Introduction
Although more severe brain injuries have long been associated with persisting neurocognitive deficits (Anderson, Catroppa, Morse, Haritou, & Rosenfeld, Reference Anderson, Catroppa, Morse, Haritou and Rosenfeld2009; Babikian et al., Reference Babikian and Asarnow2009; Fay et al., Reference Fay, Jaffe, Polissar, Liao, Rivara and Martin1994; Walz, Yeates, Taylor, Stancin, & Wade, Reference Walz, Yeates, Taylor, Stancin and Wade2010), an increasing body of literature has shown that, in general, children and adolescents with single, uncomplicated mild traumatic brain injury (mTBI) do not exhibit long-lasting neurocognitive impairments based on performance based measures, which is in contrast to persisting post-concussive symptoms (Yeates, Reference Yeates2010). This is especially true in studies that use rigorous methodologies to define a “mild” injury, discrete time points for assessing outcomes, and appropriate control groups (Asarnow et al., Reference Asarnow, Satz, Light, Zaucha, Lewis and McCleary1995; Babikian et al., Reference Babikian and Asarnow2009, 2011). Nonetheless, in some studies, including our own (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011), and through clinical experience, several children and adolescents, following what appear to be relatively mild injuries based on known clinical characteristics, exhibit persisting complaints of cognitive symptoms such as inattention or poor memory following a mTBI that are reported to result in absences from school and other activities, as well as decreased quality of life. This study reports on a minority of children with mild TBI who appear to have persisting performance based neurocognitive deficits by comparing them to a matched group of children with an injury to a body part other than the head to explore potential predictors of those who show persistent cognitive deficits.
In general, the extant literature describing the predictors of neurocognitive functioning following any severity of brain injury in childhood has focused on characteristics of the brain injury, typically including gross injury severity indices (e.g., Glasgow Coma Scale, GCS), length of impaired consciousness, and number of observable lesions (Babikian et al., Reference Babikian, Freier, Tong, Nickerson, Wall, Holshouser and Ashwal2005; Prasad, Ewing-Cobbs, Swank, & Kramer, Reference Prasad, Ewing-Cobbs, Swank and Kramer2002). Non-injury variables, including family resources and pre-injury family environment (Taylor et al., Reference Taylor, Yeates, Wade, Drotar, Klein and Stancin1999, Reference Taylor, Yeates, Wade, Drotar, Stancin and Minich2002; Yeates et al., Reference Yeates, Taylor, Drotar, Wade, Klein, Stancin and Schatschneider1997), have also been shown to predict neurocognitive outcomes; however, little if any work has been published on the predictors of neurocognitive outcomes strictly within the mild injury group.
Many patients with a mTBI complain of concussive symptoms. The time course and predictors of post-concussive symptoms have been well documented in a large longitudinal sample of young mTBI patients (Yeates, Reference Yeates2010; Yeates et al., Reference Yeates, Taylor, Rusin, Bangert, Dietrich, Nuss and Jones2009, Reference Yeates, Taylor, Rusin, Bangert, Dietrich, Nuss and Wright2012). Premorbid symptoms and behavioral adjustment have shown to predict post-injury concussive symptoms. Mild TBI, particularly accompanied by loss of consciousness, post-traumatic amnesia, or other changes in mental status (Yeates et al., Reference Yeates, Taylor, Rusin, Bangert, Dietrich, Nuss and Jones2009), was independently associated with more post-concussive symptoms within the first year of injury relative to another injury group (Yeates et al., Reference Yeates, Taylor, Rusin, Bangert, Dietrich, Nuss and Wright2012), with cognitive symptoms lasting longer than somatic symptoms. Furthermore, children with lower cognitive ability were more likely to show ongoing problems than children with better cognitive ability, suggesting a vulnerability to the effects of a brain injury in lower functioning children (Fay et al., Reference Fay, Yeates, Taylor, Bangert, Dietrich, Nuss and Wright2010). Retrospective reports of premorbid symptoms by parents also predicted post-concussive symptoms at 3 months (Fay et al., Reference Fay, Yeates, Taylor, Bangert, Dietrich, Nuss and Wright2010).
Recently, we found that, in a large sample of children and adolescents with uncomplicated mTBI ascertained from emergency room admissions, injured patients performed more poorly than healthy controls on several neurocognitive measures (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011). However, this finding was not specific to the brain injured group. The neurocognitive performance of an orthopedic control group recruited from the same emergency rooms as the children with mTBI was the same as that of children and adolescents with a brain injury, suggesting a general injury effect (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011). However, a relatively large proportion of the children and adolescents in the mTBI and orthopedic control groups (approximately one third) scored at least one standard deviation below the healthy control group's mean on 3 or more of the 10 neurocognitive measures. Only approximately 10% of the healthy control children scored in this range (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011). This parallels clinical experience for which a small subset of mTBI cases appears to have long-lasting cognitive problems. Many of the adverse outcomes, including poor neurocognitive functioning, are found in the premorbid histories of children who incur accidental injuries, including TBI (Asarnow, Satz, Light, Lewis, & Neumann, Reference Asarnow, Satz, Light, Lewis and Neumann1991; Asarnow et al., Reference Asarnow, Satz, Light, Zaucha, Lewis and McCleary1995; Bijur, Haslum, & Golding, Reference Bijur, Haslum and Golding1990). Expanding on the findings from our previous study, in this study, we determined the relative contributions of indices of injury severity and premorbid function in predicting 1-month and 1-year neurocognitive impairments in a large sample of mild pediatric head injury patients and orthopedic controls. In addition, we examined the effect of post-injury psychosocial variables (stressors and problems) in predicting 1-year neurocognitive outcomes. In doing so, the following questions were systematically addressed in this study: (1) Do clinical (injury/severity related), premorbid, and/or demographic factors predict 1-month post-injury cognitive impairment status? If so, to what degree? (2) Do clinical (injury/severity related), premorbid, demographic, and/or post-injury family or behavioral factors predict 12-month post-injury cognitive impairment status? If so, to what degree? (3) To what extent do clinical (injury/severity related), premorbid, demographic, and/or post-injury family or behavioral factors predict 12-month neurocognitive functioning in those who are identified as cognitively impaired?
Methods
The study methodology is described in greater detail elsewhere (Asarnow et al., Reference Asarnow, Satz, Light, Zaucha, Lewis and McCleary1995; Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011). In brief, children and adolescents who had incurred a mTBI were recruited from consecutive admissions to 14 different emergency rooms in the greater Los Angeles area. A control group of children with injuries other than the head [Other Injury (OI) group] was recruited from the same emergency rooms as the mTBI sample. The children in the other injury group were matched to the mTBI group on gender, age, ethnicity, socio-economic status, and injury severity level. All data were collected in accordance to our institutional guidelines for human subjects research and approved by the UCLA institutional review board. Parents consented and children and adolescents assented before entry into the study. Data collection was initiated in 1989 and completed in 1997.
As described in greater detail previously (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011), The Abbreviated Injury Scale (AIS) (Greenspan, McLellan, & Greig, Reference Greenspan, McLellan and Greig1985) was used as a measure of injury severity because this metric allowed for comparability across both the head injury and the other injury groups. Only AIS scores of 1 and 2 were included, which resulted in a relatively more uniformly “mild” spectrum of injuries within what is traditionally defined as “mTBI” in the literature. A few subjects with an AIS score of 3 who were initially enrolled were not included in these analyses to ensure the uniformity of the sample with regard to injury severity. AIS scores were derived from medical records and used to determine eligibility for enrollment in the study. Inclusion criteria for the head injury group consisted of: (a) presentation to a participating emergency room for an injury involving and resulting in an AIS score of 1 or 2; (b) no injuries above AIS level 2 at any anatomic location; (c) injury from unintentional external causes; (d) no litigation related to injury; (e) no serious injury or death of others involved in the index accident; (f) treated at 1 of 14 emergency rooms located in one of three counties within the greater Los Angeles area; (g) aged 8–17 years at the time of injury; (h) no significant pre-existing central nervous system damage or serious chronic diseases (e.g., cancer, congenital malformation); (i) parent/guardian consent; and (j) child residing with parent/guardian. Computed tomography scans were not available for review and, therefore, did not play a role in the selection of subjects for the study. Inclusion criteria for the other injury group included criteria b–j above, with AIS scores of 1 or 2 derived for injuries to a part of the body other than the head for the injury resulting in their enrollment in this study, as described in Greenspan (1985). Children with injuries that caused restricted movement of the hands/arms or discomfort during testing were excluded.
Participants were studied prospectively and assessments were conducted in the homes of the children to minimize attrition. The prospective nature of the study and relatively low attrition rates minimized methodological and sampling biases typically present in retrospective studies or samples of convenience. Initial data were collected shortly after injury (1 month) to ensure that pre-injury information (e.g., history of learning/behavioral problems and information about prior injuries) could be collected retrospectively in such a way to minimize reports biased by the subsequent course of the injury and to provide data on the acute injury characteristics. Information about injury related symptoms and injury characteristics were based on a series of multiple choice or open ended questions that parents answered at approximately 1-month post-injury in a one-on-one personal interview at their home. Pre-injury functioning was characterized using data derived from parental interviews and standardized questionnaires, including the Child Behavior Checklist (Achenbach, Reference Achenbach1991), which parents were instructed to complete with information about their child's functioning for the period roughly 6 months before the index incident.
Ten cognitive tasks were administered that fell within the following four domains of cognitive functioning shown in prior studies to be sensitive to TBI: memory (prospective, visual, and verbal memory), motor/psychomotor functioning (motor and processing speed), attention/executive functions (attention span, sustained attention, and inhibition), and general language (naming vocabulary). Table 1 lists the tasks administered for this study. Raw scores were used on all tasks with the exception of the Picture Vocabulary Test, where published norms standardized for age were used. Raw scores were used because (1) they generally provide more sensitive indices of change than scaled scores and (2) many of the tasks were developed specifically for this study and, therefore, standardized norms were not available. Age at the time of assessment was modeled in the analyses to account for normal age related differences in performance in the neurocognitive measures. A detailed description of the tasks and the scores derived from them is contained in a previous report (Asarnow et al., Reference Asarnow, Satz, Light, Zaucha, Lewis and McCleary1995) and summarized in Table 1. The same battery of neurocognitive tests was administered at the 1-month and 12-month evaluations.
Table 1 List of tests summarized for each domain, accompanied by a brief description

Note. See Asarnow (1995) for a comprehensive description of the tests and scores presented for each of the domains above. Raw scores were used on all measures with the exception of the Peabody Picture Vocabulary Test, for which age corrected standard scores were available.
Outcome Variables
A binary impaired variable (impaired vs. non-impaired) was modeled for both follow-up time points (1-month and 12-month evaluations). Injured participants were designated as neurocognitively impaired if they had scores on three or more of the 10 neurocognitive tests listed in Table 1 that were 1.5 standard deviations or more below the mean of a normative sample per age group (2-year age bands). The cutoff of 2 or fewer impairments from 10 administered tests was derived from recent literature documenting the rates of impairments on neurocognitive measures in normal healthy populations (of adults) (Schretlen, Munro, Anthony, & Pearlson, Reference Schretlen, Munro, Anthony and Pearlson2003), which was similar to literature on base rates of abnormal scores in healthy children (Brooks, 2010; Brooks, Sherman, & Iverson, Reference Brooks, Sherman and Iverson2010). The normative sample was a group of matched non-injured children that has been described in greater detail previously (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011). Further analyses were then conducted on this subset of the sample that was identified as impaired (separately for each of the two time points) based on the above criteria (n = 61 for the two injured groups combined). For this subset, cognitive functioning was characterized by a single unweighted factor score derived from a factor analysis of 6 of the 10 tests that showed most sensitivity with regard to group differences (i.e., differentiated the injured groups from the healthy control group) in a prior study (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011). The neurocognitive tests from which this summary score was derived were: Picture Memory, Prospective Memory, Word List Learning, Symbol Digit Modalities, Color Trails (Part B), and PPVT-R (see Table 1 for a description of the tests and the respective scores used in analyses). These are measures of broad cognitive abilities, including measures of memory/working memory, timed psychomotor tasks, and a measure of vocabulary. The tasks that did not differentiate between the groups and, thus, were not included were measures of pure motor speed and basic attention span.
Predictor Variables
Study variables used as predictors of outcome in the analyses were grouped as follows: (i) clinical (e.g., injury severity, type of injury, number of head injuries, severity of recent injury, number of concussive symptoms from recent injury, with the latter two referring only to the TBI group), (ii) premorbid/demographic (e.g., parent report of premorbid behavioral/emotional problems, premorbid academic achievement, parental education, history of diagnosed problems), and (iii) post-injury functioning (e.g., parent report of post-injury behavioral/emotional problems, family stress, cognitive impairment at 1-month post-injury). These are described in detail in Table 2.
Table 2 Description of predictor variables used in models

Statistical Methods
Logistic regressions were used to model a binary (impaired vs. non-impaired) outcome. Univariate and multivariate odds ratios were calculated using routines by Kundu, Aulchenko, van Duijn, and Janssens (Reference Kundu, Aulchenko, van Duijn and Janssens2011) in R version 2.13.2 (R_Development_Core_Team, 2011), which fit a standard GLM function for the logistic regression models. Brier scores were used to quantify accuracy of risk predictions by comparing predicted risks with observed outcomes at the individual level (where outcome values were either 0 “not impaired” or 1 “impaired”) (Brier, Reference Brier1950). The Brier Score is probably the most commonly used verification measure of assessing accuracy of probability forecasts. The score is the mean squared error of the probability forecasts for the verification sample; therefore, a lower score represents high accuracy with 100% accuracy for a Brier score 0 and poor accuracy with increasing Brier scores. Nagelkerke's R2, the percentage of variance of the outcome explained by the predictors in the model, was also used; it is a generation of the coefficient of determination R2 for general regression models (Nagelkerke, Reference Nagelkerke1991). Lastly, linear regressions were used to model a single continuous variable of cognitive functioning (as described above) within the subgroup of subjects identified as “impaired.” We conducted all of the analyses presented in the study separately by group to determine if our results would be different and did not see any differences in any of the analyses performed. Also, group status (TBI vs. OI) was not a predictor of our primary outcomes (impairment at 1 month or 12 months). Therefore, only the combined analyses were presented in the study. Furthermore, collinearity in the multivariate analyses reported in Tables 4 and 5 can be indexed by a collinearity index (Brun and Reichert, Reference Brun and Reichert2001), which assesses the degree of overall linear dependence between sets of parameters. Calculations of collinearity based on R package FME (Soetaert and Petzoldt, Reference Soetaert and Petzoldt2010), version 1.3, show that in no instance does any subset within Table 4 or 5 achieve an index value higher than 4.923, where the critical value for such dependence is generally taken to be at least 20. Thus, collinearity is estimated to play no central role in the reported findings.
Results
Subject Description
Table 3 summarizes participant demographics for each of the two injury groups. The sample consisted of 85 mTBI subjects and 92 Other Injury subjects at the 1-month evaluation (total N = 177) and 76 mTBI subjects and 79 Other Injury subjects at the 12-month evaluation (11% attrition for the mTBI group and 14% attrition for the Other Injury group). In general, the Other Injury group was slightly older than the mTBI group by an average of 1 year (p = .01). As a consequence, age was included as a covariate in all analyses. The groups were comparable in gender. With regard to the cognitive outcome and predictor variables, the two groups were also comparable on all of the variables with the exception of the head injury severity variables (with the mTBI group having greater severity of recent injury and a greater number of previous head injuries, as would be expected). Furthermore, parents of the mTBI group reported a higher number of years of education and the Other Injury group had a higher proportion of subjects classified as “Impaired” at 12 months (see Table 3).
Table 3 Demographic and clinical makeup of study participants

NS = not statistically significant.
Predicting 1-Month Neurocognitive Outcomes
Table 4 presents the results of both univariate and multivariate logistic regressions predicting binary (impaired vs. non-impaired) 1-month post-injury neurocognitive outcomes in the combined TBI and Other Injury groups for two categories of predictors: clinical (injury related) and demographic/premorbid problems. The clinical variables that only pertained to the head injury group (severity of head injury and extent of concussive symptoms) were evaluated only in the mTBI group.
Table 4 Predicting cognitive impairment (binary outcome of impaired/not impaired) at 1-month post injury using logistic regression models

Age was included in all of the logistic regression models. Neither age nor any of the clinical variables were predictive of the “impaired” status of a subject, including whether their injury was to the head or to another part of the body. The overall model explained less than 2% of the variance in the outcome (Table 4, ‘Clinical’ Panel). In stark contrast, in univariate analyses, premorbid academic functioning, the presence of a diagnosed learning problem, premorbid behavioral problems, and parental education were all predictive of neurocognitive impairment 1-month post-injury. In a multivariate model, all but premorbidly diagnosed learning problems individually contributed to predicting impaired neurocognitive functioning 1-month post-injury. Together, these variables explained 28.4% of the variance in the neurocognitive impairment classification (See Table 4, Demographic & Premorbid Panel).
Predicting 12-month Outcomes
Table 5 presents the results of both univariate and multivariate logistic regressions predicting impaired neurocognitive functioning at 12-months post-injury in the combined TBI and Other Injury groups for three categories of predictors: clinical, demographic/premorbid problems, and post-injury functioning variables. In addition, a fourth additional set of predictors composed of the most robust predictors of 12 month neurocognitive outcome from the above categories in univariate analyses were modeled. Again, age was included in all of the logistic regression analyses. Age was predictive of outcome (explaining less than 5% of the variance in impairment status) in both univariate and multivariate models. However, similar to the 1-month models, none of the injury severity variables were predicted which participants had neurocognitive impairments (Table 5, Clinical Panel). Of the demographic/premorbid variables, academic achievement, presence of a diagnosed learning problem, and parental education all predicted neurocognitive impairment in univariate models. In a multivariate model, only premorbid academic achievement remained a statistically significant predictor, with the entire model predicting 18% of the total variance (Table 5, Demographic and Premorbid Panel). Of the post-injury functioning variables, post-injury behavioral/emotional problems and neurocognitive performance at the 1-month evaluation predicted neurocognitive impairment in univariate models. In a multivariate model, only the 1-month neurocognitive performance was predictive of neurocognitive impairment at 12 months, explaining 54% of the variance in neurocognitive impairment classification (Table 5, Post-Injury Functioning Panel). Lastly, a fourth model that included only the most robust predictors from all of the above univariate analyses was analyzed. In this multivariate model, which included premorbid academic functioning and learning problems diagnoses, post-injury behavioral/emotional problems, and neurocognitive performance at 1-month post-injury, only the latter variable maintained independent statistical significance as a predictor, with the overall model explaining 55% of the variance (Table 5, Premorbid & Post-Injury Functioning Panel). The above analyses were repeated for only subjects (from both injury groups, n = 61) who were identified as impaired, with the outcome being an index of their overall neurocognitive functioning status (as described in Table 2: Impairment Factor). As in the larger sample, none of the injury severity variables explained a notable amount of the variance in neurocognitive performance status at 12-months post-injury in either the univariate or multivariate models. In contrast, both premorbid academic achievement and parent education predicted 12 month neurocognitive performance in both univariate and multivariate models.
Table 5 Predicting cognitive impairment (binary outcome of impaired/not impaired) at 12-months post injury using logistic regression models.

Changes in Impairment Status Over Time
Based on the binary neurocognitive impairment classification used above, 57% of the mTBI group and 50% of the Other Injury group were classified as not impaired at either time point while 20% of the mTBI and 24% of the Other Injury group was classified as impaired at both time points. Compared to those who were not classified as impaired at either time point, those with impairments at both time points came from families with lower parental education (10.9 years vs. 13.2 years; p = .002), had poorer pre-injury academic achievement (p ≤ .001), had a higher number of premorbidly diagnosed learning and/or behavioral problems (p ≤ .001), and evidenced a higher number of premorbid (p ≤ .001) and post-injury (p ≤ .001) parent reported emotional/behavioral problems. However, the two groups were not different with respect to the injury characteristics, including injury severity or whether the index injury was to the head. Very similar findings were observed when the above analyses were conducted separately by injury group (mTBI vs. Other Injury), with the exception that in the TBI group only, parent education was not different among those impaired at both time points versus those without impairment at both time points (p = .144), but differences were observed in the Other Injury group (p = .010) (Table 6). Taken collectively, these findings suggest that the neurocognitive impairments seen in children who were impaired at both 1-month and 1-year post-injury reflect premorbid characteristics of the child and family and not the effect of a mTBI.
Table 6 Changes in Impairment Status by Group over Time

Furthermore, 15% of the TBI group and 19% of the Other Injury group who were identified as impaired at the 1-month evaluation were not considered impaired at the 12-month evaluation. Since the TBI and Other Injury groups were too small to conduct subgroup analyses, they were combined. In this subsample, the only correlates of neurocognitive functioning were parent education, with a correlation of .405 (p = .040) with 1-month neurocognitive performance and a correlation of .628 (p = .001) with 12-month neurocognitive performance. None of the other clinical, demographic, or post-injury variables (including stress or number of concussive symptoms) were correlated with neurocognitive functioning at either time point (Table 6).
However, 8% of the TBI group and 6% of the Other Injury group were not classified as impaired at the 1-month evaluation but were at the 12-month evaluation. In this small subgroup only, the only variable from the clinical, demographic, and premorbid factors evaluated that was correlated with cognitive functioning at the 1-year evaluation was injury severity (r = .561; p = .073) (Table 6). After careful review of this small subset, however, the small, late decline in a few of the subjects’ scores in the first year in both groups is likely due to random variation and/or regression to the mean as the scores evaluated appeared to fall just below or just above the binary cutoff determined for a designation of “impaired.”
Discussion
The purpose of the current research was to determine the relative contributions of indices of injury severity, clinical symptomatology, demographic factors, and premorbid functioning in predicting 1-month and 1-year performance-based neurocognitive impairment in a large sample of mild pediatric head injury patients and orthopedic controls. None of the injury severity indicators or type of injury (head vs. other body part) predicted either 1-month or 12-month cognitive impairment status. Rather, premorbid variables that antedated the injury (parental education, premorbid behavior, and/or learning problems, and school achievement) predicted cognitive impairments. In summary, the best predictor of 1-month impairment classification was school achievement, followed by parent education and premorbid behavioral and academic problems, while the best predictor of 12-month impairment classification was 1-month impairment classification (Figure 1). Of interest, of the mild injury group, 97% of the parents at the 1-month evaluation reported at least one concussive symptom that was present acutely (e.g., headache, vertigo), although the presence or the severity of the concussive symptoms was not predictive of neurocognitive impairments. Although the mTBI group had a higher number of lifetime head injuries (ranging from 1 to 12 with a mean of 2) than the OI group (ranging from 0 to 3 with a mean of 0.5), raising questions about the deleterious effects of multiple impacts, the number of previous head injuries was not a predictor of outcomes in the two groups combined or when this variable was considered separately in each of the two groups.
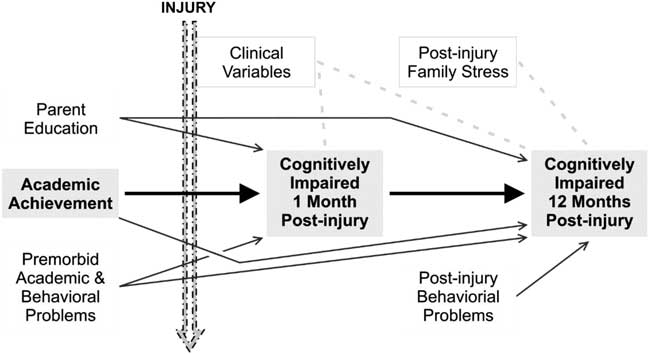
Fig. 1 Model of inter-relationships between clinical, demographic, premorbid, and post-injury functioning variables with 1-month and 12-month post injury cognitive impairment.
The implications of this research are several. For parents and care providers, it is hopeful news that, despite growing evidence of acute, transient alterations chemically, structurally, and metabolically following a TBI, a single uncomplicated mild head injury is unlikely to result in long-term neurocognitive impairment. Rather, it appears as though the cognitive impairments observed in a third of the children who have a mTBI likely predate the index injury. It is possible that learning difficulties, behavioral challenges, and/or poor school performance predating the injury may place these children at greater risk for getting injured (head or other body part), and may have come to attention only after the index injury. It is, therefore, crucial in clinical settings to acquire a sound understanding of a young patient's premorbid family background, as well as their pre-injury functioning, before attributing causality to post-injury cognitive impairments. Our study has shown that when post-injury neurocognitive impairments are observed in survivors of mild injuries (both head injury and injuries to body parts other than the head) or following uncomplicated single concussions, understanding the etiologies of these impairments is critical in designing intervention plans. A child with a developmental learning or cognitive problem is very different than a child with no history of learning or academic problems, who suddenly is faced for the first time with challenges at school or in day-to-day functioning.
Another implication of the current findings is that the acute neurocognitive evaluation is the single best predictor of chronic neurocognitive problems, highlighting the importance of using these early evaluations to identify individuals who may be at risk for long-term problems and secondly to help guide interventions, including a plan to return to school. In our sample, at least four fifths in each injury group showed consistency in their impairment classification, namely 20–25% of the sample was identified as impaired at both time points and approximately half of the subjects were classified as not impaired at both time points. A small proportion showed impairments initially but not at the 12-months post-injury follow-up. In this group, cognitive functioning at 12-months was unrelated to injury variables, but rather predicted by premorbid functioning and demographic variables (similar to the findings from the larger sample).
As observed in our study, almost all of the children with a mild injury experienced at least one concussive symptom per parent report of their acute presentation, and in many cases, several (mean number of symptoms 2.9; SD 1.2). By definition, none of the children in the other injury group were reported to have concussive symptoms. The above suggests that by the 1-month post-injury time point, there is no indication of injury related deficits in cognition based on performance based measures despite the presence of concussive symptoms, which, consistent with the works of Yeates and colleagues (2009) in children and Kashluba and colleagues (Reference Kashluba, Paniak, Blake, Reynolds, Toller-Lobe and Nagy2004) in adults, have their own ramifications on a child's return to school as well as academic, social, and behavioral functioning. For instance, initial transient problems even if not directly related to the brain injury itself, residual post-concussive type symptoms, emotional distress and stress related to a traumatic event and related medical procedures, treatments, and visits may all have consequences on a young person's life. This could involve missing classes and falling behind and performing poorly in school (even if transiently). Furthermore, since premorbid learning problems are relatively more common in these children, temporary stresses to an already stressed system could plausibly exacerbate premorbid problems and result in more pronounced deficits and problems that have real-world consequences on academic learning, emotional functioning, and general well-being. All of these risk factors and issues are relevant not only to the brain-injured group our analyses have highlighted, but additionally to the population of young patients at risk for acquiring an injury.
The focus of this study and the series of articles published from the UCLA longitudinal study of pediatric mild TBI in a well-controlled pediatric population has been neurocognition. There appears to be a dose response with regard to the severity of injury and persisting cognitive deficits, with mTBI resulting in very short-term cognitive sequelae and more severe injuries result in persisting cognitive deficits (Babikian, Reference Babikian and Asarnow2009), but the threshold of injury severity resulting in these lasting deficits is unknown. The findings from this study and our preceding study (Babikian et al., Reference Babikian, Satz, Zaucha, Light, Lewis and Asarnow2011) suggest that long-term neurocognitive impairments may be present (in approximately a third of our sample), but are unlikely to be caused by a single, uncomplicated, mild TBI. A substantial number of children who incur a mild TBI or orthopedic injury show only transient neurocognitive impairments. It is important in clinical settings to consider the effect of other aspects of a patient's neuropsychological and neurological functioning, aside from cognition, that may also have significant implications in day-to-day life for a young individual and their family.
Several limitations associated with this study are important to acknowledge. No data were collected on eligible subjects who chose not to participate, therefore, potentially contributing to a bias in the sample included in the current analyses. We also did not have access to GCS scores or neuroimaging data to better characterize the nature and/or severity of the injury. The injury severity variable (AIS) was very limited in identifying a range of acute injury severity within the mild TBI group that could potentially explain the poor outcomes in a small subset of the TBI group. Also, because only AIS scores of 1 or 2 were included in the analyses, it is important to note that the sample in this study was comprised of a relatively mild sample that included no “complicated” cases. However, the purpose of the study was to focus on single, uncomplicated injuries and exclude more severe injuries known to result unambiguously in impaired cognition. Finally, symptom reports, including measures of concussive symptoms and injury severity ratings, were based on parent report of initial presentation in the emergency room collected retrospectively at approximately 1-month post-injury and not corroborated with child report of symptoms or medical records.
Acknowledgments
There are no conflicts of interest to report. This study was funded by NINDS R01NS026801 Neurobehavioral Sequelae of Mild Brain Injury in Children; and NICHD R01HD061504 Reconnection of Neural Networks and Cognitive Recovery After Pediatric TBI.









