Introduction
Sterile crustose lichens containing a trentepohlioid photobiont have, in recent decades, often been tentatively described in genera of Arthoniomycetes such as Opegrapha and Schismatomma (e.g. James Reference James1971; Coppins & James Reference Coppins and James1989; Coppins et al. Reference Coppins, James and Hawksworth1992; Øvstedal & Schaefer Reference Øvstedal and Schaefer2013; Diederich et al. Reference Diederich, Lücking, Aptroot, Sipman, Braun, Ahti and Ertz2017). However, determining the systematic position of sterile lichens is difficult using only morphological and chemical data, rendering these generic placements highly uncertain. Important progress has been made in sequencing many fungal groups allowing the taxonomic status of sterile taxa to be resolved by placing them in phylogenetic trees. For example, in the Arthoniales, recent molecular data were used to demonstrate that the sterile Enterographa sorediata Coppins & P. James is the sorediate morph of Syncesia myrticola (Fée) Tehler (Ertz et al. Reference Ertz, Coppins and Sanderson2018a). The systematic placement of several lichenized hyphomycetes or lichens producing only pycnidia was also resolved using molecular data, with species often transferred to other, sometimes new or resurrected arthonialean genera (e.g. Ertz & Tehler Reference Ertz and Tehler2011; Frisch et al. Reference Frisch, Ohmura, Ertz and Thor2015; Ertz et al. Reference Ertz, Sanderson, Łubek and Kukwa2018c).
In the framework of molecular studies of sterile Arthoniales described from Great Britain, fresh specimens of Opegrapha multipuncta Coppins & P. James and of Schismatomma quercicola Coppins & P. James were collected. Additional specimens of both taxa were also collected during recent surveys of crustose lichens in highly oceanic forests in western and central Norway. Sequences obtained from these specimens revealed that these two species do not belong to the Arthoniomycetes, but instead to the Lecanoromycetes. The present study aims to clarify their systematic position.
Materials and Methods
Voucher specimens are deposited in the herbaria BR and TRH, and the private herbarium of N. A. Sanderson. The external morphology was studied and measured using an Olympus SZX12 stereomicroscope. Macroscopic photographs were taken using a Keyence VHX-5000 digital microscope and a VH-Z20R/W/T lens. Lichen secondary metabolites were identified using thin-layer chromatography (TLC) in solvent B (Orange et al. Reference Orange, James and White2001).
Molecular techniques
Well-preserved and freshly collected specimens lacking any visible symptoms of fungal infection were used for sequencing. A group of 4–6 soredia was used for direct PCR as described in Ertz et al. (Reference Ertz, Tehler, Irestedt, Frisch, Thor and van den Boom2015). Amplification reactions were prepared for a 50 μl final volume containing the lichen material as explained in Ertz et al. (Reference Ertz, Sanderson, Łubek and Kukwa2018c). A targeted fragment of c. 0·8 kb of the mitochondrial ribosomal RNA small subunit (mtSSU) was amplified using primers mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The yield of the PCRs was verified by running the products on a 1% agarose gel using ethidium bromide. Both strands were sequenced by Macrogen® using amplification primers. Sequence fragments were assembled with Sequencher v. 5.3 (Gene Codes Corporation, Ann Arbor, Michigan). Sequences were subjected to GenBank ‘megablast’ searches to verify their closest relatives and to detect potential contaminations.
Taxon selection and phylogenetic analyses
Five new mtSSU sequences were obtained for this study, three for Opegrapha multipuncta (MK990614, MK990615, MK990616) and two from Schismatomma quercicola (MK990617, MK990618). Their closest matches based on ‘megablast’ searches were retrieved from GenBank. For the placement of Opegrapha multipuncta additional species of Porina were selected from Grube et al. (Reference Grube, Baloch and Lumbsch2004), Baloch & Grube (Reference Baloch and Grube2006), Nelsen et al. (Reference Nelsen, Lücking, Andrew, Aptroot, Cáceres, Mercado-Díaz, Rivas Plata and Lumbsch2014) and Orange (Reference Orange2015). Coenogonium luteum (Dicks.) Kalb & Lücking, C. pineti (Ach.) Lücking & Lumbsch and Gyalecta ulmi (Sw.) Zahlbr were chosen as an outgroup based on the phylogenetic trees of Nelsen et al. (Reference Nelsen, Lücking, Andrew, Aptroot, Cáceres, Mercado-Díaz, Rivas Plata and Lumbsch2014) and Orange (Reference Orange2015). Available sequences of mtSSU for members of the Graphidaceae were selected for the placement of Schismatomma quercicola, mainly from Frisch et al. (Reference Frisch, Kalb and Grube2006), Miadlikowska et al. (Reference Miadlikowska, Kauff, Hofstetter, Fraker, Grube, Hafellner, Reeb, Hodkinson, Kukwa and Lücking2006), Mangold et al. (Reference Mangold, Martín, Lücking and Lumbsch2008), Nelsen et al. (Reference Nelsen, Lücking, Rivas Plata and Mbatchou2010) and Rivas Plata et al. (Reference Rivas Plata, Hernández, Lücking, Staiger, Kalb and Cáceres2011, Reference Rivas Plata, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernández, Cáceres, Kalb and Sipman2013). Phaeographis intricans (Nyl.) Staiger and Thalloloma anguinum (Mont.) Trevis. were chosen as an outgroup based on the phylogenetic tree of Rivas Plata et al. (Reference Rivas Plata, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernández, Cáceres, Kalb and Sipman2013; selection within the ‘Graphideae’ clade). In total, 95 sequences were retrieved from GenBank for the two phylogenetic analyses (Figs 1 & 2). The sequences were aligned using MAFFT v. 6.814b (Katoh et al. Reference Katoh, Misawa, Kuma and Miyata2002) within Geneious (5.1.7) (https://www.geneious.com) and improved manually using Mesquite 3.04 (Maddison & Maddison Reference Maddison and Maddison2015). Terminal ends of sequences and ambiguously aligned regions were delimited manually and excluded from the datasets.
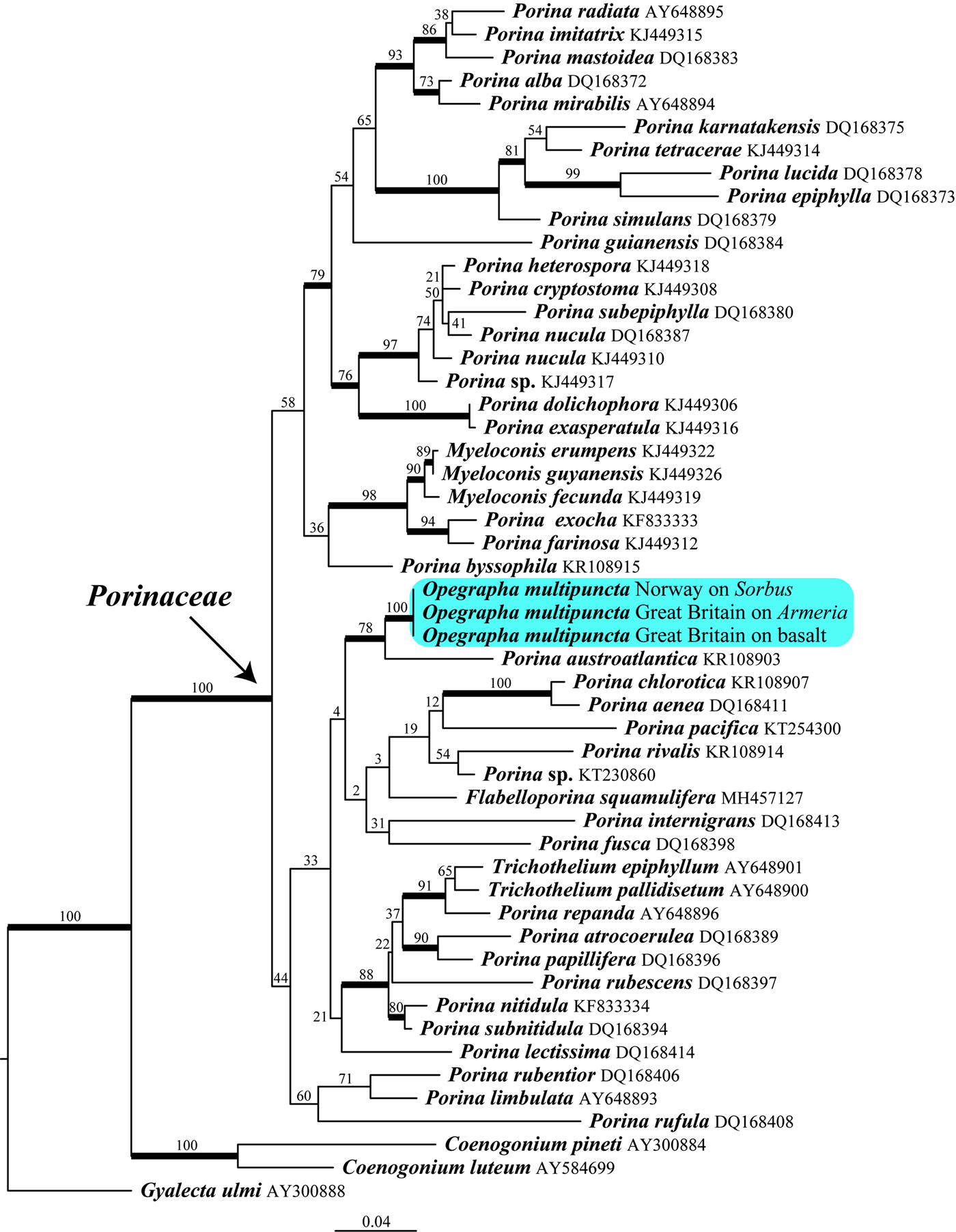
Fig. 1. Phylogeny of Porinaceae based on a dataset of mtSSU sequences obtained from a RAxML analysis. Maximum likelihood bootstrap values are shown above or near internal branches. Internal branches, considered strongly supported by both the RAxML and Bayesian analyses, are represented by thicker lines. The newly sequenced samples of Porina (‘Opegrapha’) multipuncta are highlighted. The outgroup consists of Coenogonium luteum, C. pineti and Gyalecta ulmi. In colour online.

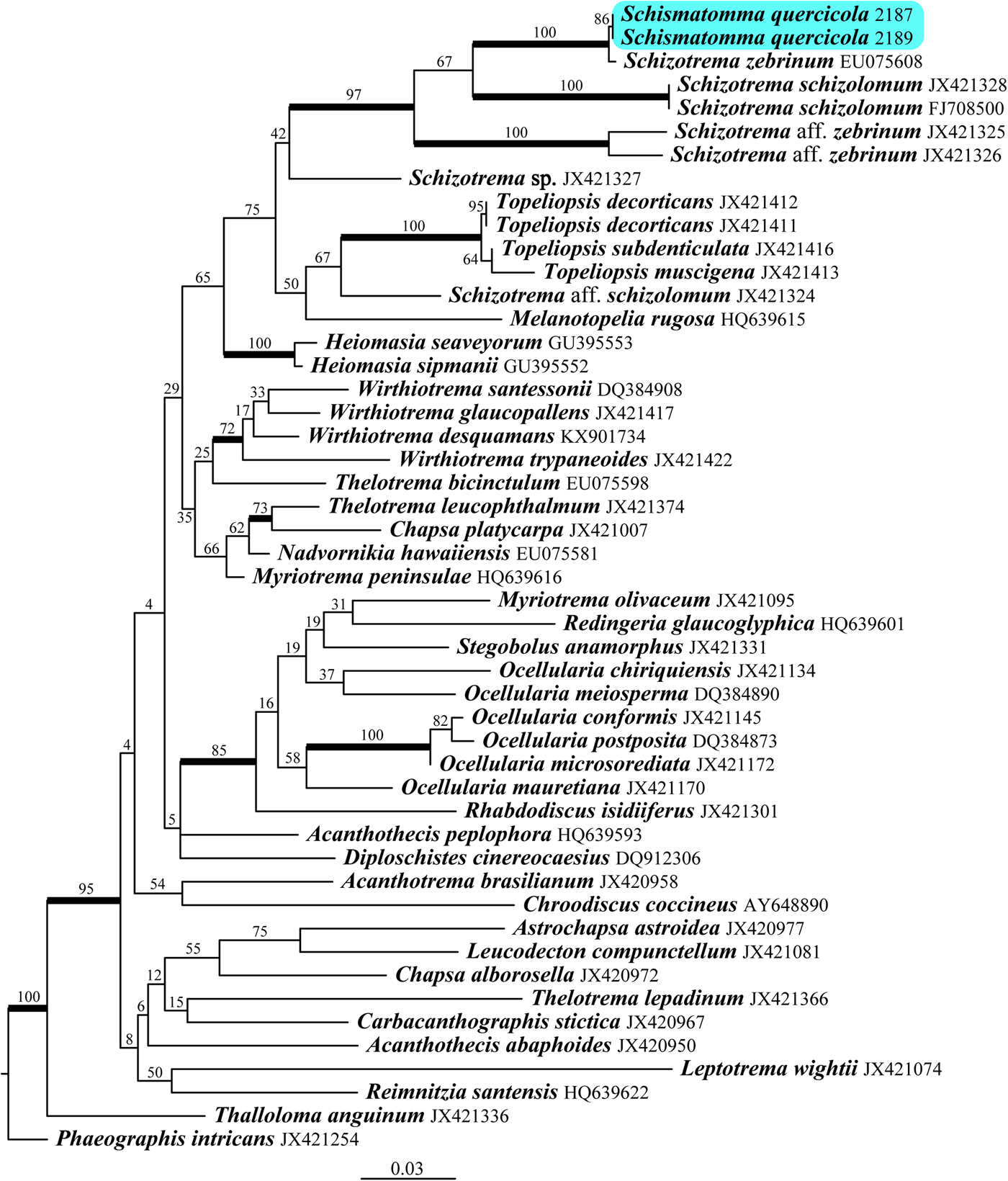
Fig. 2. Phylogeny of Graphidaceae based on a dataset of mtSSU sequences obtained from a RAxML analysis. Maximum likelihood bootstrap values are shown above or near internal branches. Internal branches, considered strongly supported by both the RAxML and Bayesian analyses, are represented by thicker lines. The newly sequenced samples of Schizotrema (‘Schismatomma’) quercicola are highlighted. The outgroup consists of Phaeographis intricans and Thalloloma anguinum. In colour online.
Bayesian analyses were carried out on the dataset using the Metropolis-coupled Markov chain Monte Carlo (MCMCMC) method in MrBayes v. 3.2.6 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001; Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) on the CIPRES web portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Best-fit evolutionary models were estimated using the Akaike Information Criterion (AIC; Akaike Reference Akaike, Petrov and Csaki1973) as implemented in jModelTest2 v. 2.1.6 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012). The GTR + I+G model was selected for both the ‘Opegrapha multipuncta’ and the ‘Schismatomma quercicola’ datasets. For each dataset, two parallel MCMCMC runs were performed, each using four independent chains and 40 million generations, sampling trees every 1000th generation. Tracer v. 1.6 (Rambaut & Drummond Reference Rambaut and Drummond2007) was used to ensure that stationarity was reached by plotting the log-likelihood values of the sample points against generation time, making sure that the ESS values were higher than 200. Convergence between runs was also verified using the PSRF (Potential Scale Reduction Factor), where all values were equal or close to 1·000. Posterior probabilities (PP) were determined by calculating a majority-rule consensus tree generated from the 60 002 post-burn-in trees of the 80 002 trees sampled by the two MCMCMC runs, using the sumt option of MrBayes for the two datasets. In addition, a maximum likelihood (ML) analysis was performed on the CIPRES web portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) using RAxML-HPC2 v.8.2.10 (Stamatakis Reference Stamatakis2014) with 1000 ML bootstrap iterations (ML-BS) and the GTRGAMMA model.
The RAxML tree did not contradict the Bayesian tree topology for the strongly supported branches. Therefore, only the RAxML tree is shown here, with the bootstrap support values added above the internal branches (Figs 1 & 2). ML-BS ≥ 70 and PP ≥ 95 were considered to be significant. Internal branches considered strongly supported by both the RAxML and Bayesian analyses are represented by thicker lines (Figs 1 & 2). Phylogenetic trees were visualized using FigTree v. 1.4.2 (Rambaut Reference Rambaut2012).
Results
The mtSSU dataset for Opegrapha multipuncta consisted of 52 specimens and 597 unambiguously aligned sites, while the mtSSU dataset for Schismatomma quercicola consisted of 49 specimens and 756 unambiguously aligned sites.
Despite our phylogenetic analyses revealing a lack of support for the backbone of the Porinaceae tree (Fig. 1), several well-supported clades emerged, such as the Porina subnitidula to Trichothelium epiphyllum clade, the P. aenea-chlorotica clade, the Porina farinosa to Myeloconis erumpens clade (all with muriform ascospores), and the P. exasperatula to P. radiata clade (including the generic type P. nucula and three well-supported subclades). Our three specimens of Opegrapha multipuncta growing on three different substrata (basalt rock, dead stem of Armeria maritima and trunk of Sorbus) have identical mtSSU sequences and form a well-supported clade with Porina austroatlantica P. M. McCarthy & Fryday, a saxicolous species recently described from the Falkland Islands (McCarthy & Fryday Reference McCarthy and Fryday2009). Therefore, Opegrapha multipuncta is newly combined below in the genus Porina.
In our phylogenetic tree of the Graphidaceae (Fig. 2), only a small number of clades are strongly supported by both analyses, corresponding to the genera Heiomasia, Topeliopsis and Wirthiotrema, and the Rhabdodiscus isidiiferus to Myriotrema olivaceum clade, the Chapsa platycarpa and Thelotrema leucophthalmum clade, and the Schizotrema aff. zebrinum to Schismatomma quercicola clade. Schismatomma quercicola is closely related to Schizotrema zebrinum Mangold, the type species of Schizotrema. Therefore, Schismatomma quercicola is newly combined below in the genus Schizotrema.
Taxonomy
Porina multipuncta (Coppins & P. James) Ertz, Coppins & Frisch comb. nov.
MycoBank No.: MB 831216
Opegrapha multipuncta Coppins & P. James, in Coppins et al., Lichenologist 24: 365 (Reference Coppins, James and Hawksworth1992); type: Great Britain, Scotland [‘Caledonia’], Zetlandia (V.C.112), Mainland, between Voe and Gonfirth, HU/374618, abundant on Salix aurita by small stream, June 1980, P. W. James & W. Syratt (BM—holotype).
(Fig. 3A–D)

Fig. 3. Sequenced specimens of Opegrapha multipuncta (A–D) and Schismatomma quercicola (E & F). A & B, fresh thallus of O. multipuncta with bright orange soralia on dead stem of Armeria maritima (Coppins s. n.); C & D, fresh thallus of O. multipuncta with bright orange soralia on basalt sea-cliff (Coppins s. n.). E & F, thallus of S. quercicola with pale greyish soralia on bark of Quercus petraea (Sanderson 2187). Scales: A, C & E = 1 mm; B, D & F = 500 μm. In colour online.
Descriptions
See Coppins et al. (Reference Coppins, James and Hawksworth1992), Pentecost & James (Reference Pentecost, James, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009), and Tønsberg (Reference Tønsberg1992).
Distribution and ecology
Known from the Azores (Aptroot & Rodrigues Reference Aptroot and Rodrigues2005), France (Aptroot et al. Reference Aptroot, Jordaens, Sparrius, Spier and Van den Broeck2007; Bricaux Reference Bricaud2007), Great Britain (Coppins et al. Reference Coppins, James and Hawksworth1992), Italy (Tretiach Reference Tretiach2004) and Norway (Tønsberg Reference Tønsberg1992), where it grows on various trees (e.g. Callistemon, Juglans, Malus, Populus, Pyrus, Quercus), shrubs, rocks and stems of plants (e.g. Armeria) in areas characterized by intense precipitation and high air humidity.
Notes
1) Our phylogenetic results place Opegrapha multipuncta in the genus Porina. 2) Porina multipunctata G. Merr. ex R. Sant. is a name based on a different type and a synonym of Strigula multipunctata (G. Merr. ex R. Sant.) R. C. Harris, a foliicolous lichen. The species name ‘multipunctata’ is similar to that of the new combination (‘multipuncta’). Therefore, the name of the new combination might have to be treated as a homonym following Art. 53.2. However, we consider that the two species names are sufficiently different not to be confused so we prefer to introduce a new combination rather than a new name. 3) Opegrapha multipuncta is characterized by a thin, dull or dark red-brown thallus with bright orange soralia and an absence of lichen secondary products. The species name was first published in an identification key to the genus Opegrapha in Great Britain as O. multipuncta Coppins & P. James (ined.) (Pentecost & Coppins Reference Pentecost and Coppins1983), and later recorded from the Faroe Islands (Alstrup & Alstrup Reference Alstrup, Alstrup, Hojgaard, Johansen and Odum1989) before being validly published in Coppins et al. (Reference Coppins, James and Hawksworth1992).
Sequenced specimens
Great Britain: Scotland: V.C. 82, East Lothian, Tyninghame, Baldred's Cradle, Grid NT637813, 10 m, on dead stems of Armeria maritima on cliff top, 21 viii 2017, Coppins s. n. (E; GenBank no MK990615); ibid., on E-facing, basalt sea-cliff, 21 viii 2017, Coppins s. n. (E; GenBank no MK990616).—Norway: Trøndelag: Åfjord, Lauføya, Plassahaugen, 63°55·883′N, 09°56·681′E, on the trunk of Sorbus aucuparia, 14 m, 2015, Frisch 15/No17 (TRH; GenBank no MK990614).
Schizotrema quercicola (Coppins & P. James) Ertz, Frisch & Sanderson comb. nov.
MycoBank No.: MB 831217
Schismatomma quercicola Coppins & P. James, Lichenologist 21: 237 (Reference Coppins and James1989); type: Great Britain, England, V.C.11, Hampshire, New Forest, c. 500 m NE of Rufus Stone, Grid 41/274.127, on Quercus, 3 December 1979 (published as ‘3 Iulius 1978’), F. Rose (BM—holotype; E, UPS—isotypes).
Descriptions
See Coppins & James (Reference Coppins and James1989) and Wolseley & Hawksworth (Reference Wolseley, Hawksworth, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009).
Distribution and ecology
Known from Great Britain (Coppins & James Reference Coppins and James1989) and France (Roux Reference Roux2014), and recorded here as new to Norway from five localities in Hordaland and Sogn og Fjordane. In Great Britain, it grows most frequently on the acid bark of mature Quercus trees. The species can, however, also be locally common on Alnus, Ilex, Betula and Fagus, and can occasionally colonize other phorophytes such as Castanea, Corylus, Crataegus, Larix, Pinus sylvestris, Prunus padus, Salix and Sorbus aucuparia (Coppins & James Reference Coppins and James1989; British Lichen Society 2019). Occasionally it has been found on Quercus lignum. In Great Britain, it is essentially a species of ancient or old-growth woodland and is used as an indicator of ecological continuity (Sanderson et al. Reference Sanderson, Wilkins, Bosanquet and Genney2018). It is most frequent in the extensive old-growth woodlands of the New Forest (Hampshire, England), where it has also been noted colonizing adjacent, more disturbed woods. Here Sanderson (Reference Sanderson2001) recorded it on 60 and 69 trees per hectare in two sampled little disturbed old-growth stands, and on 17–30 trees per hectare within three 19th century oak plantations. The obligate lichenicolous fungus Arthonia invadens Coppins was recorded growing on Schizotrema quercicola only in the sites with 60 or more occupied trees per hectare, where the parasite occurred on 3–5 trees per hectare. Beyond the New Forest, Schizotrema quercicola is a much less frequently encountered species in Britain. In Norway, the species was mainly found on Scots pine in highly oceanic, coastal pine forests but also at the base of an old oak in agricultural landscape.
Notes
1) The species was tentatively described in the genus Schismatomma based on its morphological resemblance to S. decolorans (Erichsen) Clauzade & Vězda (Coppins & James Reference Coppins and James1989). Schismatomma quercicola is characterized by a thin, pale brownish grey to greyish white, crustose thallus with scattered, discrete, pink-grey soralia when fresh, becoming whitish in the herbarium, and a chemistry with fumarprotocetraric acid, ±protocetraric acid and an unidentified substance (soralia Pd+ orange). Our phylogenetic results, based on material sequenced from the type locality (New Forest), prove that S. quercicola is a member of the genus Schizotrema. 2) In the original description of Schismatomma quercicola (Coppins & James Reference Coppins and James1989), the holotype specimen from BM is dated ‘3 Iulius 1978’, but the collection date of the holotype specimen on JSTOR (specimen BM000975908) is ‘3 December 1979’. We consider that there was an error in the original publication. 3) Arthonia invadens is an obligate lichenicolous fungus on Schizotrema quercicola, endemic to Great Britain and Ireland. However, because its host lichen is known only as a sterile crust, the lichenicolous habit of A. invadens was questioned in the original description since it is possible that its ascomata could be those of the host (Coppins Reference Coppins1989). Our phylogenetic results showing the placement of ‘Schismatomma’ quercicola in the family Graphidaceae-genus Schizotrema, definitively prove that the arthonioid ascomata belong to a lichenicolous fungus. Moreover, sequences of A. invadens obtained recently from the type locality (New Forest) place the species in the Coniocarpon-Reichlingia clade (as defined by Van den Broeck et al. Reference Van den Broeck, Frisch, Razafindrahaja, Van de Vijver and Ertz2018) in the Arthoniaceae, where several other lichenicolous fungi confined to Graphidaceae hosts occur (D. Ertz, unpublished data).
Sequenced specimens
Great Britain: England: V.C.11, New Forest, Sunny Bushes, Grid Ref SU25946 14250, Grid2 41 21, Quercus-Fagus-Ilex pasture woodland, acid bark on old Quercus petraea, 2016, Sanderson 2187 (hb. Sanderson; GenBank no MK990617); ibid., Coppice of Linwood, Grid Ref SU25418 14222, Grid2 41 21, Quercus-Fagus-Ilex pasture woodland, acid bark on old Quercus petraea, 2016, Sanderson 2189 (hb. Sanderson; GenBank no MK990618).
New localities
Norway: Hordaland: Fusa, Holmefjord, Eikhaugen, 60°17·922′N, 05°39·847′E, at the base of an old oak, 37 m, 2018, Frisch 18/No60 (TRH); Os, Strøno, Svensvikmyrane, 60°10′39·0″N, 05°20′58·6″E, on Pinus sylvestris in coastal pine forest, 35–50 m, 2018, Frisch 18/No56 & Klepsland (TRH). Sogn og Fjordane: Gulen, Sygnefest nordøst, 61°04′16·6″N, 05°06′23·3″E, on Pinus sylvestris in coastal pine forest, 20–50 m, 2018, Frisch TSD S13-2-Ps4-2 & Klepsland (TRH); Florø, Svanøya, Vågsfjellet nord, 61°29′23·0″N, 05°04′56·7″E, on Pinus sylvestris in coastal pine forest, 25–50 m, 2018, Frisch TSD S14-1-Ps2-1 & Klepsland (TRH); ibid., on Sorbus aucuparia, 25–50 m, 2018, Frisch TSD S14-1-Sa2-7 & Klepsland (TRH); ibid., Storefjellet nordvest, 61°40′00·5″N, 05°00′02·2″E, on Pinus sylvestris in coastal pine forest, 35–70 m, 2018, Frisch 18/No96 & Klepsland (TRH).
Discussion
Our molecular data using mtSSU sequences prove that Opegrapha multipuncta and Schismatomma quercicola do not belong to the Arthoniomycetes, where these species were originally described, but to the Lecanoromycetes (Figs 1 & 2). The sequencing of sterile Arthoniales usually changes the generic or family position within the order (e.g. Schismatomma cretaceum was moved from the Roccellaceae to the Arthoniaceae; Frisch et al. Reference Frisch, Ohmura, Ertz and Thor2015), but examples where sterile taxa have been transferred from one class to another are rare. Among them, Herpothallon antillarum (Vain.) Aptroot et al. and H. sipmanii (Aptroot et al.) Nelsen et al. (Arthoniomycetes: Arthoniaceae) were placed in Diorygma and Heiomasia (Lecanoromycetes: Graphidaceae) respectively (Nelsen et al. Reference Nelsen, Lücking, Rivas Plata and Mbatchou2010, Reference Nelsen, Lücking, Andrew, Rivas Plata, Chaves, Cáceres and Ventura2012), while Buellia violaceofusca (Lecanoromycetes: Caliciaceae) was shown to represent the trebouxioid photomorph of Lecanographa amylacea (Arthoniomycetes: Lecanographaceae) (Ertz et al. Reference Ertz, Guzow-Krzemińska, Thor, Łubek and Kukwa2018b).
Our phylogenetic analyses prove that Opegrapha multipuncta belongs to the Porinaceae (Ostropales), with P. austroatlantica being the closest relative (Fig. 1). As a consequence, we combined this species in Porina (see Results). However, the generic placement remains somewhat uncertain because Porina is a paraphyletic genus. Indeed, phylogenetic analyses placed the genera Myeloconis (Nelsen et al. Reference Nelsen, Lücking, Andrew, Aptroot, Cáceres, Mercado-Díaz, Rivas Plata and Lumbsch2014), Flabelloporina (Sobreira et al. Reference Sobreira, Cáceres, Maia and Lücking2018) and Trichothelium (Baloch & Grube Reference Baloch and Grube2006) within Porina, rendering the generic delimitations within Porinaceae unclear. This is also evident in our phylogenetic analyses with species of Trichothelium being sister to Porina repanda and species of Myeloconis sister to Porina exocha + P. farinosa, while Flabelloporina squamulifera is included in the clade Porina fusca-P. chlorotica despite this latter position being weakly supported (Fig. 1). There is little evidence for a splitting of the genus Porina because recent phylogenies recovered several proposed segregate genera of Porina as non-monophyletic (Baloch & Grube Reference Baloch and Grube2006; Nelsen et al. Reference Nelsen, Lücking, Andrew, Aptroot, Cáceres, Mercado-Díaz, Rivas Plata and Lumbsch2014), but a larger sampling and the sequencing of more genes are needed to re-evaluate the status of Porina s. lat. Porina multipuncta is distantly related to the type species of Porina (P. nucula). Its closest known relative, P. austroatlantica, is a saxicolous species recently described from the Falkland Islands and characterized by a thin, rimose to sparingly areolate, off-white to pale greenish, esorediate thallus, brownish black to black, prominent perithecia with a variably developed reddish brown involucrellum and small 3-septate ascospores (McCarthy & Fryday Reference McCarthy and Fryday2009). Our phylogenetic analyses confirm that Porina multipuncta is distinct from all the other sequenced species of the genus (Fig. 1). Therefore, P. multipuncta does not appear to represent a sorediate morph of another known Porina, at least with the molecular data available. The species disperses strictly by means of soredia. A strictly sterile dispersal strategy is rare in the family Porinaceae, an example being Porina distans Vězda & Vivant that disperses by means of isidia (Vězda Reference Vězda1994), but the status of this species is still unclear as it might represent the sterile isidioid morph of another, usually fertile species (Lücking Reference Lücking2008). Within Porinaceae, some species of the tropical genus Myeloconis are also known to form brightly coloured soralia (McCarthy & Elix Reference McCarthy and Elix1996). However, these lichens are all known to be fertile, despite M. erumpens P. M. McCarthy & Elix usually being sterile. Their medulla and soralia contain bright yellow or orange pigments (viz. leucomyeloconone, myeloconone and myelocoterpene), while P. multipuncta is not known to contain secondary metabolites.
In contrast with Opegrapha multipuncta, the new generic position of Schismatomma quercicola leaves no doubt. Our molecular data clearly place S. quercicola in the genus Schizotrema, close to its type species S. zebrinum (Fig. 2). The two mtSSU sequences of S. quercicola are identical and very similar (3 different nucleotides) to that of S. zebrinum, a corticolous species endemic to south-east Australia (Australian mainland, Tasmania and Lord Howe Island) and New Zealand, where it occurs in temperate and subtropical rainforests (Mangold et al. Reference Mangold, Elix, Lumbsch and McCarthy2009; Lumbsch et al. Reference Lumbsch, Divakar, Messuti, Mangold and Lücking2010; McCarthy Reference McCarthy2018). Schizotrema zebrinum is characterized by an almost endophloeodal, smooth, greyish thallus lacking vegetative propagules, perithecioid to indistinctly apothecioid ascomata with a layered margin and moderately large, transversely septate ascospores. Some specimens have a secondary chemistry of the protocetraric acid chemosyndrome, thus similar to the chemistry of Schismatomma quercicola. Others have the stictic acid chemosyndrome or produce acids of both chemosyndromes. Except for the mode of reproduction, the thallus and the chemistry of some specimens of S. zebrinum are thus similar to those of S. quercicola. A larger sampling and ITS sequences would be needed to verify if S. zebrinum might represent the fertile morph of S. quercicola (in that case, S. zebrinum would become a synonym of S. quercicola), but this is unlikely considering their disjunct distribution in Europe and Australasia. With the new systematic position of S. quercicola, the genus Schizotrema is newly reported from Europe, the other species of the genus being so far known only from the Southern Hemisphere, China and the Caribbean (Mangold et al. Reference Mangold, Elix, Lumbsch and McCarthy2009; Zefeng & Lücking Reference Zefeng and Lücking2019). A strictly sterile dispersal strategy is known from different taxa in the family Graphidaceae (e.g. the genus Heiomasia and the species Diorygma antillarum, Myriotrema frondosolucens Lücking and M. maroense Lücking), and might be more widespread because sterile trentepohlioid lichens are frequent in tropical regions but are still poorly studied.
We wish to warmly thank Cyrille Gerstmans for his technical assistance with the figures. We are also grateful to Myriam Dehaan and Wim Baert for their help with the molecular work. Financial support by the Norwegian Biodiversity Information Centre to AF and JK (species project: ‘Three storied diversity: mapping and barcoding crustose lichens and lichenicolous fungi in the Norwegian rainforests’) is kindly acknowledged.