INTRODUCTION
Haemogregarines (Apicomplexa: Adeleorina) are parasites of the circulating blood cells of both ectothermic and endothermic vertebrates, found in a wide range of environments. They are grouped into several haemogregarine genera, but those that occur in reptiles include Haemogregarina Danilewsky, 1885, Karyolysus Labbé, 1894, Hepatozoon Miller, 1908, and Hemolivia Petit et al. Reference Petit, Landau, Baccam and Lainson1990 (see Telford, Reference Telford2009). The cladistic review of haemogregarines presented by Siddall (Reference Siddall1995) indicated that all species infecting chelonians fall within the single genus Haemogregarina (senus stricto) and are leech transmitted. For example, when the terrapin Emys orbicularis (Linnaeus, 1758), is bitten by the leech Placobdella catenigera Blanchard, 1893, parasitized by Haemogregarina stepanowi Danilewsky, 1885, the terrapin presents with secondary erythrocytic meronts, along with gamonts in the peripheral blood (see Telford, Reference Telford2009). However, in more recent taxonomic evaluations haemogregarine species infecting chelonians have been separated into two genera, Haemogregarina and Hemolivia (see Cook et al. Reference Cook, Smit and Davies2009; Telford, Reference Telford2009).
Currently, the only known life cycle for a land tortoise haemogregarine is that of Hemolivia mauritanica (Sergent and Sergent, Reference Sergent and Sergent1904) (see Landau and Paperna, Reference Landau and Paperna1997). First described as Haemogregarina mauritanica Sergent and Sergent, Reference Sergent and Sergent1904, this organism was renamed Hepatozoon mauritanicum (Sergent and Sergent, Reference Sergent and Sergent1904) by Michel (Reference Michel1973) following his re-examination of the material of Brumpt (Reference Brumpt1938), before being assigned its current name by Landau and Paperna (Reference Landau and Paperna1997). Hemolivia mauritanica has been proven experimentally to have a definitive host tick vector and tortoise intermediate hosts, namely two Palaearctic species Testudo graeca Linnaeus, 1758 and Testudo marginata Schoepff, 1792 (see Brumpt, Reference Brumpt1938; Michel, Reference Michel1973; Landau and Paperna, Reference Landau and Paperna1997; Široký et al. 2007). Formation of oocysts occurs within the tick, Hyalomma aegyptium Linnaeus, 1758, and sporogony within the tick intestinal cells leads eventually to the formation of sporocysts containing sporozoites (Široký et al. 2007). Ingestion of the tick by a tortoise is considered the transmission mechanism for this protozoan (see Široký et al. 2004, 2007). Following ingestion, meronts occur in cells of the reticulo-endothelial system and in erythrocytes, particularly in tissues, while cystic forms exist in parenchymatous organs such as kidney, spleen and liver. Gamonts occur in erythrocytes and on maturity develop stain-resistant capsules (Široký et al. Reference Široký, Kamler, Frye, Fictum and Modrý2007).
South Africa has the greatest number of species of land tortoises in the world (Branch, Reference Branch2008). Cook et al. (Reference Cook, Smit and Davies2009) found the haemogregarine, Haemogregarina (sensu lato) fitzsimonsi Dias, Reference Dias1953 (Adeleorina: Haemogregarinidae), the most prevalent species of tortoise haematozoan in South Africa. Blood films from 154 wild and captive tortoises, of five species, across four South African provinces, revealed ∼31% of tortoises to be parasitized by trophozoites, possible dividing forms, and immature and mature gamont stages of this haemogregarine. Cook et al. (Reference Cook, Smit and Davies2009) observed a lack of leeches on tortoises, but the presence of two tick species, Amblyomma marmoreum Koch, 1844 and Amblyomma sylvaticum (de Geer, 1778), led to the hypothesis that the arthropods could be potential vectors of H. fitzsimonsi in light of the findings of Široký et al. (2004, 2007). The association of the ticks with tortoises, and the high prevalence of parasitism among tortoises across South Africa, conflicted with the characteristics of chelonian Haemogregarina spp. as described by Siddall (Reference Siddall1995) and Telford (Reference Telford2009). These discrepancies led Cook et al. (Reference Cook, Smit and Davies2009) to question whether the haemogregarine found in blood of South African land tortoises was a species of Haemogregarina, or in fact a species of Hepatozoon or Hemolivia, which both use ticks as definitive hosts.
The current study was performed to clarify the taxonomic position of H. fitzsimonsi and to identify the haemogregarine genus to which it should be allocated. This involved detailed examination of blood films from a broader range of tortoise hosts across South Africa, as well as careful assessment of ticks found on the tortoises. Both traditional light microscopy techniques and molecular analyses were employed, and results demonstrate that H. fitzsimonsi is best placed in the genus Hepatozoon.
MATERIALS AND METHODS
Collection of tortoises, tortoise blood and tick ectoparasites
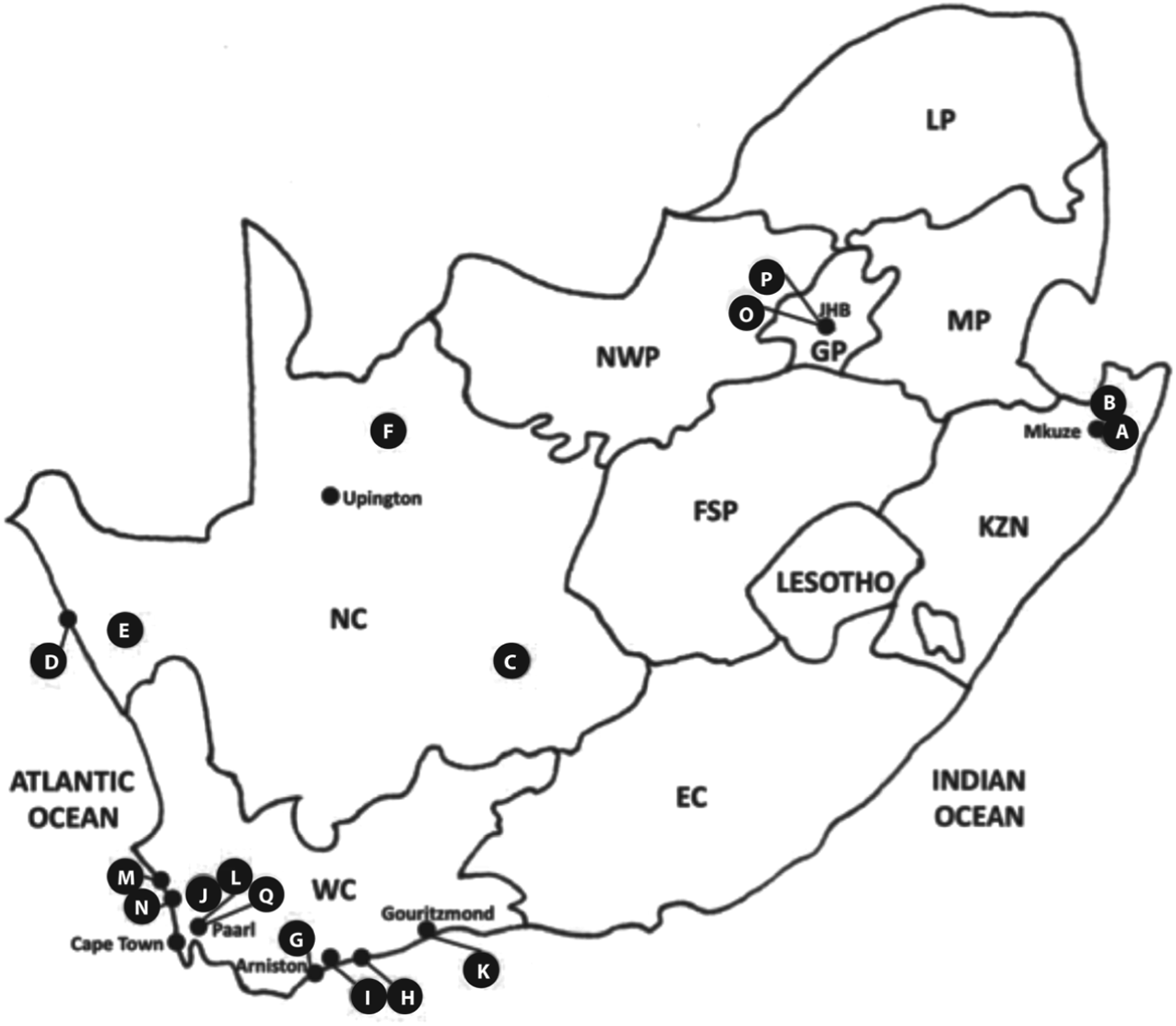
Land tortoises (n = 275), from 2009–2011, were examined from 4/9 South African provinces (Fig. 1, Table 1) including Gauteng (GP), KwaZulu-Natal (KZN), the Northern Cape (NC) and the Western Cape (WC) and, since these chelonians are protected by law, permit grants allowed only examination of live animals. Wild tortoises (n = 195) were collected from 16 sites across three provinces, while captive (n = 80) tortoises were examined from four sites in two provinces (Fig. 1).

Fig. 1. Collection sites for wild and captive tortoises. Provinces are shown, top left to right, LP: Limpopo, NWP: North West, GP: Gauteng, MP: Mpumalanga, NC: Northern Cape, FSP: Free State, KZN: KwaZulu-Natal, WC: Western Cape, EC: Eastern Cape. Wild tortoises were sampled at sites in three provinces: (A) Mkuze Nature Reserve and adjacent Bonamanzi Private Reserve, (B) Pongola Nature Reserve (KZN); (C) Britstown, (D) De Beer's Diamond Route Conservancy, (E) Namaqualand Conservancy, (F) Tswalu Kalahari Private Nature Reserve (NC); (G) Arniston (Waenhuiskrans), (H) De Hoop Nature Reserve, (I) De Mond Nature Reserve, (J) Elandsberg Private Nature Reserve, (K) Gouritzmond, (L) Paarl, (M) Paternoster, (N) West Coast Conservancy (WC). Captive tortoises were sampled at sites in two provinces: (O) Johannesburg Zoological Gardens, (P) Johannesburg private collections (GP); (Q) Butterfly World, Paarl (WC). Redrawn and adapted from Cook et al. (Reference Cook, Smit and Davies2009).
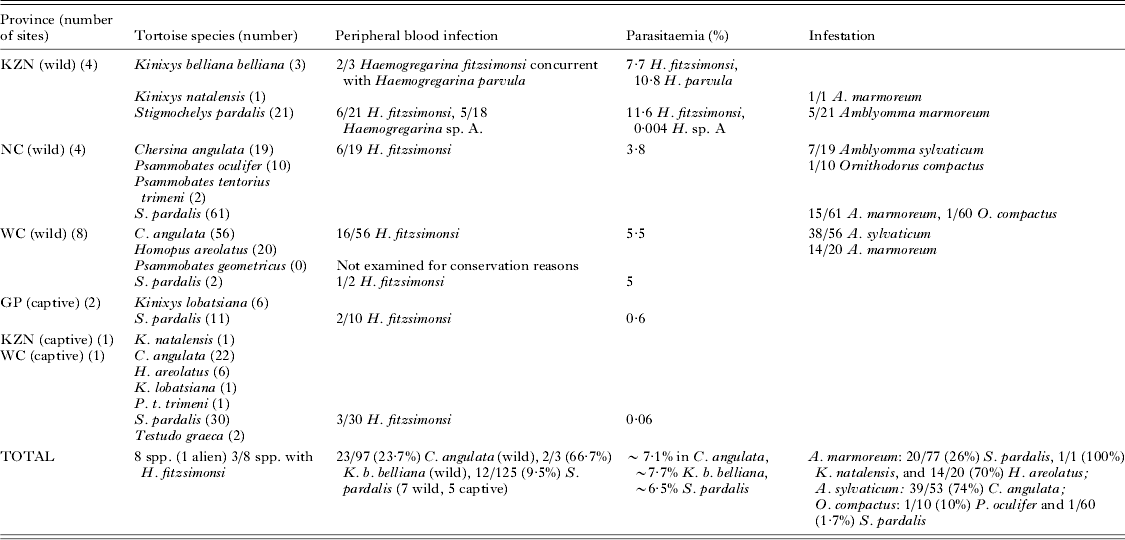
Table 1. Wild and captive tortoises collected and examined for haematozoans. Recorded below is the province, sampling site, tortoise species, haematozoan recorded, average parasitaemia (%) for each tortoise species at each site, as well as the tortoise tick species and its prevalence. Ticks were collected from only wild tortoises, as captive tortoise establishments removed ectoparasites immediately on receiving the animals

Tortoises were identified to species level using field guides (Branch, Reference Branch1998, Reference Branch2008; Boycott and Bourquin, Reference Boycott and Bourquin2000). Tortoises were of 10 species in six genera: Chersina angulata (Schweigger, 1812), (angulate tortoise); Homopus areolatus (Thunberg, 1787) (parrot-beaked padloper); Kinixys belliana belliana Gray, 1830 (Bell's hinged tortoise); Kinixys lobatsiana (Power, 1927) (Lobatse hinged tortoise); Kinixys natalensis Hewitt, 1935 (Natal hinged tortoise); Psammobates geometricus (Linnaeus, 1758) (geometric tortoise); Psammobates oculiferus (Kuhl, 1820) (Kalahari tent tortoise); Psammobates tentorius trimeni (Boulenger, 1886) (Trimen's tent tortoise); Stigmochelys pardalis (leopard tortoise); and Testudo graeca (Mediterranean spur-thighed tortoise), a species alien to South Africa.
Blood was collected from the subcarapacial sinuses of tortoises as described by McArthur et al. (Reference McArthur, Wilkinson and Meyer2004) (ethically approved by the Academic Ethics Committee of the Faculty of Science, University of Johannesburg, Reg. No. 920203595). Thin blood smears were prepared, air-dried, fixed in absolute methanol (Merck) for 10 min and stained in the field, or at the site of tortoise captivity, for 20 min in a modified solution of Giemsa's stain (Sigma) and stored in a dustproof container.
Ticks of three species, A. marmoreum, A. sylvaticum and Ornithodorus compactus Walton, 1962, were collected from the softer areas of the neck and rear of tortoises, by extending the legs of the reptiles outwards. Some ticks were also collected from the plastron. Ticks which were damaged during removal were killed immediately for impression smears (see below). Live ticks were sorted during collection into larvae, nymphs, and adult males and females. In total, 82 sets of ticks were collected from individual tortoises, including 45 sets of A. sylvaticum, 35 sets of A. marmoreum and two of O. compactus (Table 1), and most ticks were collected during summer (October–February) with only a few in spring (September). Sets from individual tortoises comprised either all three stages (larval, nymphal and adult stages), a combination of two stages, or a single stage. Live ticks were placed on ice to reduce activity and desiccation, and to increase their longevity. At a later stage, immediately after fixation in 70% molecular grade ethanol, ticks were identified to species level using Howell et al. (Reference Howell, Walker and Nevill1978) and keys provided by H. Heyne of the Onderstepoort Tick Museum, South Africa.
Screening of samples
Entire stained blood smears were screened with an Olympus CX21FS1 field light microscope (Olympus, Hamburg, Germany), and images were captured later in the laboratory with a Zeiss Axiocam digital camera attached to a Zeiss Axioplan 2 photomicroscope (Carl Zeiss, Jena, Germany) with a 100× oil immersion objective. Measurements (μm) were taken using AxioVision Release 4·3 (11–2004) software (Carl Zeiss), calibrated to a stage micrometer. Parasite prevalence for each tortoise type was estimated, and parasitaemias/parasite intensities, following examination of entire smears, were calculated per 100 red blood cells, with ∼104 erythrocytes examined per blood film (Cook et al. Reference Cook, Smit and Davies2009, Reference Cook, Smit and Davies2010; Van As et al. Reference Van As, Davies and Smit2013).
Adult, fixed ticks were dissected according to the method of Edwards et al. (Reference Edwards, Goddard and Varela-Stokes2009), and visceral impressions were prepared on clean slides. Fixed larvae and nymphs, on the other hand, were squashed whole. Adult, larval and nymphal preparations were left to air dry, fixed in absolute methanol (Merck) for 10 min and then stained for 20 min in Giemsa's stain for detection of parasite stages. Screening was done using the Olympus CX21FS1, and if sporocysts were found, further Giemsa-staining for 10 min was usually necessary for the sporozoites to become visible within each sporocyst. Sporogonic stages were identified and confirmed through comparison to other reptile apicomplexan blood parasite vector sporocysts, such as the findings of Michel (Reference Michel1973) and Telford et al. (Reference Telford, Wozniak and Butler2001). Images of the infective stages were captured using the Axioplan 2 photomicroscope, as for blood films. Sporocysts were measured following Telford et al. (Reference Telford, Wozniak and Butler2001). Numbers of sporozoites within each sporocyst were estimated by counting sporozoite nuclei. When possible, free sporozoites were measured, that is, their length and width, as for haemogregarine gamonts (see Cook et al. Reference Cook, Smit and Davies2009).
DNA extraction, PCR and sequence analysis
For the purposes of molecular work, parasitized blood was derived only from C. angulata, since Cook et al. (Reference Cook, Smit and Davies2009) and the current study demonstrated that this was the only tortoise species parasitized solely by H. fitzsimonsi (Table 1). Methanol-fixed, Giemsa-stained blood smears taken from two wild specimens of C. angulata from Arniston and De Mond (see Fig. 1), with H. fitzsimonsi parasitaemias of 3·3 and 0·8% respectively, were placed on separate sterile foil strips and each slide was then scraped with an individual sterile scalpel blade. Scrapings were then collected and transferred to individual sterile 1·5 mL Eppendorf tubes, and DNA was extracted from the two samples using a DNeasy Animal Tissue Kit (QIAGEN Ltd, UK).
Using similar methods, two methanol-fixed, Giemsa-stained impression slides of A. sylvaticum adult male ticks with sporocyst infections, collected from two C. angulata from De Mond, were scraped from slides and treated as above. The first was a tick collected feeding on a tortoise with a peripheral blood parasitaemia of 0·8%, the impression slide presenting with a high sporocyst number (>30 sporocysts per slide), and the second from a tortoise with no observable peripheral blood infection, the impression slide presenting with a low (<30) sporocyst number. Also processed were four partially dissected ethanol-fixed, sporocyst-infected whole ticks, and a fifth, a fresh non-sporocyst-infected tick; two of these were A. sylvaticum (a male from a tortoise with no observable peripheral blood infection and a female from a tortoise with a peripheral blood parasitaemia of 0·4%) from two C. angulata from De Mond respectively, both ticks with high sporocyst numbers, and three others A. marmoreum (all three male, one with a high, the other a low sporocyst number and the last with no observable sporocysts) from a leopard tortoise S. pardalis from Britstown, which showed no observable peripheral blood infection (Fig. 1). Tick material was transferred to separate sterile 1·5 mL Eppendorf tubes and DNA was extracted using the same DNeasy method as above.
Hepatozoon specific fragments of the apicomplexan 18S rDNA was amplified from the total DNA extracted from tortoise and tick samples, using PCR protocols and conditions as described by Perkins and Keller (Reference Perkins and Keller2001) for the HEMO1/2 primer sets and by Ujvari et al. (Reference Ujvari, Madsen and Olsson2004) for the HepF300 and HepR900 primer sets. The PCR reactions in all cases were performed using 12·5 μL of DreamTaq™ PCR master mix (2×DreamTaq buffer, 0·4 mm of each dNTP, 4 mm MgCl2) and at least 1–2 ng μL−1 of DNA with final reactions made up to 25 μL with PCR grade water. Reactions were performed using a Veriti 96 well thermal cycler (Applied Biosystems™) PCR machine and only 5 μL of each amplicon was visualized on a 1% agarose gel stained with gel red (Bioline) under UV.
The remaining 20 μL of positive PCR products were used for DNA sequencing which was performed at the DNA sequencing facility of the Natural History Museum, London, using fluorescent dye terminator sequencing kits (Applied Biosystems™); these reactions were then run on an Applied Biosystems 3730KL automated sequencer. Resulting sequences were visualized, assembled and edited in Bioedit 7.5.0.2 (Hall, Reference Hall1999) and initially identified as those of Hepatozoon using the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/blast/Blast/). The nucleotide sequence generated is available from the GenBank database with the following accession number: KJ702453.
Only amplicons using the HepF300 and HepR900 primers (Ujvari et al. Reference Ujvari, Madsen and Olsson2004) yielded usable DNA sequences of approximately 530 bp once edited for phylogenetic analysis. The sequences generated within this study were aligned with published 18S sequences (accession numbers as seen in Fig. 4) representing 47 Hepatozoon species derived from reptiles, amphibians, mammals, birds and ticks. Owing to the limited availability of published sequences, the only two sequences of Hemolivia species available, the only available sequences of Haemogregarina which coincidently represent species parasitizing turtles and a Dactylosoma sequence (GenBank HQ224958) from a frog were incorporated into the analysis, with the 18S of Adelina bambarooniae (GenBank AF494058) and Klossia helicina (GenBank HQ224956) used consistently as an outgroup. Sequences were aligned using the MUSCLE sequence alignment tool (http://www.ebi.ac.uk), visualized in Bioedit 7.5.0.2 (Hall, Reference Hall1999), and phylogenetic analyses were performed using MEGA5 (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Maximum likelihood (ML) trees were constructed under the conditions of the Tamura 3-parameter+Gamma model (T92+G). The T92+G model was also identified in MEGA5 (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011), based on having the lowest Bayesian information criteria relative to other models. The ML phylogeny with the highest log likelihood (ln −1984·703) was selected as being the most accurate and best supported reconstruction. Additionally, maximum parsimony (MP) phylogenies were constructed. Final phylogenetic interrelationships were inferred from 11 648 most parsimonious trees and were obtained using the Close-Neighbour-Interchange algorithm with a search level 2, in which the initial trees were obtained with the random addition of sequences (3000 replicates). In all phylogenetic analyses performed, nodal supports were calculated using 1000 bootstrap replicates but only those greater than 50% are shown.
RESULTS
Prevalence, host range and morphology of H. fitzsimonsi in tortoise peripheral blood smears
Collectively, 39/275 (14·2%) of wild and captive tortoises had haemogregarines, including 36/275 (13·1%) with H. fitzsimonsi (Fig. 2A–E), 2/275 (0·7%) with Haemogregarina parvula Dias, Reference Dias1953 (Fig. 2F) (see Cook et al. Reference Cook, Smit and Davies2009), and with 5/275 (1·8%) an unknown Haemogregarina sp. A. (Cook et al. Reference Cook, Smit and Daviesin press) (Table 1). Haemogregarina fitzsimonsi parasitized C. angulata (all wild), K. b. belliana (all wild) and S. pardalis (5 captive, 7 wild), and in KZN samples, it formed mixed infections with H. parvula (Fig. 2E) in K. b. belliana and with Haemogregarina sp. A in S. pardalis (see Cook et al. unpubl. data). The collection sites, prevalences and parasitaemias are recorded in Table 1.

Fig. 2. Light micrographs of Giemsa-stained stages of Haemogregarina fitzsimonsi Dias, Reference Dias1953, Haemogregarina parvula Dias, Reference Dias1953 and probable sporogonic stages of H. fitzsimonsi within the haemocoel of dissected Amblyomma marmoreum Koch 1844, from Stigmochelys pardalis recorded during the study. (A, B) Rarely found stages (arrows), recorded only once from a single Kinixys belliana belliana Gray 1830 from KwaZulu-Natal (KZN), possibly trophozoites or merozoites, note the vacuolation at the poles (arrows). (C) Double infection of immature gamonts, recurved at both poles. (D) Mature gamonts, with possible extracellular gamonts (left hand top corner). (E) Double infection of a single erythrocyte by H. fitzsimonsi and H. parvula from a concurrent infection of a K. b. belliana from KZN, note what appears to be a double infection of H. parvula, with two gamonts superimposed, as indicated by their nuclei (arrow and arrowhead). (F) H. parvula, commonly occurring as a double infection within erythrocytes at high parasitaemias. (G) Single sporocyst, containing sporozoites, some with a slightly visible outline. (H) Clustered sporocysts, either naturally ruptured from an oocyst or ruptured during dissection, note sporocyst impressions. (I) Sporozoites from ruptured sporocysts, each with a central rounded nucleus. Scale bar = 10 μm.
Stages of H. fitzsimonsi observed in Giemsa-stained peripheral blood films from tortoises during this study (2009–2011) were compared with those reported for H. fitzsimonsi by Cook et al. (Reference Cook, Smit and Davies2009). Both included trophozoites or merozoites (Fig. 2A and B respectively), measuring 4·7±1·0 (4·2–5·9) μm long by 2±0·1 (1·9–2·2) μm wide (n = 3), immature (Fig. 2C) and mature gamonts (Fig. 2D and E), measuring 16·2±1·7 (14–18·2) μm long by 2·1±0·6 (1·5–2·8) μm wide (n = 5) and 17·2±0·2 (17–17·5) μm long by 3·8±0·3 (3·4–4·2) μm wide (n = 15) respectively. All were intraerythrocytic with the exception of extracellular gamonts, although the latter were not commonly observed and appeared to be membrane-encased (Fig. 2D). Less often, when parasitaemias involved between 14–60% of erythrocytes, paired gamonts occurred in single erythrocytes (Fig. 2C), however, most often mature gamonts occurred singly within mature erythrocytes (Fig. 2D).
Tick distribution and morphology of parasite life stages within them
Ticks were collected only from wild tortoises. Amblyomma marmoreum was taken from H. areolatus, K. natalensis and S. pardalis, A. sylvaticum from C. angulata, and O. compactus from P. oculiferus and S. pardalis. Amblyomma marmoreum was found at: Mkuze and Pongola (KZN) on S. pardalis and K. natalensis; Britstown (NC) and Tswalu (NC) on S. pardalis; and Paarl (WC) on H. areolatus (see Table 1 for prevalences at these sites). Amblyomma sylvaticum was located only on C. angulata at Arniston (WC), De Hoop (WC), De Mond (WC), Paternoster (WC) and West Coast Conservancy (WC) (see Table 1 for prevalences). Ornithodorus compactus was collected from P. oculiferus and S. pardalis from Tswalu (NC) (Table 1). All life stages including the larval, nymphal and adult stages were observed for A. marmoreum and A. sylvaticum, but only adults for O. compactus. Tick infestations ranged from ∼5 to >50 per tortoise, the high infestations comprising mostly larvae. It was difficult to count exact numbers of ticks per tortoise, especially on large tortoises, due to their strength when handling and habit of retreating into their shells. However, the average number collected per infested tortoise was estimated at ∼20, including all life stages.
No intact oocysts were observed in any tick stage, but ticks found to be infected with sporocyst and sporozoite stages included both adult males and females of A. sylvaticum, and males of A. marmoreum, from H. fitzsimonsi-parasitized and, based on entire blood smear examination, apparently non-parasitized tortoises. Sporocysts in these two tick species were found in impressions of intact viscera, suggesting that these developmental stages occur in the haemocoel. Two O. compactus taken from uninfected tortoises revealed no sporocysts, and squashes of larvae and nymphs taken from haemogregarine-parasitized and seemingly non-parasitized tortoises, also showed no sporocysts.
Of 18/45 (40%) sets of A. sylvaticum collected from H. fitzsimonsi-infected tortoises (Table 1), 3/18 (17%) revealed sporocysts and two of these ticks, a male and a female from De Mond (WC), were subjected to molecular analysis. Two further male A. sylvaticum from four ticks collected at De Mond, but from 1/4 C. angulata lacking an obvious peripheral blood infection (Table 1), were also infected with sporocysts and used for subsequent molecular analysis. Of the 35 sets of A. marmoreum collected, a male tick taken at Britstown (WC), from an S. pardalis, showing no observable H. fitzsimonsi peripheral blood infection (Table 1), revealed no sporocysts, whereas two further male A. marmoreum from the same tortoise had sporocysts; all three ticks were processed for molecular analysis. Further details of the morphometrics of sporocysts and sporozoites appear below.
Sporocysts
Found in impressions of intact adult tick viscera, they were usually free from host tissue; they were narrowly to broadly oval, and measured 27·9±1·9 (25·8–29·6) μm long and 11·2±0·06 (9·1–13·3) μm wide (n = 10). Single sporocysts had an apparently double-layered, thick, non-staining capsule (Fig. 2G and H). Sporocysts were also seen in clusters, staining more readily, and possibly released recently from oocysts, either naturally, or during dissection when fragile oocysts may have ruptured (Fig. 2H). There appeared to be at least 16–18 (n = 10) sporozoites within each sporocyst, estimated by counting sporozoite nuclei. Outlines of sporozoites were visible in some sporocysts (Fig. 2G).
Sporozoites
Also in impressions of intact tick viscera, a few elongate and slender sporozoites were observed free of sporocysts and host tissue, and measuring 13·6±0·3 (13·2–14·1) μm long and 1·9±0·2 (1·8–2·3) μm wide (n = 8). These were slightly curved, with pale blue-stained cytoplasm and a small blue-stained, almost centrally located, rounded nucleus measuring 1·5±0·2 (1·2–1·8) μm long and 1·9±0·1 (1·8–2) μm wide (n = 22). Purple-stained structures were evident each side of the nucleus (Fig. 2I), perhaps corresponding to the crystalline bodies observed in other apicomplexan sporozoites such as those of Cryptosporidium parvum (see Tetley et al. Reference Tetley, Brown, McDonald and Coombs1998).
Sequence identification, alignment and phylogenetic analysis of tortoise peripheral blood H. fitzsimonsi and tick sporocyst stages
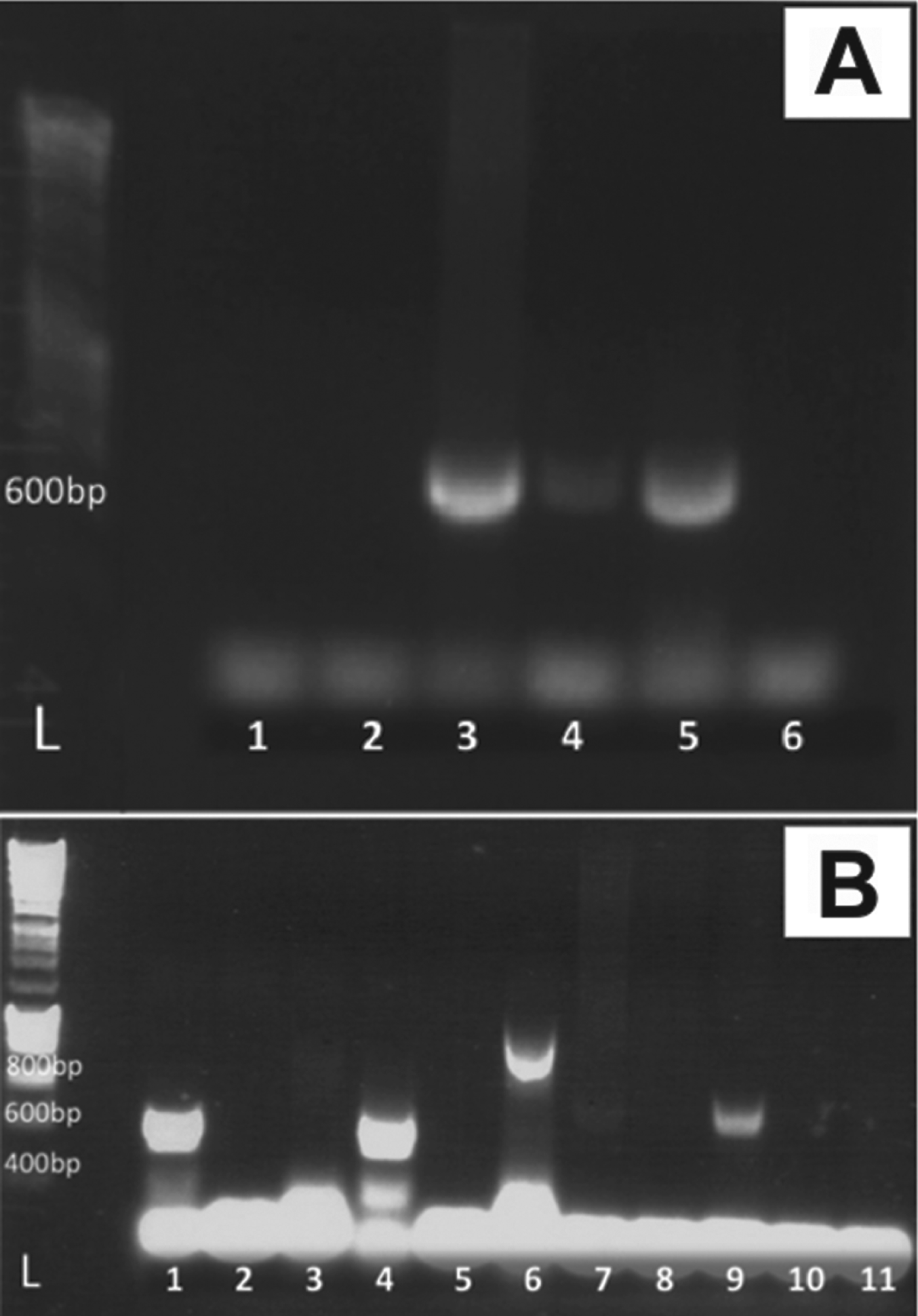
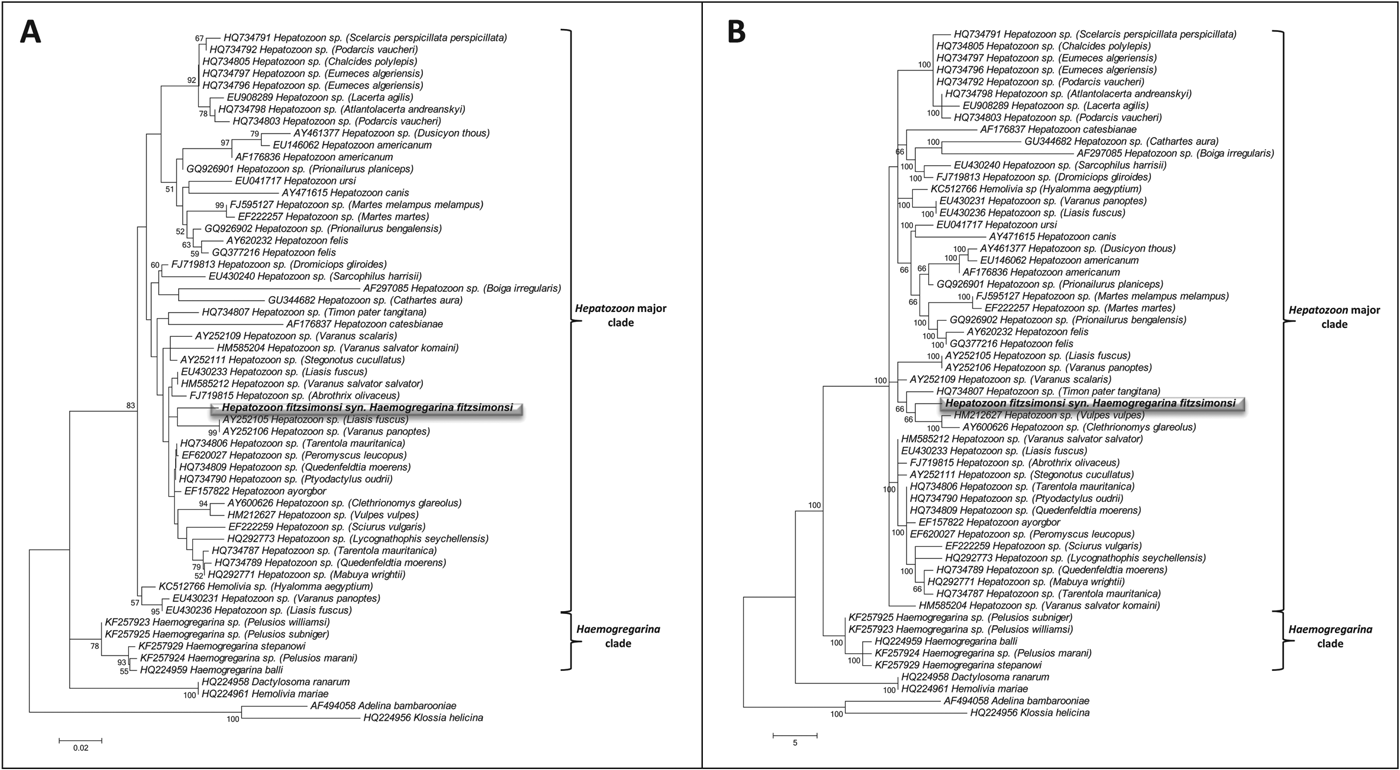
Amplicons of ∼900 and ∼600 bp respectively were derived using both primer sets HEMO1 and HEMO2, and HEPF300 and HEPR900, with the H. fitzsimonsi blood smear with the higher parasitaemia (3·3%) yielding a stronger band on gels than the lower parasitaemia (0·8%) (Fig. 3). Both ML and MP phylogenetic analysis produced trees containing a distinct major hepatozoon clade and a separate clade containing only Haemogregarina species. In both ML and MP analysis H. fitzsimonsi fell into the major Hepatozoon clade being distinctly separated from the clade containing the Haemogregarina species, whereas the two Hemolivia species did not cluster with each other with Hemolivia mariae appearing to be closely related to Dactylosoma ranarum and KC512766 Hemolivia sp. consistently falling within the major clade containing all Hepatozoon species. There is a considerable lack of congruence between the ML and MP phylogenies with the true phylogenetic relationship between each of the parasite species within the Hepatozoon and Haemogregarina clades remaining unclear and are therefore considered to be unresolved; however, both phylogenetic analyses do produce discreet Hepatozoon and Haemogregarina clades and show that H. fitzsimonsi is a species within the genus Hepatozoon and not Haemogregarina or Hemolivia.

Fig. 3. Amplified partial 18S rDNA fragments, using HEPF300 and HEPR900 primers, from Giemsa-stained blood smears containing Haemogregarina fitzsimonsi Dias Reference Dias1953, and sporocyst-containing tissues of both Amblyomma marmoreum Koch 1844 and Amblyomma sylvaticum (de Geer 1778). (A) L: DNA marker, lanes 3–4: ∼600 bp fragment from Giemsa-stained C. angulata blood smears of H. fitzsimonsi, with parasitaemias of 3·3% (strong band) and 0·8% (faint band) respectively, lane 5: unfixed impression slide of A. marmoreum (strong band) with no visible infection from an apparently uninfected S. pardalis; (B) L: DNA marker, lanes 7 and 9: ∼600 bp fragments of dissected high parasitaemia (>30 sporocysts per slide) sporocyst-containing A. marmoreum (faint band) and A. sylvaticum (stronger band) respectively.

Fig. 4. Phylogenetic analysis, implemented in MEGA5, for Haemogregarina fitzsimonsi Dias, Reference Dias1953 (highlighted) against species of Hepatozoon, Haemogregarina and Hemolivia, with Adelina bambarooniae and Klossia helicina as an outgroup. Nodal support is provided by bootstrap values with only those >50 shown. (A) Maximum likelihood analysis under the conditions of the Tamura 3-parameter model. The tree with the highest log likelihood (−1984·703) is shown. (B) Maximum parsimony analysis inferred from 11 648 most parsimonious trees.
In terms of ticks derived from South African tortoises, gel bands of ∼600 bp were observed from DNA extracted from infected and seemingly uninfected impression slides and dissected A. marmoreum males (Fig. 3) using HEPF300 and HEPR900 primers. A similar band was produced with a sporocyst-infected adult female of A. sylvaticum. Unfortunately, the sequenced products were unsatisfactory in all cases and therefore could not be compared readily with those derived from H. fitzsimonsi-infected blood and from published Hepatozoon sequences.
DISCUSSION
As noted earlier, in Siddall's (Reference Siddall1995) partial revision of the haemogregarine complex, H. fitzsimonsi was placed with the haemogregarines of aquatic terrapins, implying it is one of a number of Haemogregarina (sensu stricto). Such a haemogregarine would undergo division in peripheral erythrocytes and a leech vector would likely transmit it, this process probably occurring during blood feeding. However, H. fitzsimonsi rarely has peripheral blood division stages and the probability of its land tortoise hosts being bitten by infected leech vectors is very low, given the dry terrain most of these tortoises inhabit, and the fact that they are commonly tick, rather than leech infested. Even though several apicomplexan haematozoans are transmitted by dipteran vectors (see Telford et al. Reference Telford, Wozniak and Butler2001; Telford, Reference Telford2009), no notable dipteran vectors were observed feeding on tortoises during this study or previous studies (see Cook et al. Reference Cook, Smit and Davies2009), suggesting the tick, particularly in light of the sporogonic stages found in the ticks, to be a more probable vector for H. fitzsimonsi.
Sporocysts and sporozoites were found in the haemocoel of the tick species A. sylvaticum and A. marmoreum collected from H. fitzsimonsi-infected and seemingly uninfected tortoises and these are most probably the sporogonic stages of the haemogregarine. Although, as yet, it has not been possible to generate specific sequences from ticks using molecular methods, others have been successful in acquiring Hemolivia and Hepatozoon sequences from these invertebrates (see Vilcins et al. Reference Vilcins, Ujvari, Old and Deane2009; Herbert et al. Reference Herbert, Godfrey, Bull and Menz2010; Harris et al. Reference Harris, Gracia, Jorge, Maia, Perera, Carretero and Gimenez2013). Future research into the experimental infection of tortoises by feeding of sporocyst-containing ticks, as carried out by Široký et al. (2007), would be beneficial in elucidating the efficacy of these sporocysts in causing a H. fitzsimonsi infection. Since intact oocysts were not observed in the current study, it is difficult to differentiate the development stages in the ticks from those of Haemogregarina spp. which have oocysts containing eight or more naked sporozoites (Davies and Johnston, Reference Davies and Johnston2000; Telford, Reference Telford2009), or the oocysts of Hemolivia which are characteristically stellate in form (Landau and Paperna, Reference Landau and Paperna1997; Paperna, Reference Paperna2006). However, if the sporozoites observed are those of H. fitzsimonsi, they are covered by a thick, non-staining sporocyst wall and are thus very different from those of typical Haemogregarina (see Davies and Johnston, Reference Davies and Johnston2000). Furthermore, in Hemolivia the sporocyst stages occur within gut cells of ticks, and this is very different from observations made during this study of the likely stages of H. fitzsimonsi in A. sylvaticum and A. marmoreum, where sporocysts occur in the tick haemocoel. To add weight to these conclusions, H. fitzsimonsi from the tortoise host Chersina angulata is clearly distinct phylogenetically from Haemogregarina (GenBank HQ224959) and Dactylosoma (GenBank HQ224958), these two genera being considered closely related to each other (Barta, Reference Barta1991; Barta et al. Reference Barta, Ogendengbe, Martin and Smith2012). The current phylogenetic analyses also placed H. fitzsimonsi at some distance from a species of the genus Hemolivia (GenBank KC512766) which supports its taxonomic separation from this genus.
All evidence from this study indicates that the genus Hepatozoon, a haemogregarine genus commonly infecting reptiles (see Smith, Reference Smith1996; Telford, Reference Telford2009), is the genus to which H. fitzsimonsi should be assigned. A characteristic of the genus is that it lacks, or forms few division stages in the peripheral blood of the intermediate host and may form oocysts and sporocysts in the definitive host haemocoel (see Davies and Johnston, Reference Davies and Johnston2000). These features appear to apply to the stages seen in tortoises and the ticks fed on H. fitzsimonsi-parasitized tortoises. Added to this, genetic analyses proved successful in linking the 18S rDNA apicomplexan sequences derived from H. fitzsimonsi Giemsa-stained C. angulata blood films with sequences from other Hepatozoon species. Although H. fitzsimonsi has yet to be sequenced from other tortoise hosts, owing to the problems of concurrent infections, the sequences from C. angulata closely matched Hepatozoon species, particularly within a small clade comprising two Australian reptiles, one a python (GenBank AY252105) and the other a monitor lizard (GenBank AY252106) in the ML tree (Fig. 4A). Furthermore, the above Hepatozoon spp. are transmitted by ticks of Amblyomma spp. (see Vilcins et al. Reference Vilcins, Ujvari, Old and Deane2009). Thus it seems evident that H. fitzsimonsi is not a species of Haemogregarina, or of Hemolivia, but one of Hepatozoon, likely transmitted by ticks of the genus Amblyomma (see Fig. 4A and B). This finding may help support the hypothesis of Barta et al. (Reference Barta, Ogendengbe, Martin and Smith2012) that haemogregarine parasites have co-evolved with their definitive hosts.
Except for the renaming of Haemogregarina mauritanica Sergent and Sergent, Reference Sergent and Sergent1904 from land tortoises, by Michel (Reference Michel1973) as Hepatozoon mauritanicum (Sergent and Sergent, Reference Sergent and Sergent1904), which has since been assigned to the genus Hemolivia (see Landau and Paperna, Reference Landau and Paperna1997; Telford, Reference Telford2009), no other Hepatozoon species have been, or are, reported from chelonians, especially terrestrial tortoises. Thus, the findings of the work presented here are particularly significant and novel. Consequently, together with the work of Dias (Reference Dias1953) and Cook et al. (Reference Cook, Smit and Davies2009), the results of this study lead to the recommendation of the following nomenclatural correction: Hepatozoon fitzsimonsi (Dias, Reference Dias1953) (syn. Haemogregarina fitzsimonsi Dias, Reference Dias1953) in the terrestrial tortoises Kinixys belliana belliana (syn. Kinixys belliana zuluensis) (type host), Chersina angulata, Kinixys lobatsiana, Kinixys natalensis and Stigmochelys pardalis and the probable tick vectors Amblyomma marmoreum and Amblyomma sylvaticum.
ACKNOWLEDGEMENTS
We are grateful to Professor Jorge Eiras, University of Porto, Portugal, for translating the relevant sections of Dias (Reference Dias1953) from Portuguese. We would also like to thank Johannesburg Zoological Gardens, Paarl Butterfly Park, De Hoop, De Mond, and Mkuze Nature Reserves, and Bonamanzi and Tswalu Private Reserves for allowing us to examine their tortoises. We would also like to thank Mrs J. Llewellyn–Hughes and her staff for performing the DNA sequencing reactions at the Molecular Sequencing Facility at the Natural History Museum, London, UK.
Courtney Cook, Nico Smit and Scott Lawton would like to acknowledge their cherished colleague and friend Professor Angela Davies, who passed away suddenly on 28 December 2013. Her dedication and expert knowledge will be deeply missed.
FINANCIAL SUPPORT
This work was partially funded by a University of Johannesburg Sasol Fund Research Grantas well as a North West University post-doctoral grant to Courtney Cook. The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged (project IFR2011040100022). Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF.