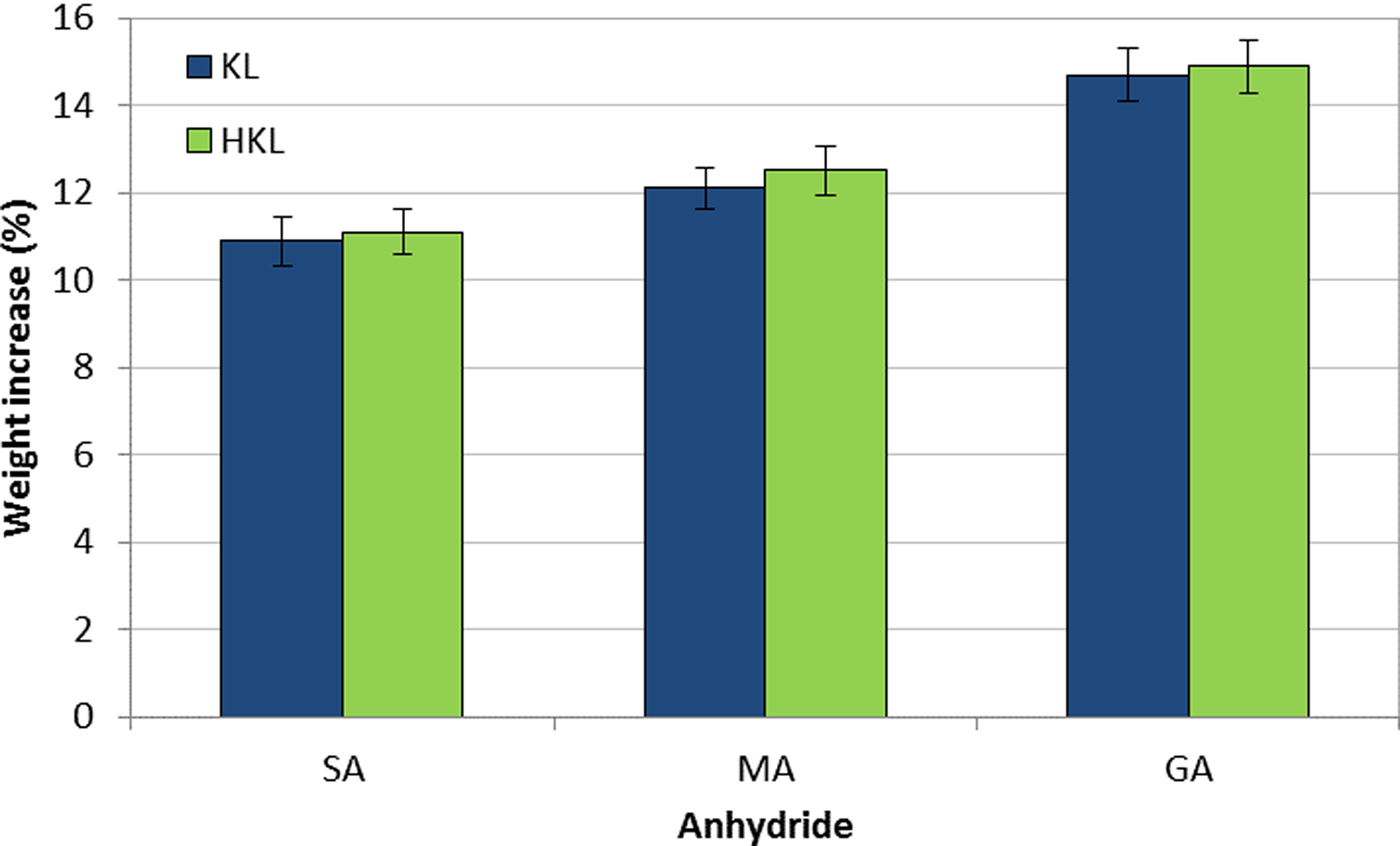
Fig. 2. Weight increase (WI %) of KL and HKL upon esterification (SA: succinic anhydride, MA: maleic anhydride, GA: glutaric anhydride).
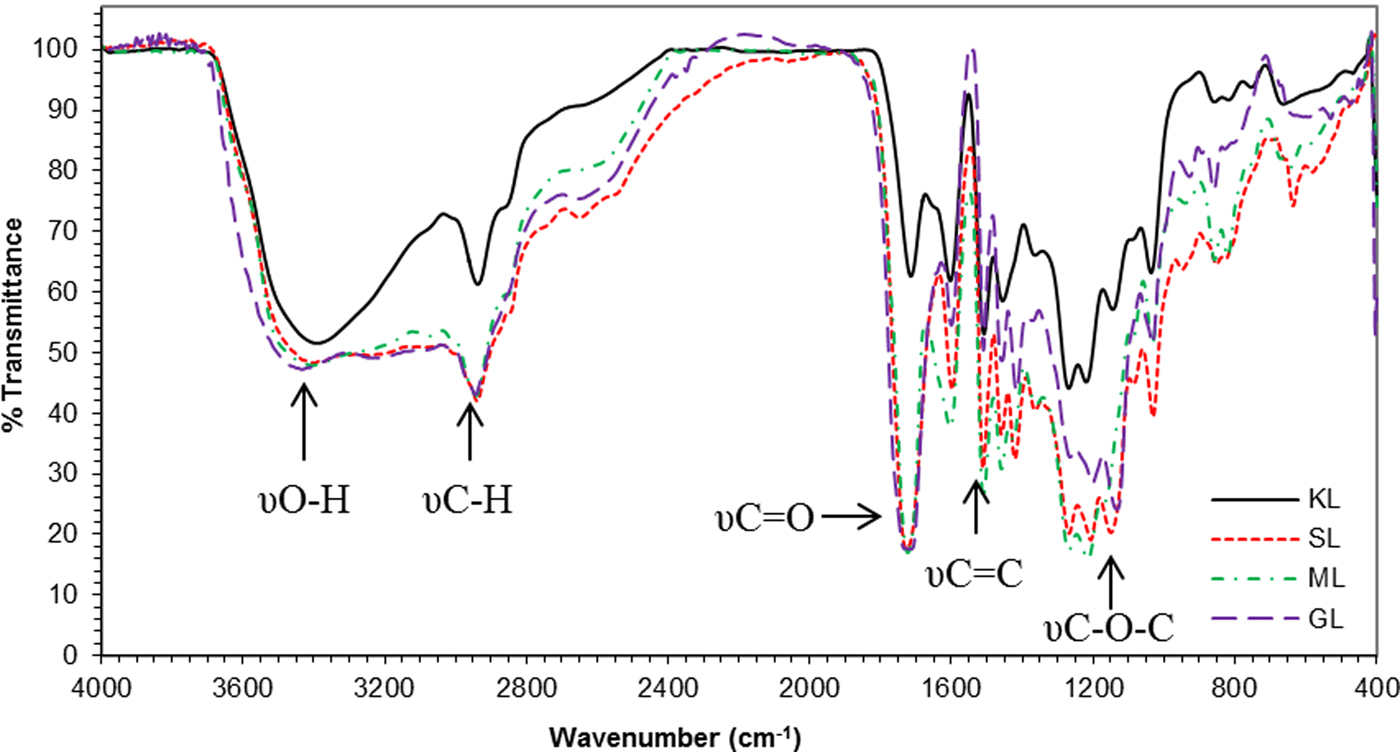
The esterification of KL was confirmed by FTIR spectroscopy (Fig. 3). The FTIR spectra of the neat KL, and the KL derivatives show the typical signals described in similar studies (Nada et al., Reference Nada, El-Sakhawy and Kamel1998; Rana et al., Reference Rana, Langenfeld-Heyser, Finkeldey and Polle2010; Toledano et al., Reference Toledano, Erdocia, Serrano and Labidi2013). A broad band at ~3400 cm−1 (hydroxyl groups stretching), as well as the bands appearing at 2941 and 2846 cm−1 (C-H stretching in methyl and methylene groups), were observed. In addition, the bands at 1595, 1510 and 1427 cm−1 are attributed to the vibrations of the aromatic skeleton, and the bands between 1000 and 1250 cm−1 are assigned to the C–O stretching of methoxyl groups and of alcohols.
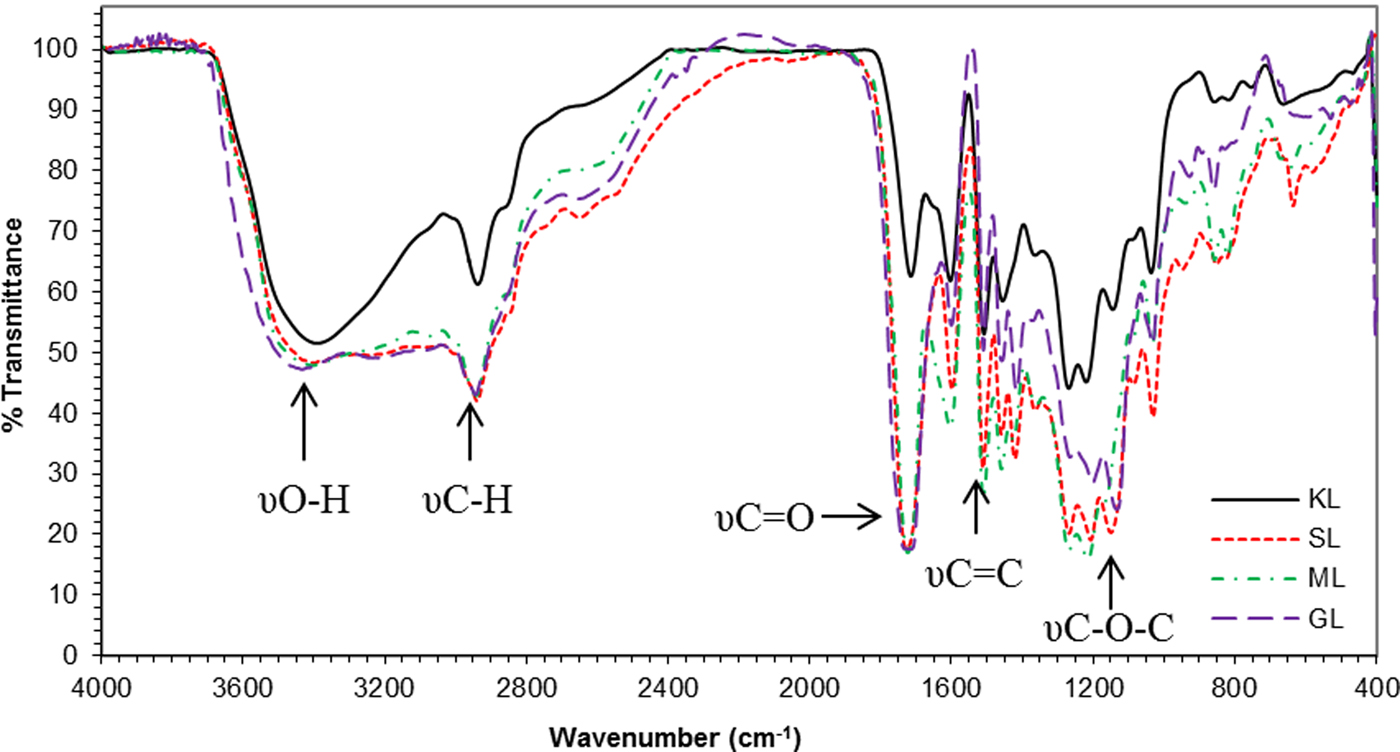
Fig. 3. FTIR spectra of KL and KL derivatives (SL: succinated lignin, ML: maleilated lignin, GL: glutarated lignin).
The KL also shows a typical band (1700 cm−1) attributed to carbonyl groups, commonly associated with the acid precipitation process used for the isolation of the lignin from the black liquor. Such apparent degradation of lignin has been also reported by Boeriu et al. (Reference Boeriu, Bravo, Gosselink and Van Dam2004) as a consequence of the oxidation.
The spectra of the esterified KL-based products (SL, ML and GL) show a sharp band at ~1725 cm−1, corresponding to carbonyl groups. The signals of the ester groups (1750 cm−1), and carboxylic acids (1712 cm−1) overlap. The intensity of the aforementioned band is greater than that of the KL. Such spectral differences confirm that esterification was successful (Xiao et al., Reference Xiao, Sun and Sun2001; Thielemans & Wool, Reference Thielemans and Wool2005; Maldhure et al., Reference Maldhure, Chaudhari and Ekhe2011). The increase in absorption at ~1250–1100 cm−1 (stretching of the C–O bond of esters and carboxylic acids), as well as a slight change in the aliphatic CH tension signal intensity (2950–2870 cm−1), and the flexural C–H vibration (1460 cm−1) are additional insights into the lignin esterification.
The results are in accord with Xiao et al. (Reference Xiao, Sun and Sun2001), who performed the chemical modification of lignin with SA using conventional heating (1–12 h), and with Maldhure et al. (Reference Maldhure, Chaudhari and Ekhe2011), who achieved the esterification of lignin with MA using microwaves in short reaction times (20 min). The modified products showed similar structural features as shown by FTIR. However, the use of microwaves allowed the preparation of esters from lignin in a single step in very short reaction times (60–80 s).
Dispersing properties of lignin and esterified lignin
The stability, dispersibility and rheological properties of aqueous bentonite (2 wt. %/v) were evaluated by varying the concentration of the dispersants (lignin and esterified lignin) between 0 and 5000 mg L−1.
Stability
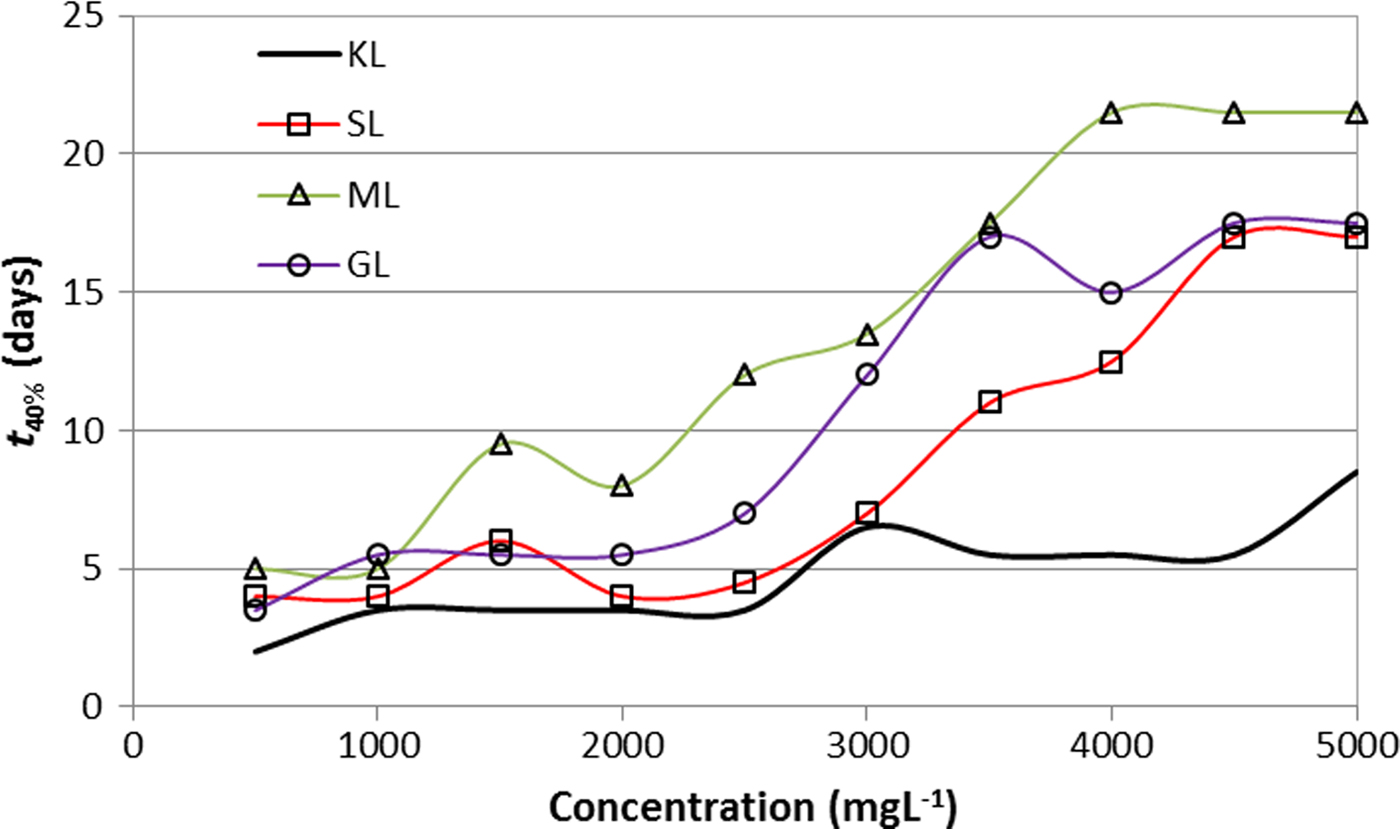
The stability was evaluated taking into account the time for separating 40% of the total volume of the suspension (t 40%). Variation in t 40% as a function of KL concentration and of the esterified derivative is shown in Fig. 4. The esterified derivatives-based suspension took longer to separate than those in which KL was used, indicating that derivatives have better dispersing properties of bentonite than the unmodified lignin. The result is in accord with previous works where greater surface activity of esterified lignins was reported (Delgado et al., Reference Delgado, Ysambertt, Chávez, Bravo, Márquez and Bullón2012, Reference Delgado, Ysambertt, Padilla, Chávez, Bravo and Márquez2014).
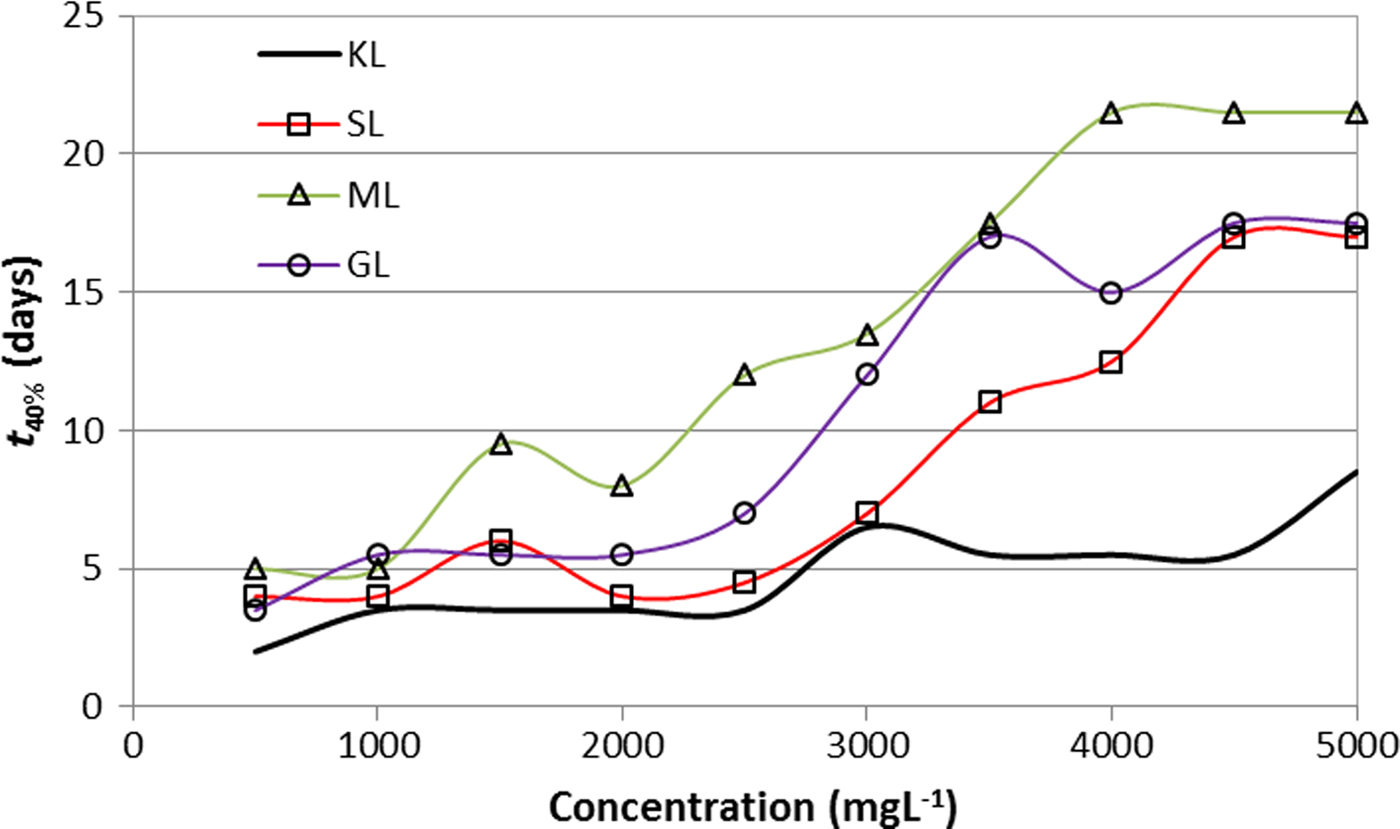
Fig. 4. Stability of aqueous suspensions of bentonite as a function of the concentration of KL and esterified derivatives (2 wt. %/v bentonite; T = 25°C) t 40%: time for separating 40% of the total volume of the suspension.
Stable suspensions were prepared with ML as a dispersant, apparently due to the unsaturated chain. The α,β-unsaturated carboxylate group confers a rigid constraint in comparison to the rest of the derivatives. Some evidence suggests that structural constraints of surface-level arrangements stabilize or destabilize the dispersing system. Similar results were obtained by Zürcher & Graule (Reference Zürcher and Graule2005) who compared the dispersing properties of palmitic acid, stearic acid and oleic acid. Dispersions with oleic acid showed lower viscosity compared to the other two fatty acids listed. This difference, despite the similarity in the chain length, is probably due to the presence of the double bond in the oleic acid chain, which increases the polarizability of the molecule.
Rheological behaviour
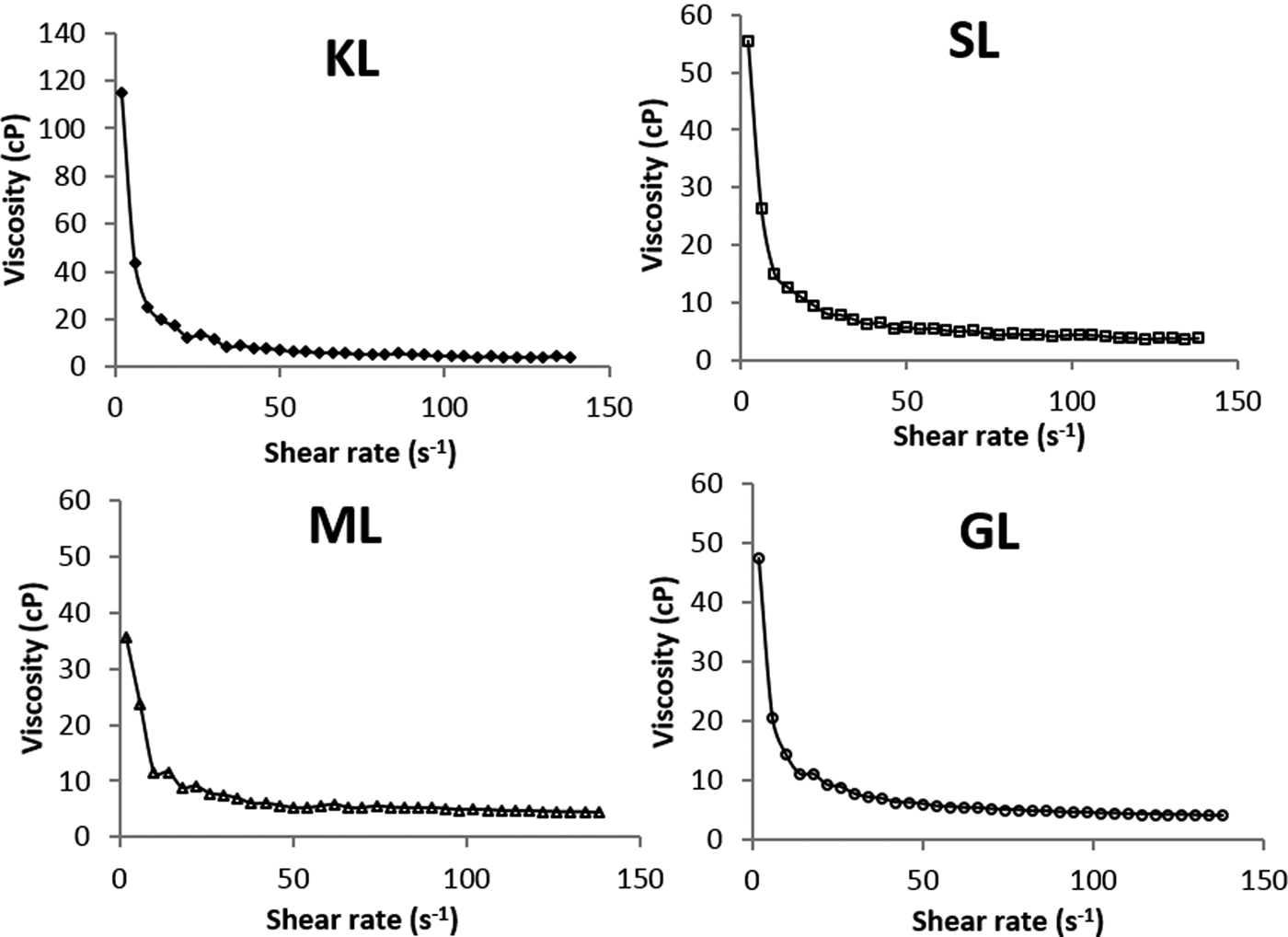
The viscosity of bentonite suspensions decreases with increasing shear rate for KL- and esterified derivatives (Fig. 5), suggesting that the bentonite suspensions behave as non-Newtonian fluids (pseudoplastic type). This is because the derivatives-based suspensions show lower viscosity than suspensions prepared with unmodified lignin.
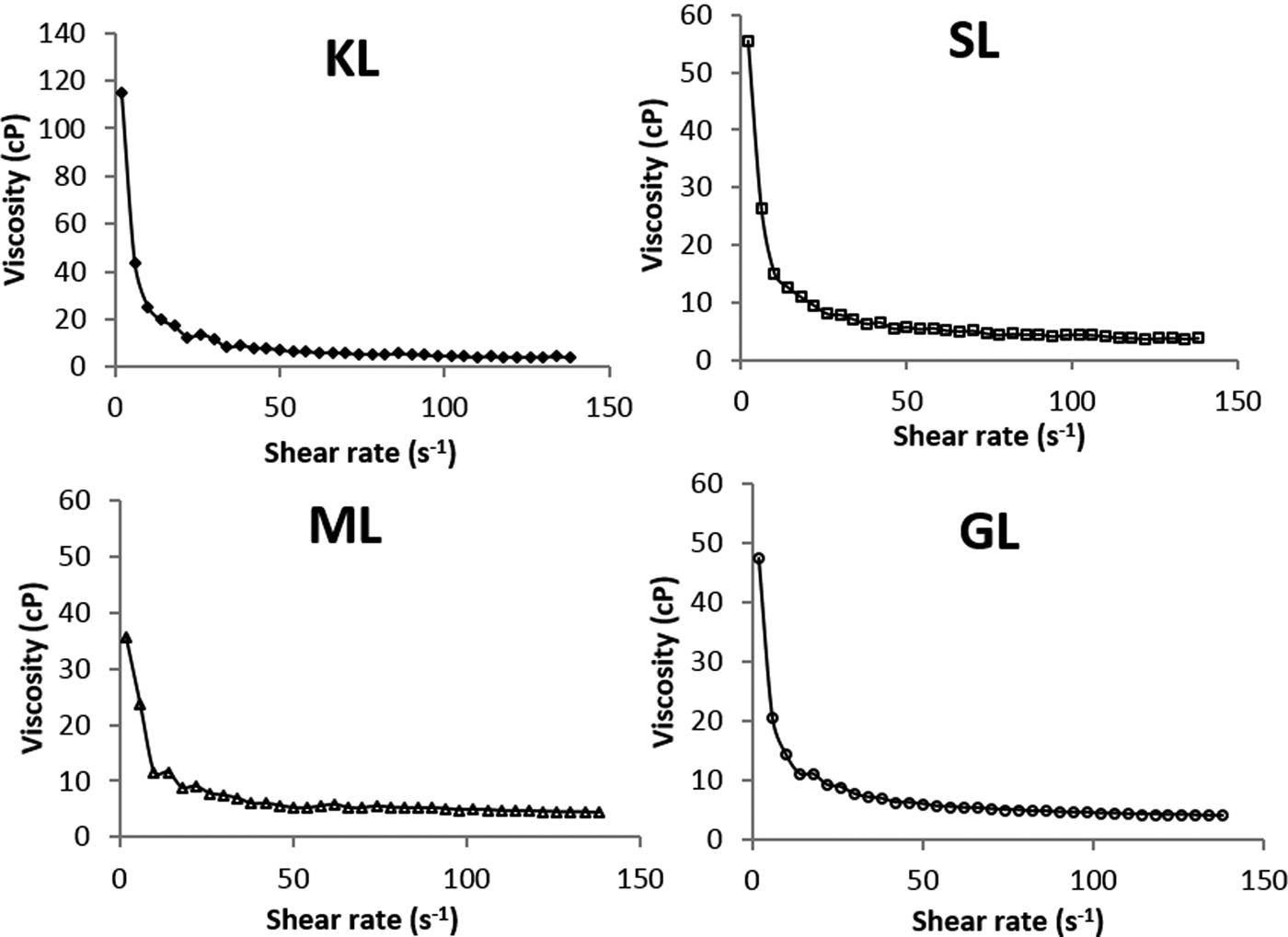
Fig. 5. Variation of viscosity for aqueous bentonite formulated with KL and esterified derivatives (2 wt. %/v of bentonite; 5000 mg L−1 of KL or KL-based derivatives; T = 25°C).
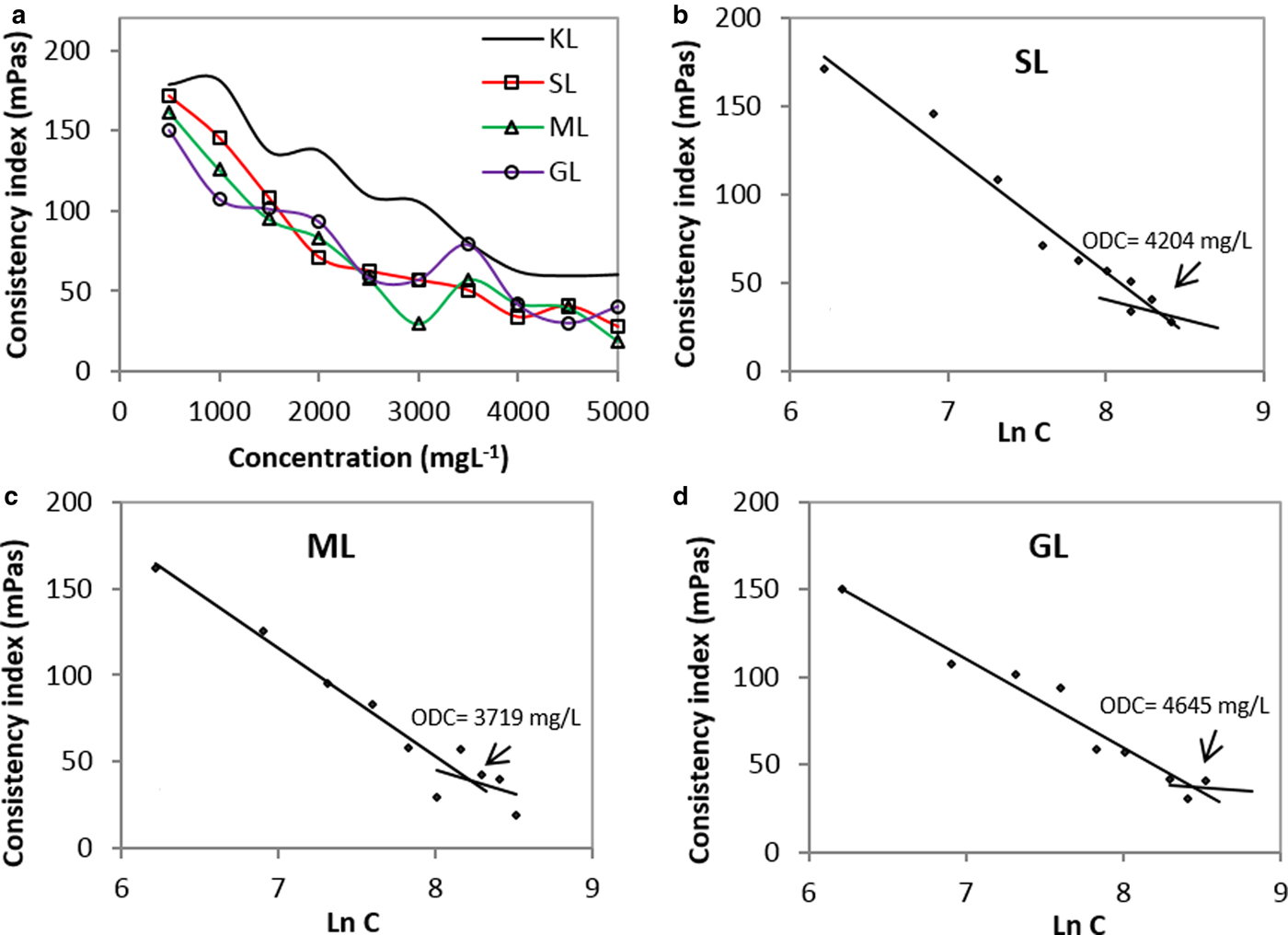
The consistency index decreases as the concentration of the dispersants increases, as shown in Fig. 6a. The results suggest that both KL and the esterified derivatives show bentonite-dispersing properties. Also, the viscosity of the derivatives-based suspensions was lowest. In addition, the stability trends confirm that the esterified derivatives are better dispersants than the unmodified lignin.
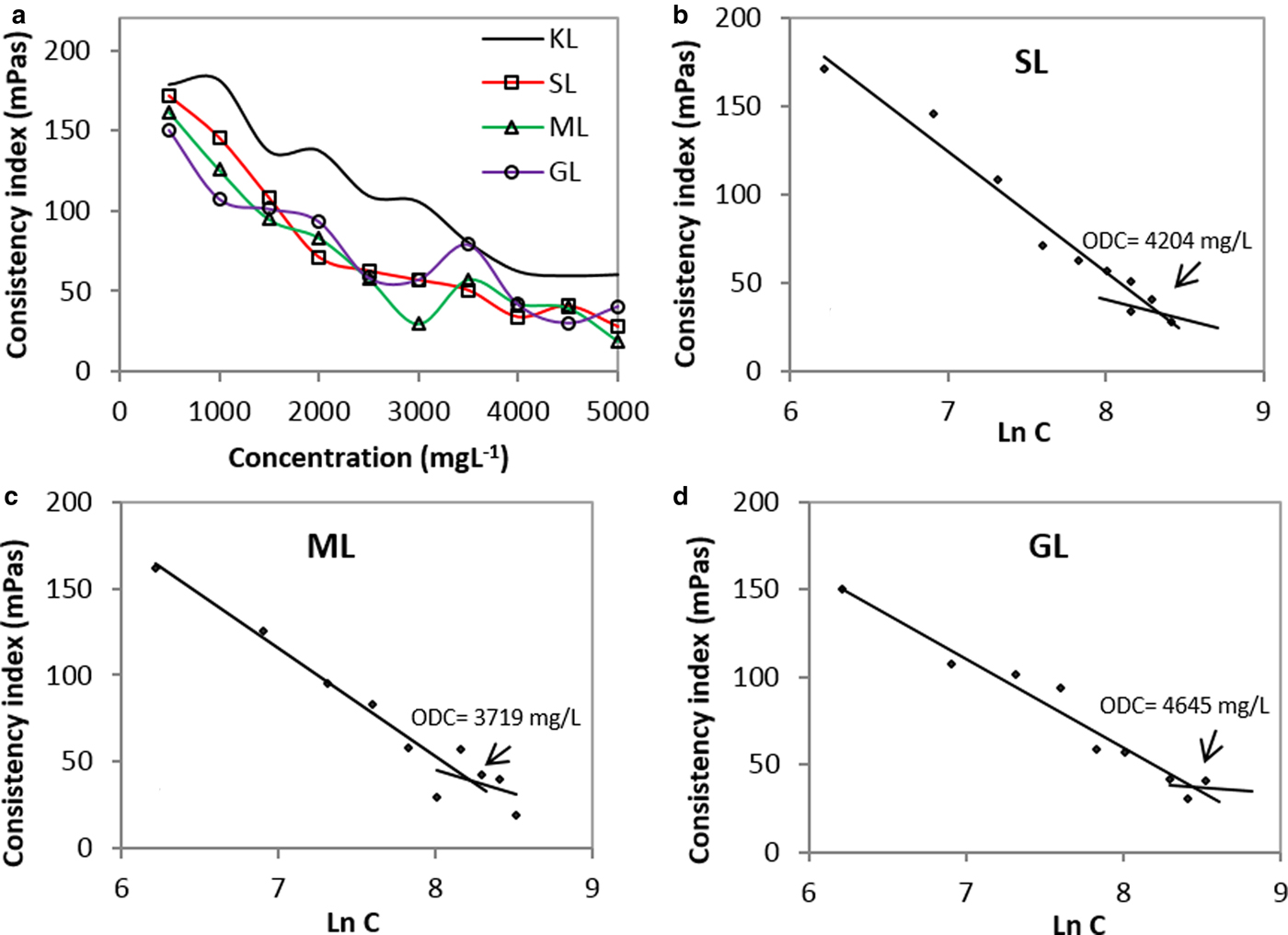
Fig. 6. Consistency index as a function of concentration for: (a) KL and the esterified derivatives; (b) SL; (c) ML; (d) GL (2 wt.%/v of bentonite; T = 25°C).
The surface tension as a function of the concentration is illustrated in Fig. 6a. In order to linearize the trend, the data were transformed by a logarithmic model. The variation of the consistency index is shown in Fig. 6(b–d). The results allow the approximate point at which the viscosity reduction trend tends to be constant (optimum dispersion concentration, ODC) to be determined. Such a value corresponds to: (1) the point at which the surface of the bentonite particles is saturated with the dispersant; and (2) the concentration at which the dispersant shows maximum effectiveness. The ML derivative displays minimum ODC, indicating that a smaller amount of ML dispersant would be necessary to achieve comparable viscosity by decreasing the SL- and GL-based dispersants.
Dispersibility
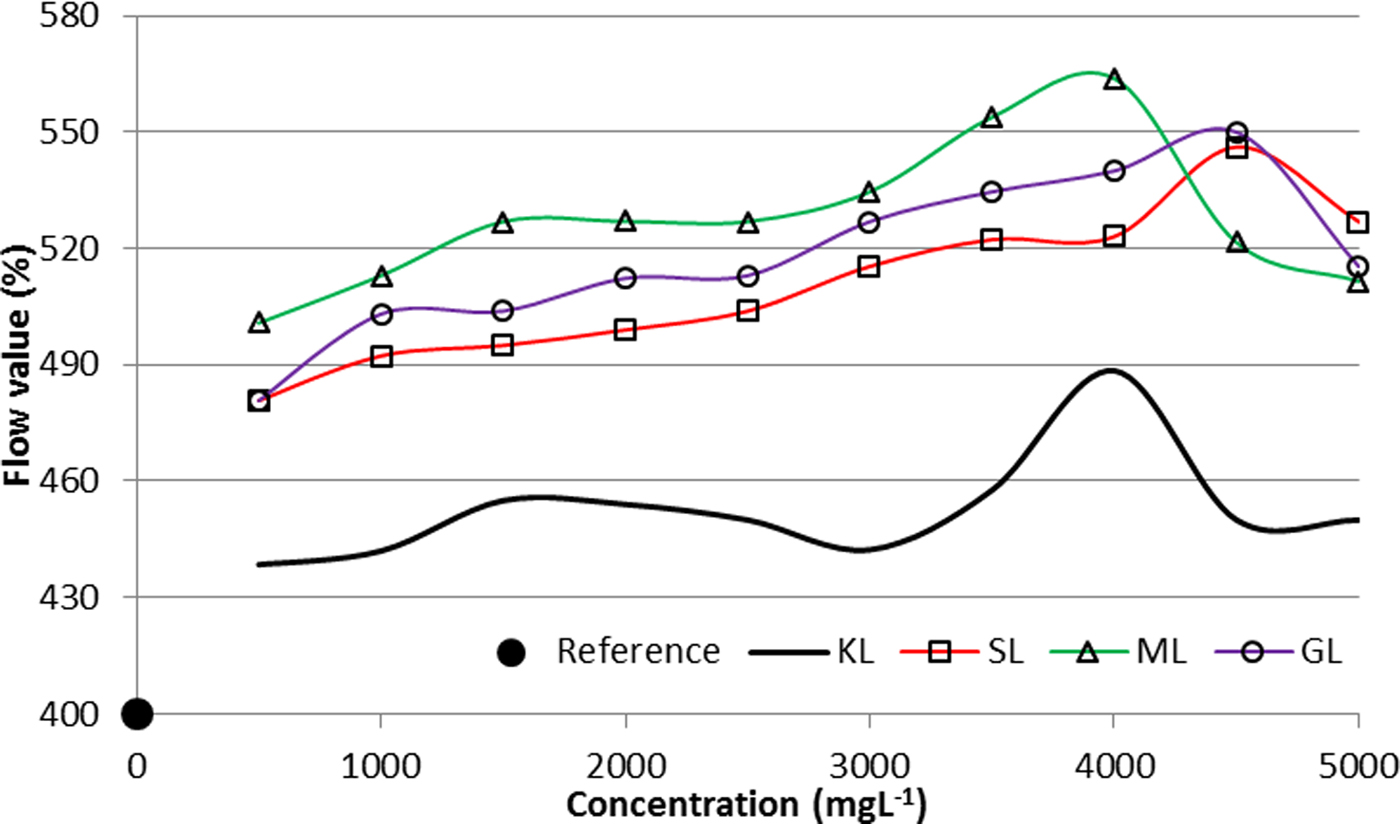
The variation of the flow value as a function of the concentration is shown in Fig. 7. In addition, the flow value of the referential suspension prepared without any dispersant is provided.
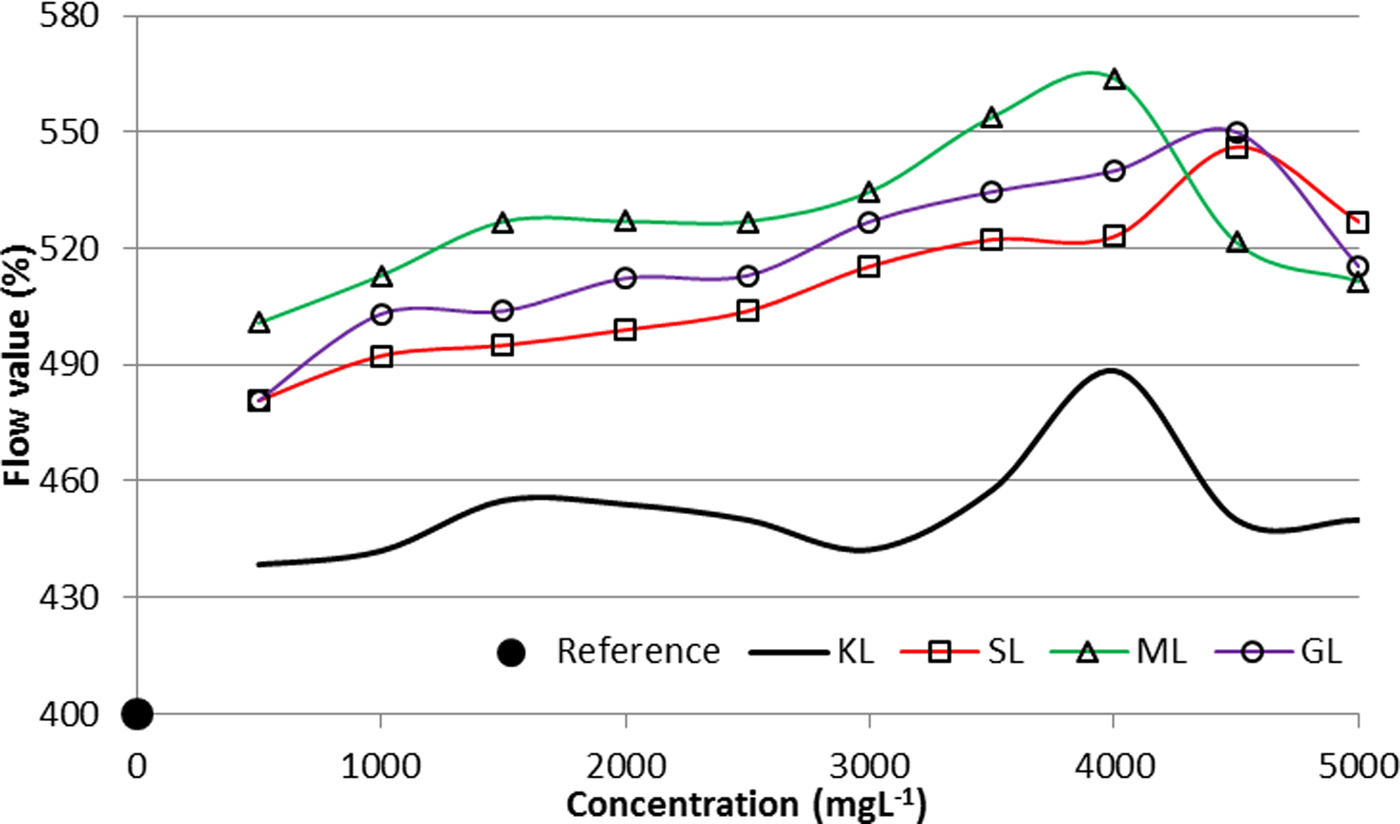
Fig. 7. Dispersibility of aqueous suspensions of bentonite prepared with KL and the corresponding esterified derivatives (2 wt.%/v of bentonite; T = 25°C).
The maximum flow value is close to 4500 mg L−1, 4000 mg L−1 and ~4500 mg L−1 for SL, ML and GL, respectively, confirming that at the aforementioned concentrations the derivatives show a greater dispersing capacity. Although the ODC was calculated from a mathematical model, the results show a good correlation with the experimentally calculated dispersibility.
The suspensions prepared with the esterified derivatives have better dispersing properties than the unmodified lignin. The ML derivative induces the most stable and least viscous suspensions, with a greater dispersibility. Both lignin and the esterified derivatives provide bentonite dispersing properties considering the flow value of the referential suspensions.
The double bond from the carboxylic chain of the derivatives appears to have a favourable effect on the dispersing properties, as the ML-based derivatives show a more stabilizing effect of the suspensions (lower ODC).
Comparison of the dispersing properties
It is well known that high-molecular-weight lignin and lignosulfonates show better dispersing properties than their corresponding unfractionated counterparts (Zhou et al., Reference Zhou, Qiu, Yang, Lou and Ouyang2007; Yang et al., Reference Yang, Qiu, Zhou and Lou2007; Li & Ge, Reference Li and Ge2011). Therefore, the dispersing results were compared with those obtained using a high-molar-weight lignin fraction (HKL) and the corresponding esterified derivatives.
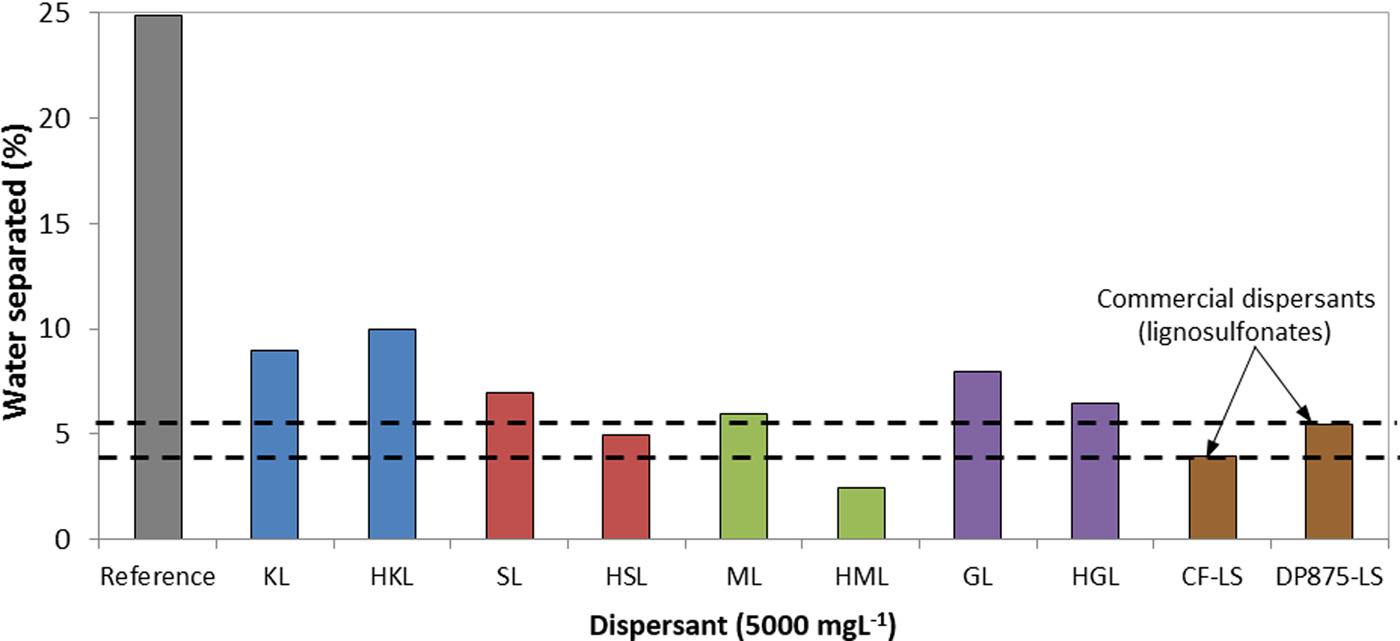
The HKL-based suspensions were more stable than those obtained using unfractionated derivatives. In addition, only the high-molecular-weight derivatives showed stability results comparable to those obtained using the commercial dispersants. In fact, the HML-based suspensions were highly stable in comparison to the commercial dispersants (Fig. 8).
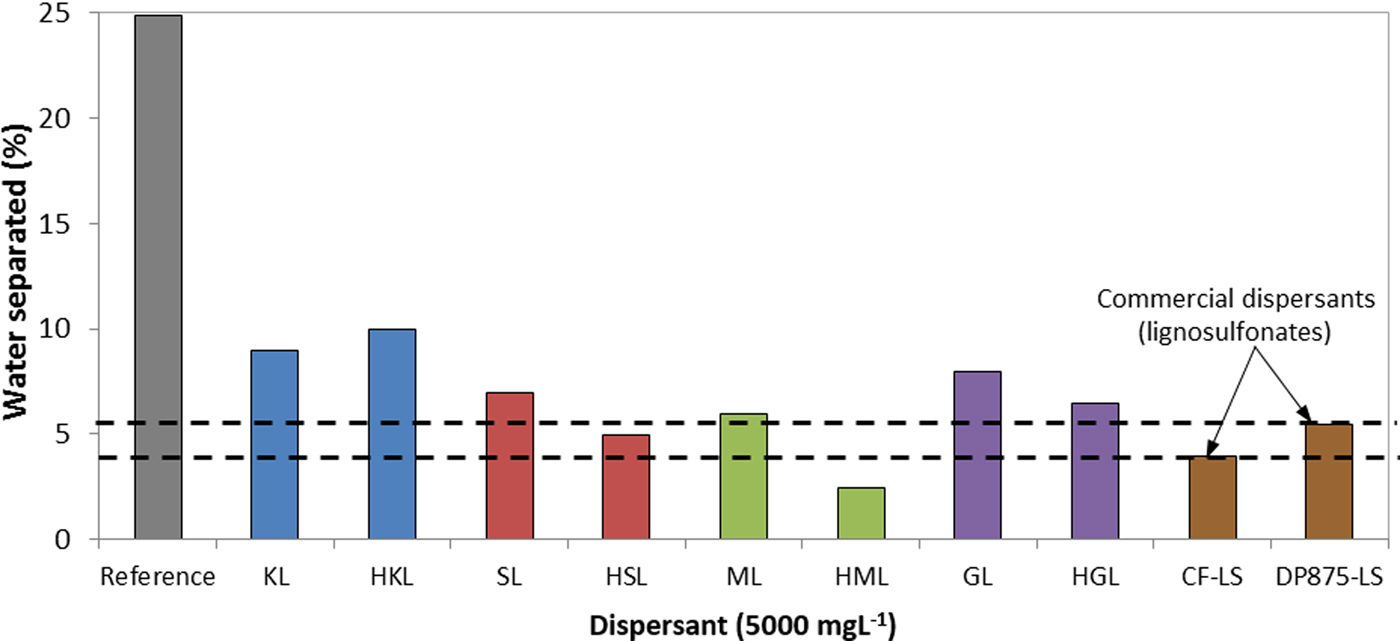
Fig. 8. Percentage of separated aqueous phase in bentonite suspensions prepared with several dispersants (7 wt.%/v of bentonite, t = 30 days, T = 25 °C) CF-LS: commercial sodium lignosulfonate, DP875-LS: commercial ammonium lignosulfonate.
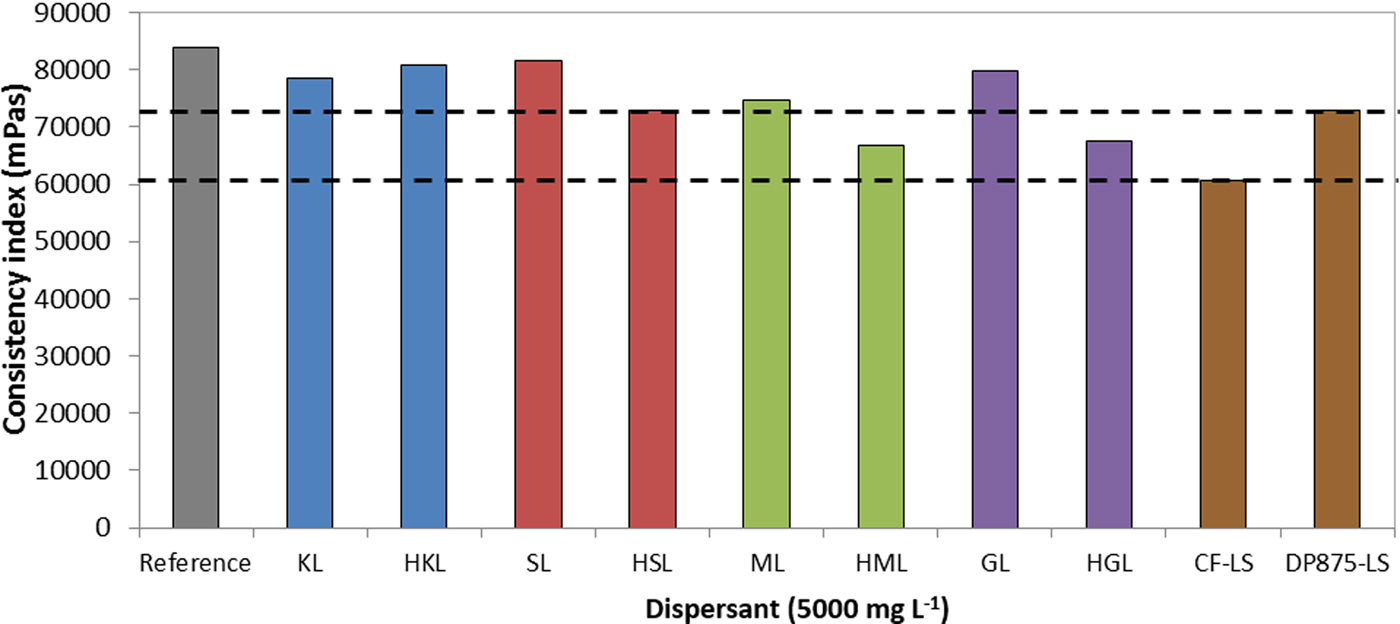
Suspensions formulated with high-molecular-weight polyphenol-based dispersants were less viscous than those formulated with the unfractionated counterparts (Fig. 9). This is in accordance with the finding about stability. In addition, the viscosity of the HML- and HGL-based systems is lower than that of the DP875-LS reference, and slightly higher than that of the CF-LS counterpart.
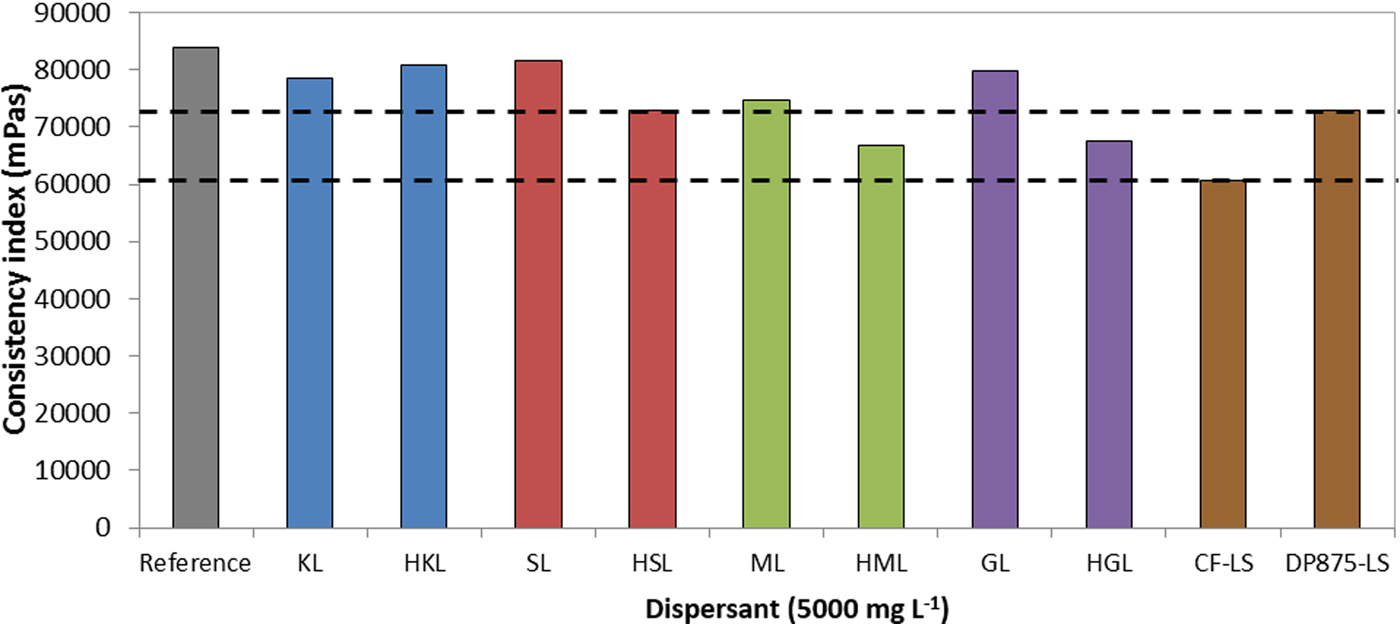
Fig. 9. Consistency index of bentonite suspensions prepared with different dispersants (7 wt.%/v of bentonite; T = 25°C) CF-LS: commercial sodium lignosulfonate, DP875-LS: commercial ammonium lignosulfonate.
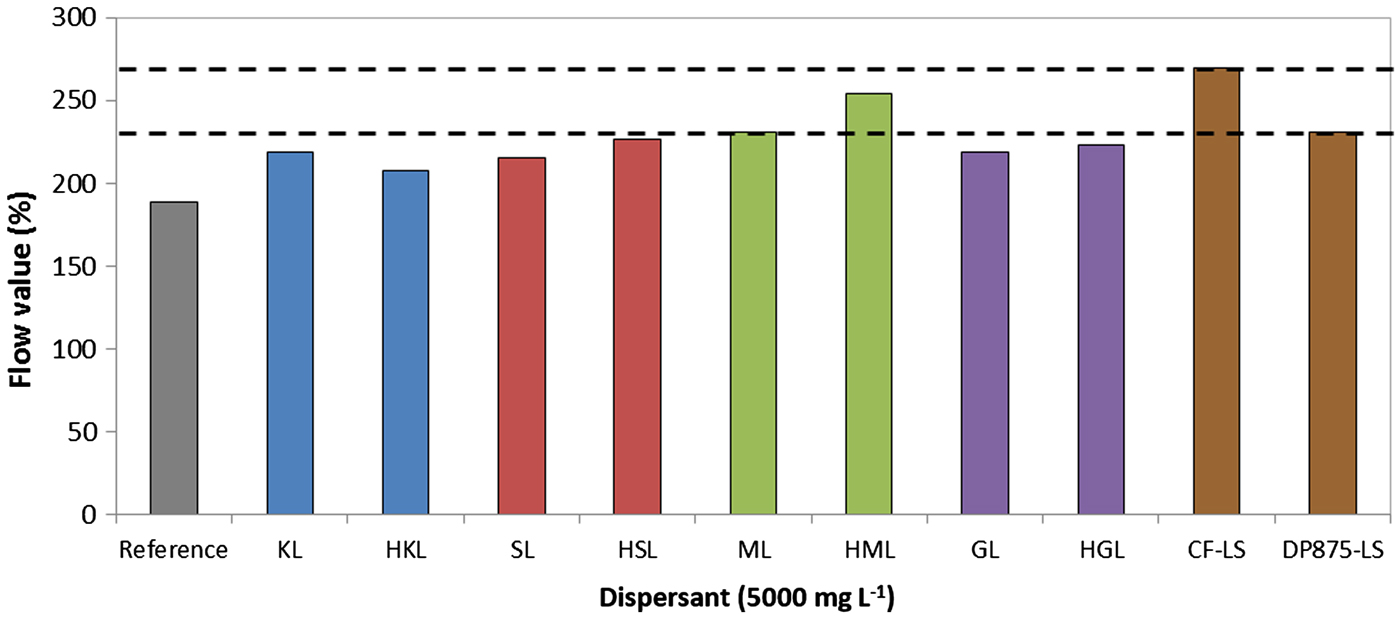
All systems formulated with high-molecular-weight lignin fraction are more fluid than their unfractionated counterparts (Fig. 10). The fluidity of the systems formulated with dispersants of high molar weight is comparable to that of the DP875-LS dispersant, but slightly lower than that of the CF-LS dispersant.
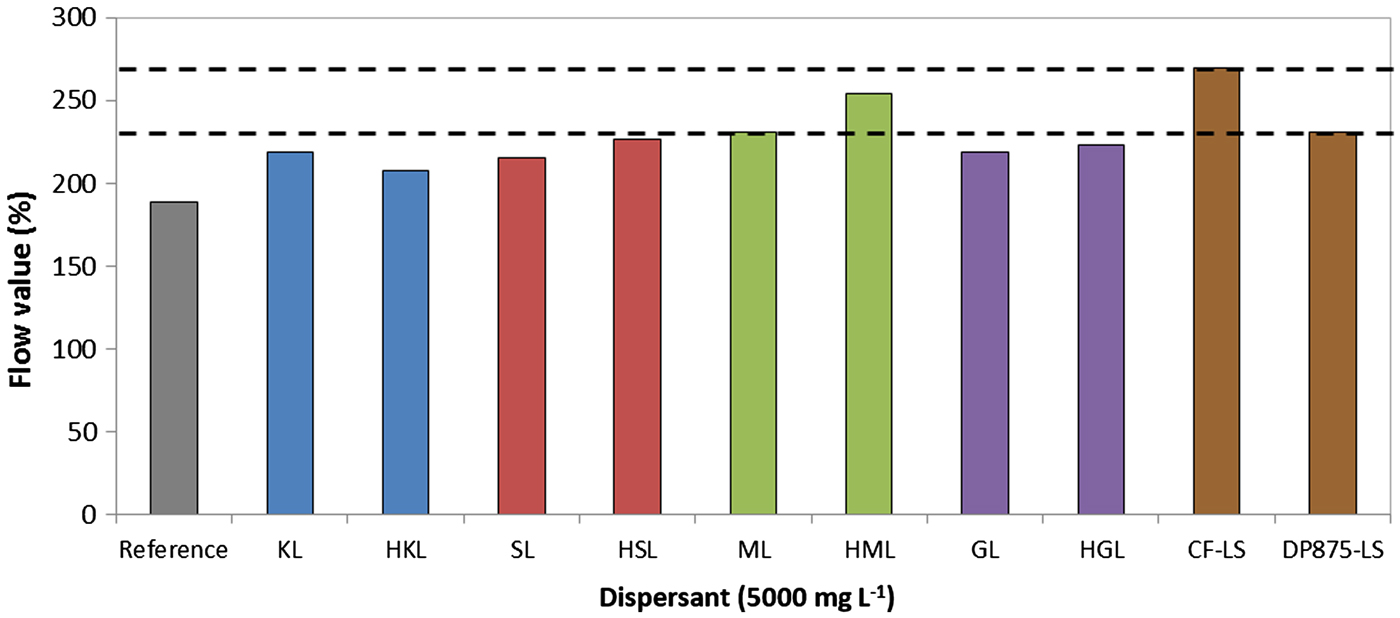
Fig. 10. Dispersibility of bentonite suspensions prepared with several dispersants (7 wt.%/v of bentonite; T = 25°C) CF-LS: commercial sodium lignosulfonate, DP875-LS: commercial ammonium lignosulfonate.
In general, the high-molecular-weight esterified derivatives possess better dispersing properties than their unmodified counterparts. This result may be explained by the effect of steric hindrance on the stabilization of the suspensions. In fact, the high-molecular-weight derivatives appear to prevent flocculation/aggregation due to the molecular size.
On the other hand, the HML derivative shows the most stable and least viscous suspensions. Finally, the effectiveness of the high-molecular-weight dispersants was similar to the commercial products (DP875-LS, CF-LS). These results indicate that esterification is a viable alternative to be used in tailoring the dispersing properties of lignin for the formulation of bentonite drilling muds in the oil industry.
CONCLUSIONS
Lignin and high-molecular-weight fractions of lignin were modified with cyclic anhydrides under microwave radiation. The esterification was proven by the increase in mass, as well as by FTIR spectroscopy. The lignin esterified derivatives synthesized show better dispersing properties of bentonite than unmodified lignin. The ML-based derivative exhibited better dispersant properties in terms of the higher stabilizing capacity of suspensions, and the lower ODC. Structural features may be the main reason for this remarkable behaviour.
The high-molecular-weight fractionated lignin derivatives were more efficient dispersants than the non-fractioned lignin derivatives fraction. The high dispersity of such natural polyphenol affects significantly the molecular properties of the resulting bio-based dispersant.
The fractionation and esterification of pine lignin may contribute to the design of bio-based chemicals for drilling-mud technologies.
ACKNOWLEDGEMENTS
The authors are grateful to “Proyecto Basal PFB-27”, Technological Develop Unit (UDT), Concepción University, Chile. The Laboratory of Mixing, Separation and Industrial Synthesis (LMSSI) of the Faculty of Engineering, University of Los Andes (ULA), Mérida-Venezuela, especially Johnny Bullón, is acknowledged for technical support (tangential UF equipment).