Introduction
Rare Cr-bearing oxides mcconnellite, CuCrO2, and ellinaite, β-CaCr2O4, were found in varicoloured marbles of the pyrometamorphic Hatrurim Complex in the Tulul Al Hammam area, Daba-Siwaqa, central Jordan in association with a recently discovered new garnet of the bitikleite group – priscillagrewite-(Y), (Ca2Y)Zr2Al3O12 (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Zeliński and Prusik2021). The results of previous investigations of both mcconnellite, described more than 40 years ago (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976), and a new mineral ellinaite (Sharygin, Reference Sharygin2019; Sharygin et al., Reference Sharygin, Britvin, Kaminsky, Wirth, Nigmatulina, Yakovlev, Novoselov and Murashko2020) allow for some important questions to be made.
Mcconnellite (CuCrO2, R $\bar{3}$![]() m, a = b = 2.983(4) Å, c = 17.16(3) Å) was known from only one locality – Merume River, Kamakusa, Potaro-Siparuni Region, Guyana, in hydrothermal Cr-bearing ore, so called, ‘merumite’ type (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). The mcconnellite description contains incomplete chemical data and its identification was based on a few lines from a diffraction pattern coinciding with the lines of a synthetic analogue (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). Mcconnellite was simultaneously described in association with three other new minerals: grimaldiite, α-CrOOH; guyanaite, β-CrOOH; and bracewellite, γ-CrOOH (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). Mcconnellite, according to the authors’ opinion, occurs in epitaxial intergrowth with isostructural grimaldiite, α-CrOOH (R $\bar{3}$
m, a = b = 2.983(4) Å, c = 17.16(3) Å) was known from only one locality – Merume River, Kamakusa, Potaro-Siparuni Region, Guyana, in hydrothermal Cr-bearing ore, so called, ‘merumite’ type (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). The mcconnellite description contains incomplete chemical data and its identification was based on a few lines from a diffraction pattern coinciding with the lines of a synthetic analogue (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). Mcconnellite was simultaneously described in association with three other new minerals: grimaldiite, α-CrOOH; guyanaite, β-CrOOH; and bracewellite, γ-CrOOH (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). Mcconnellite, according to the authors’ opinion, occurs in epitaxial intergrowth with isostructural grimaldiite, α-CrOOH (R $\bar{3}$![]() m, a = b = 2.973(2) and c = 13.39(1) Å), forming zones unevenly enriched in Cu to ~10 μm in thickness in grimaldiite (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). A phase similar to mcconnellite has been identified in a historical Cu–Pb-slag from Tsumeb, Namibia (Ettler et al., Reference Ettler, Johan, Křibek, Šebek and Mihaljevič2009). Recently, synthetic CuCrO2 and other materials with a delafossite-type structure have been studied extensively because of their thermoelectric, optoelectric, electric, magnetic and superconductor properties (Kumar et al., Reference Kumar, Marinel, Miclau and Martin2012; Monteiro et al., Reference Monteiro, Jurelo and Siqueira2017; Majee and Bhobe, Reference Majee and Bhobe2020). Synthetic delafossite with the general formula CuR 3+O2 is prepared at different temperatures by performing a solid-state reaction, usually at temperatures >850°C, by the sol-gel method, with a high-temperature post treatment (>650°C) or by hydrothermal synthesis at 180°C (Kim et al., Reference Kim, Kim, Choi and Youn2020).
m, a = b = 2.973(2) and c = 13.39(1) Å), forming zones unevenly enriched in Cu to ~10 μm in thickness in grimaldiite (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). A phase similar to mcconnellite has been identified in a historical Cu–Pb-slag from Tsumeb, Namibia (Ettler et al., Reference Ettler, Johan, Křibek, Šebek and Mihaljevič2009). Recently, synthetic CuCrO2 and other materials with a delafossite-type structure have been studied extensively because of their thermoelectric, optoelectric, electric, magnetic and superconductor properties (Kumar et al., Reference Kumar, Marinel, Miclau and Martin2012; Monteiro et al., Reference Monteiro, Jurelo and Siqueira2017; Majee and Bhobe, Reference Majee and Bhobe2020). Synthetic delafossite with the general formula CuR 3+O2 is prepared at different temperatures by performing a solid-state reaction, usually at temperatures >850°C, by the sol-gel method, with a high-temperature post treatment (>650°C) or by hydrothermal synthesis at 180°C (Kim et al., Reference Kim, Kim, Choi and Youn2020).
Ellinaite is a new mineral, approved in 2020, was described from two localities and studied using only a few grains: a μm-sized inclusion in diamond from Brazil (co-type, Kaminsky et al., Reference Kaminsky, Wirth and Schreiber2015) and inclusions of 20 μm in pyrrhotite from gehlenite-bearing breccia with phosphides (holotype; Sharygin Reference Sharygin2019; Sharygin et al., Reference Sharygin, Britvin, Kaminsky, Wirth, Nigmatulina, Yakovlev, Novoselov and Murashko2020). A large specimen of gehlenite-bearing breccia was found ex situ by Mikhail Murashko in 2005 at the dry Zohar Wadi in the Negev Desert (Hatrurim Basin), Israel (Galuskin et al., Reference Galuskin, Galuskina, Vapnik and Murashko2020). The small size and limited amount of materials did not allow for investigation into the detailed physical properties and to also accurately determine structural parameters of ellinaite from this occurrence (Kaminsky et al., Reference Kaminsky, Wirth and Schreiber2015; Sharygin, Reference Sharygin2019; Sharygin et al., Reference Sharygin, Britvin, Kaminsky, Wirth, Nigmatulina, Yakovlev, Novoselov and Murashko2020). For ellinaite from Israel, the following structural data were obtained: an orthorhombic crystal system, Pnma space group, a = 8.868(9), b = 2.885(3), c = 10.355(11) Å and V = 264.9(5) Å3 (Sharygin et al., Reference Sharygin, Britvin, Kaminsky, Wirth, Nigmatulina, Yakovlev, Novoselov and Murashko2020). The cell parameters of the ellinaite inclusion in diamond were obtained based on electron diffraction (HRTEM): orthorhombic, Pnma, a = 9.017, b = 2.874, c = 10.170 Å and V = 263.6 Å3 (Kaminsky et al., Reference Kaminsky, Wirth and Schreiber2015). These parameters differ distinctly from the cell parameters obtained by us for ellinaite from a new location in Jordan: Pnma, a = 9.0875(2), b = 2.96980(10), c = 10.6270(3) Å and V = 286.80(2) Å3, which are close to the parameters of the synthetic β-CaCr2O4 (Pnam, a = 9.083(2), b = 10.629(2), c = 2.971(1)Å and V = 286.8(1) Å3, Zhai et al., Reference Zhai, Yin, Shieh, Shan, Xue, Wang, Yang and Higo2016). The chemical composition and Raman spectroscopy data of Israeli ellinaite (Sharygin, Reference Sharygin2019) prove that this is a natural analogue of β-CaCr2O4.
In the present paper, we provide new data on the composition and structure on mcconnellite and ellinaite from Jordan. In addition, novel Raman data are provided, together with optical images of these minerals showing their real colour, which is also diagnostic property.
Methods of investigation
Composition and morphology
The morphology and composition of ellinaite, mcconnellite and associated minerals (Tables 1,2,3) were studied using optical microscopy and scanning electron microscopes (Phenom XL and PhilipsXL30/EDAX, Institute of Earth Sciences, Faculty of Natural Sciences, University of Silesia, Sosnowiec, Poland) and electron microprobe analysis (Cameca SX100, Institute of Geochemistry, Mineralogy and Petrology, University of Warsaw, Warszawa, Poland). Chemical analyses were carried out in the WDS-mode (wavelength-dispersive X-ray spectroscopy, settings: 15 keV, 10 nA and ~1 μm beam diameter) using the following lines and standards: CaKα and MgKα – diopside, AlKα – orthoclase, TiKα – rutile, FeKα – Fe2O3, SrLα – celestine, CuKα – cuprite, ZnKα – sphalerite and CrKβ – Cr2O3. Contents of other chemical elements, including some of those determined in the holotype ellinaite i.e. Na, V and Mn, are below the detection limit.
Table 1. Chemical composition (wt.%) of ellinaite from Jordan.

S.D. – standard deviation
Table 2. Chemical composition (wt.%) of mcconnellite from Jordan.

S.D. – standard deviation
Table 3. Chemical composition of minerals associated with ellinaite and mcconnellite: cuprite and Cr-spinel (core – zincochromite, rim – magnesiochromite).

n.d. – not detected
Raman spectroscopy
Raman spectra of ellinaite and mcconnellite were recorded on a WITec alpha 300R Confocal Raman Microscope (Institute of Earth Science, Faculty of Natural Sciences, University of Silesia, Sosnowiec, Poland) equipped with an air-cooled solid-state laser (488 nm) and a charge-coupled device (CCD) camera operating at −61°C. An air Zeiss LD EC Epiplan-Neofluan DIC-100/0.75NA objective (Carl Zeiss AG, Jena, Germany) was used. Raman scattered light was focused on a broadband single-mode fibre with an effective pinhole size of ~30 μm and a monochromator with a 600 g/mm–1 grating was used. The power of the laser at the sample position was ca. 20 mW. Integration times of 5 s with an accumulation of 20 scans and a resolution 3 cm–1 were chosen. The monochromator was calibrated using the Raman scattering line of a silicon plate (520.7 cm−1).
X-ray diffraction and crystal structure determination
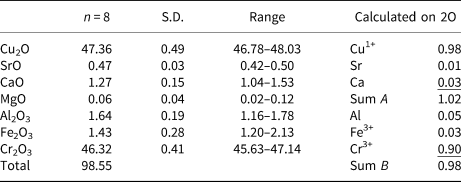
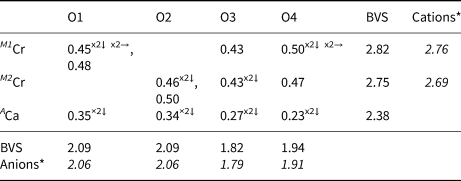
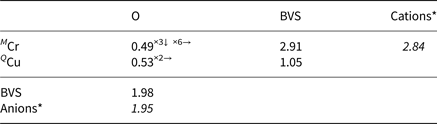
X-ray data were collected at the University of Warsaw Biological and Chemical Research Centre, using an SuperNova four-circle diffractometer equipped with an EOS CCD detector (Rigaku Oxford Diffraction), the detector-to-crystal distance was 45.8 mm. MoKα radiation (λ = 0.71073 Å) was used at 50 kV and 0.8 mA. Ellinaite and mcconnellite structures are refined based on diffraction data collected on crystals 7 × 10 × 28 μm and 8 × 10 × 18 μm, respectively (Table 4). Composition and Raman spectra were measured on the same crystals. A frame-width of 1° in ω scans and a frame time of 240 s and 100 s were used for mcconnellite and ellinaite, respectively. The full Ewald sphere was collected up to θ = 34° with R int values of 0.0208 for mcconnellite and up to 32° with R int = 0.0495 for ellinaite. Reflection intensities were corrected for Lorentz, polarisation and absorption effects and converted to structure factors using CrysalisPro® software (Rigaku-Oxford Diffraction). A numerical absorption correction based on Gaussian integration over a multifaceted crystal model was used. The crystallographic information files of both minerals have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below). Fractional atomic coordinates and displacement parameters, selected bond distances and bond valence sums (BVS) for ellinaite are given in Tables 5–7 and for mcconnellite in Tables 8–10. Both structures were solved with a dual-space iterative phasing algorithm implemented in ShelXT (Sheldrick, Reference Sheldrick2015a) that located all the positions of cations (all labelled as Ca) and O anions. Correct element assignment for cations was based upon compositional data obtained by electron microprobe analysis (EMPA) and crystal-chemical reasoning, comprising site-scattering, coordination and bond lengths. The model was refined with the least squares minimisation using Shelxl (Sheldrick, Reference Sheldrick2015b), within Olex2 (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009) as the graphical interface. When more than one element occupies the same position in the asymmetric unit, constraints for equal atom coordinates and equal anisotropic displacement parameters for these groups of atoms within each unique site were applied. The occupancies of M1 and M2 in ellinaite (Table 5) and M in mcconnellite (Table 8), were constrained to 1 and refined as M 1(Cr vs. Al), M 2(Cr vs. Mg) and M(Cr vs. Al). The A site (ellinaite, Table 5) and Q site (mcconnellite, Table 8) were refined as fractional occupancy of ACa vs. vacancy (□) and fractional occupancy of QCu vs. □, respectively.
Table 4. Crystal data, X-ray measurement and refinement parameters for ellinaite and mcconnellite.

Table 5. Fractional atomic coordinates and displacement parameters (Å2) for ellinaite.
U12=U23=0 for all atoms
* Refined fractional Cr vs. Al occupancies at M1 is xCr = 0.833(18); for Cr vs. Mg at M2 is xCr = 0.858(8); for Ca vs. ◻ at A is xCa = 0.965(4)
Table 6. Selected bond distances (Å) of ellinaite.

Table 7. Bond-valences+ and bond-valence sums (BVS) in valence units for ellinaite.

+ Calculated using the bond-valence parameters of Brown and Altermatt (Reference Brown and Altermatt1985)
* Calculated using occupancies assigned from EMPA for M1, M2, equally distributed for both sites
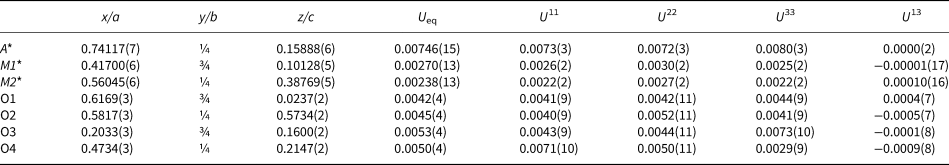
Table 8. Atom coordinates, equivalent-isotropic and anisotropic displacement parameters (Å2) of mcconnellite.

U13=U23=0 for all atoms
* Refined fractional Cr vs. Al occupancies at M is xCr = 0.833(18); for Cu vs. □ at Q is xCu = 0.946(8)
Table 9. Selected bond distances (Å) for mcconnellite.

Table 10. Bond-valences+ and bond-valence sums (BVS) in valence units for mcconnellite.

+ Calculated using the bond-valence parameters of Brown and Altermatt (Reference Brown and Altermatt1985)
* Calculated using occupancies assigned from EMPA for M.
Occurrence and material description
Mcconnellite and ellinaite were found in a sample of spurrite marble (Fig. 1a) collected from one of quarries (31°32′31′′N, 36°10′19′′E) in the Tulul Al Hammam area, which is in the pyrometamorphic field of the Daba-Siwaqa area, central Jordan. The varicoloured marble belongs to the upper part of the Maastrichtian–Paleogene Muwaqqar Chalk–Marl Unit (Khoury et al., Reference Khoury, Sokol, Kokh, Seryotkin, Nigmatulina, Goryainov, Belogub and Clark2016; Sokol et al., Reference Sokol, Kozmenko, Khoury, Kokh, Novikova, Nefedov, Sokol and Zaikin2017; Khoury, Reference Khoury2020). This unit was transformed to varicoloured marble by pyrometamorphism, and is, therefore, included in the Hatrurim Complex (Mottled Zone of Picard, Reference Picard1931). The Daba–Siwaqa area embraces numerous outcrops of the Hatrurim Complex rocks located on the Transjordan plateau south of Amman (Novikov et al., Reference Novikov, Vapnik and Safonova2013; Khoury et al., Reference Khoury, Sokol, Kokh, Seryotkin, Nigmatulina, Goryainov, Belogub and Clark2016; Khoury, Reference Khoury2020).
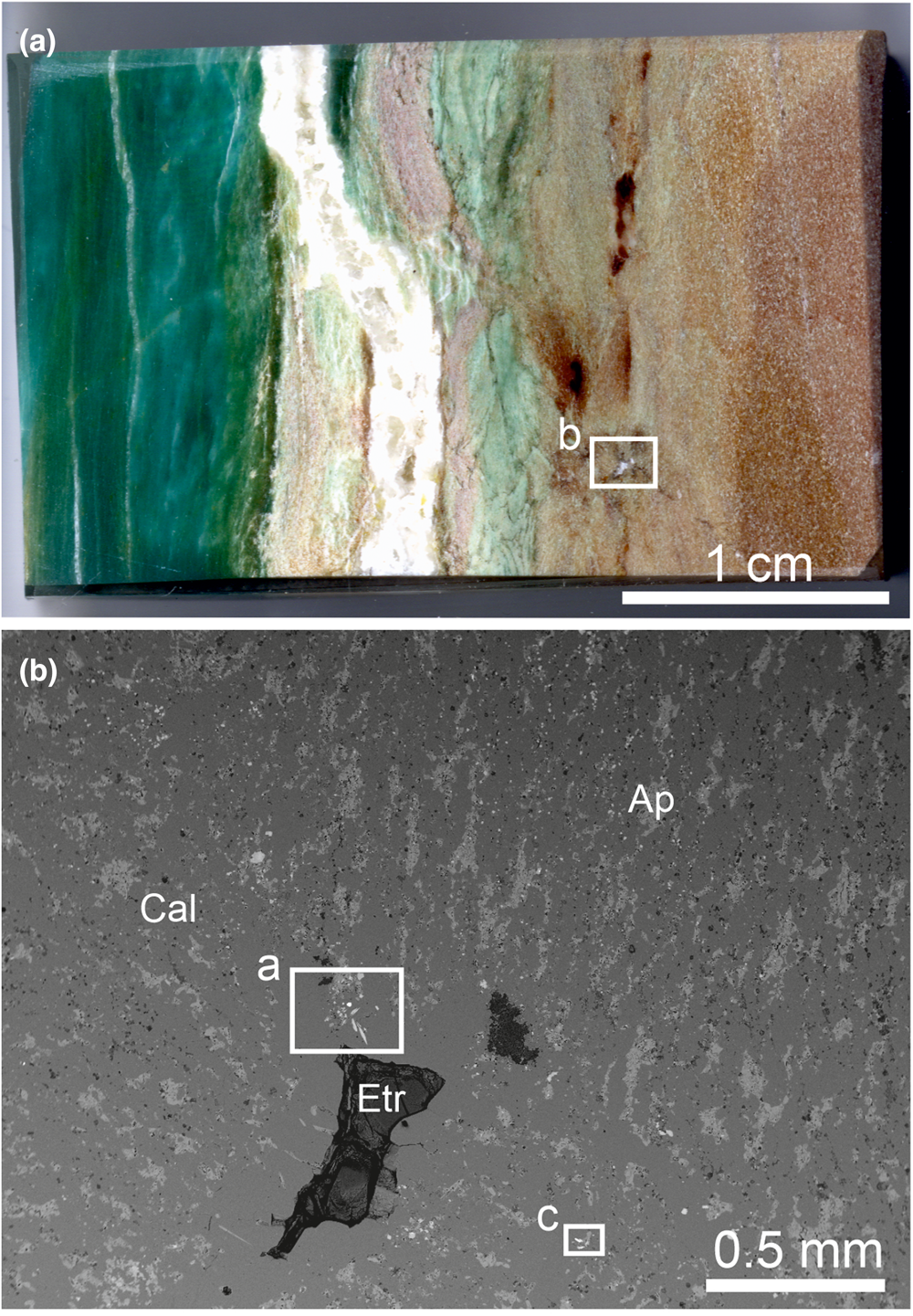
Fig. 1. (a) Polished section of varicoloured marble, in which in brown calcite zone mcconnellite and ellinaite were found and the green fluorapatite zone in which priscillagrewite-(Y) was detected. The white frame shows the area magnified in Fig. 1b. (b) Channel in marble of partially infilled ettringite, near which ellinaite (frame a) and mcconnellite (frame c) occur. Fragments in frames are magnified in Fig. 2a and 2c, respectively. Ap = fluorapatite, Etr = ettringite and Cal = calcite.

Fig. 2. (a, b) Ellinaite associated with minerals of the magnesiochromite–zincochromite series and cuprite. (a) Back-scattered electron image, (b) optical image – the characteristic bright-green colour of ellinaite is visible. (c, d) Series of sub-parallel mcconnellite crystals. (c) BSE image, (d) optical image – the characteristic purple colour of mcconnellite is visible in thin crystals. Ap = fluorapatite, Cal = calcite, Cpr = cuprite, Ell = ellinaite, Mcr = magnesiochromite–zincochromite and Mcc = mcconnellite.
The sample of spurrite marble shown in Fig. 1a contains green zones enriched in fluorapatite crystals. In these zones a new garnet, priscillagrewite-(Y), {Ca2Y}[Zr2](Al3)O12 has been found (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Zeliński and Prusik2021). In the brown part of the marble, whose colour is mainly caused by minerals of the brownmillerite–srebrodolskite series (Fig. 1a), mcconnellite and ellinaite crystals are present near a channel filled with later ettringite (Fig. 1b). In spurrite marble, besides calcite, spurrite, fluorapatite, minerals of the brownmillerite–srebrodolskite series and priscillagrewite-(Y), the following accessory minerals are found: fluormayenite, lakargiite, baghdadite, hematite, sphalerite, zincite, garnet of the andradite–grossular series, tululite, vapnikite, minerals of the lime–monteponite and magnesiochromite–zincochromite series, cuprite and Y-bearing and Y-free perovskite (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Zeliński and Prusik2021). Cuprite contains Cr and Ca impurities (Table 3). Zincochromite (ZnCr2O4 ≈ 54%) is the predominant end-member in the core of Cr-bearing spinel, the rim is composed of magnesiochromite (MgCr2O4 ≈ 77%; Table 3).
Elongated crystals of ellinaite up to 40 μm in length, and up to 8 μm in width have a bright-green colour, and occur in calcite (Fig. 2a, b). Ellinaite from Jordan has Ti4+, Fe3+, Al, Sr and Mg impurities and slightly enhanced Ca content in comparison with the ideal formula (Table 1). The empirical formula of ellinaite calculated on 4 oxygens is: (Сa1.00Sr0.01)Σ1.01(Cr3+1.79Al0.07Fe3+0.04Ca0.04Ti4+0.03 Mg0.03)Σ2.00O4, which gives a CaCr2O4 end-member content of ~90%. Ellinaite from inclusions in diamond (co-type) also has slight non-stoichiometry: (Ca0.97Mg0.02Mn0.02)Σ1.01(Cr1.71Ca0.11Fe3+0.06V0.06Ti0.03Al0.03)Σ2.00O4 (Kaminsky et al., Reference Kaminsky, Wirth and Schreiber2015). Sharygin (Reference Sharygin2019) assigned the crystal chemical formula: (Ca0.967Fe2+0.017Na0.010)Σ0.994(Cr3+1.696V3+0.201Ti3+0.080Al0.024Ti4+0.005)Σ2.006O4, to ellinaite from phosphide-bearing rock (holotype), balancing a charge excess by Ti3+ (Sharygin, Reference Sharygin2019). This ellinaite is characterised by relatively high V content.
Mcconnellite forms a series of parallel crystals in calcite, the largest of which is ~20 μm long and 10 μm thick (Fig. 2c). Mcconnellite in thin crystals shows a characteristic purple colour (Fig. 2d). The mcconnellite studied from Jordan has the crystal chemical formula (Cu0.98Ca0.03Sr0.01)Σ1.02(Cr3+0.90Al0.05Fe3+0.03)Σ0.98O2, that corresponds to 90% of the end-member CuCrO2 (Table 2). Accurate data on the holotype mcconnellite composition from Guyana are absent (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976).
Raman spectroscopy data of ellinaite and mcconnellite
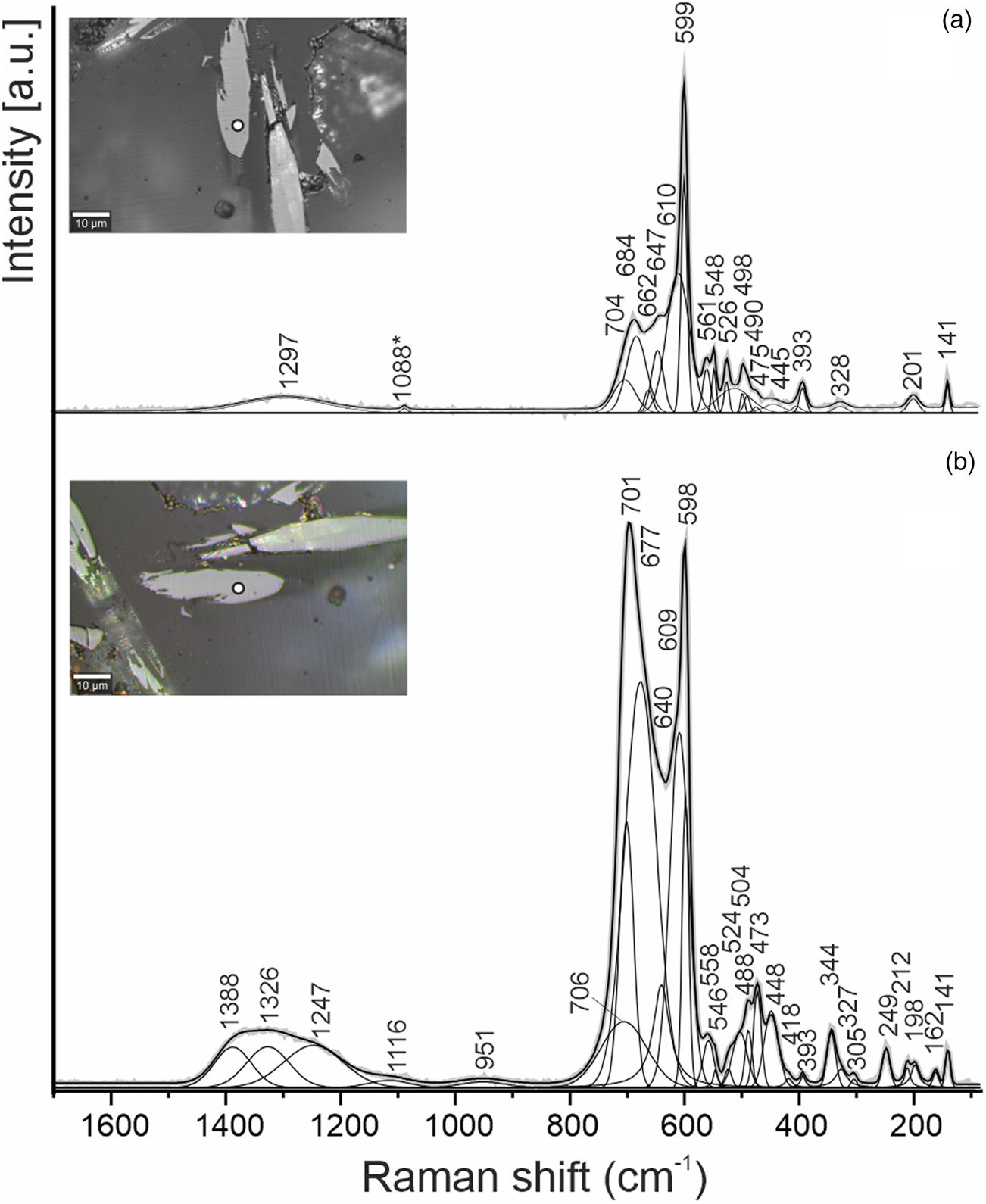
The spectral features of both ellinaite and mcconnellite are dependent on the crystal orientation (Figs 3, 4). Raman spectra of ellinaite from Jordan are similar to the spectra of the holotype ellinaite from Israel (Sharygin, Reference Sharygin2019) and its synthetic analogue (Zhai et al., Reference Zhai, Yin, Shieh, Shan, Xue, Wang, Yang and Higo2016). The main bands in the Raman spectrum of ellinaite are the following [Fig. 3a, interpretation by analogy with harmunite, CaFe2O4 (Kolev et al., Reference Kolev, Iliev, Popov and Gospodinov2003; Galuskina et al., Reference Galuskina, Vapnik, Lazic, Armbruster, Murashko and Galuskin2014)]: a series of bands in the range 1400–1200 cm–1 [combination of first-order phonons Ag (701 cm–1) + Ag (598 cm–1), 2 × Ag (701cm–1), 2 × Ag (598 cm–1)]; 701, 598, 558 cm–1 (Ag, stretching modes); 488, 473, 448 (B2g/Ag, bending and tilting modes); 393, 344, 327 (Ag/B1g/B3g, rotation mode); 249, 212, 198 (Ag), 141 (Ag, Ca-related vibration). The main band at 598 cm–1 in the Raman spectrum of ellinaite is related to symmetric vibrations of Cr–O in octahedron, in the eskolaite Cr2O3 spectrum the analogous band is at a lower frequency – 548 cm–1 (Shim et al., Reference Shim, Duffy, Jeanloz, Yoo and Iota2004). The band at ~700 cm–1 in the ellinaite spectrum is polarised and has large full widths at half maximum and a complex nature (Fig. 3). It is probably attributed to symmetric vibrations in the Cr–O–Cr group and asymmetric vibrations of Cr–O.
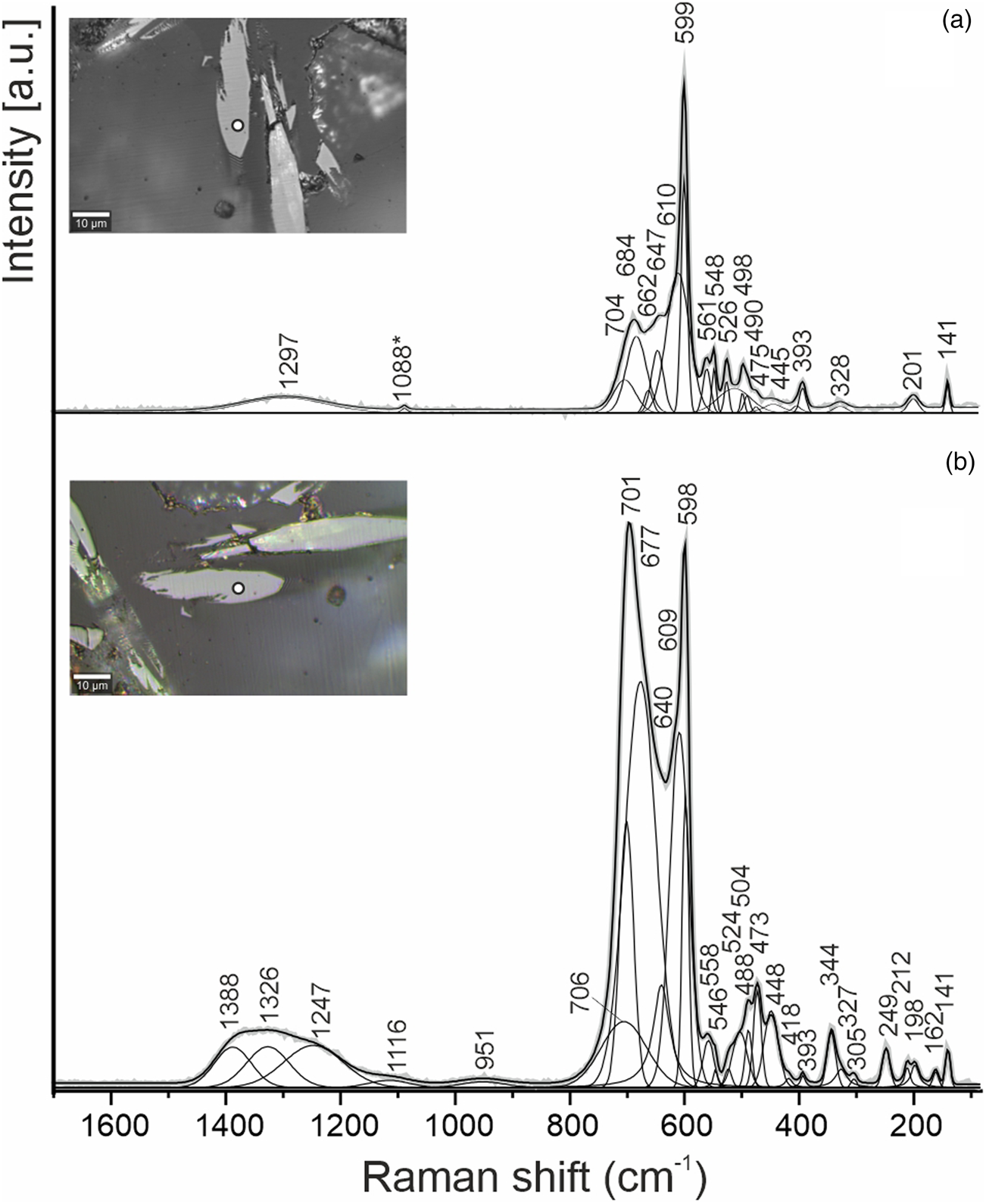
Fig. 3. Raman spectra of ellinaite obtained in a random section at the two orientations relative to a polarised laser beam. The point location for the spectra is shown as white circles in the inset optical images.

Fig. 4. Raman spectra of mcconnellite obtained at different orientations of the crystal. The point location for the spectra is shown as white circles in the inset BSE image.
For the first time we provide the Raman spectra of mcconnellite (Fig. 4), which are similar to the spectra of synthetic CuCrO2, for which depending on orientation and/or composition modification significant changes of intensity of the main bands are observed (Aktas et al., Reference Aktas, Truong, Otani, Balakrishnan, Clouter, Kimura and Quirion2012; Elkhouni et al., Reference Elkhouni, Amami, Strobel and Ben Salah2013; Monteiro et al., Reference Monteiro, Jurelo and Siqueira2017, Reference Monteiro, Siqueira, Vallis, de Andrade, Barcote and Jurelo2018; Han et al., Reference Han, Lu, Liu, Hu, Chen, Jiang, Zhang and Li2020). In the Raman spectra of mcconnellite there are six main bands (Fig. 4b, cm–1): 105, 210 (Ag), 458 (Eg), 538 (P1), 556 (P1′) and 709 (A1g) (Monteiro et al., Reference Monteiro, Siqueira, Vallis, de Andrade, Barcote and Jurelo2018). The nature of the strong band at 538 cm–1 (P1) and the shoulder at 556 cm–1 (P1′) is currently disputable and is connected with structural defects of mcconnellite, which interferes with an action of the Raman selection rule (Majee and Bhobe, Reference Majee and Bhobe2020; Han et al., Reference Han, Lu, Liu, Hu, Chen, Jiang, Zhang and Li2020).
Crystal structure of ellinaite and mcconnellite
Ellinaite from Jordan, CaCr2O4 (Pnma, a = 9.0875(2), b = 2.9698(1), c = 10.6270(3) Å and V = 286.80(2) Å3), belongs to the post-spinel of the CF (calcium ferrite) structural type. Harmunite, CaFe2O4 (Pnma; a = 9.2183(3), b = 3.0175(1), c = 10.6934(4) Å, Z = 4 and V = 297.45(2) Å3), described from larnite rocks of the Hatrurim Complex, Jabel Harmun, Palestine (Galuskina et al., Reference Galuskina, Vapnik, Lazic, Armbruster, Murashko and Galuskin2014) and wernerkrauseite with the defect structure, Ca2/3(Fe3+4/3Mn4+2/3)Σ2O4 (Pnma, a = 9.0548(2), b = 2.8718(1), c = 10.9908(2) Å and V = 285.80(1) Å3), found in altered xenoliths within alkaline basalts of the Bellerberg volcano, Eifel, Rhineland-Palatinate, Germany (Galuskin et al., Reference Galuskin, Krüger, Krüger, Blass, Widmer and Galuskina2016) are the natural low-pressure and high-temperature members of CF-type ferrites. The ellinaite structure is composed of two types of symmetrically independent distinct corner sharing (Cr3+O6) distorted octahedra. Each of these octahedra forms double chains ∞1[Cr2O6], linked by shared edges in a rutile-type manner (Fig. 5). The double chains connect together by common oxygen corners and build a tunnel-framework with large trigonal prismatic cavities occupied by Ca along [010] (Fig. 5). Octahedra (Cr3+O6) forming rutile-like chains in ellinaite are distorted with average distances M 1Cr–O = 2.004(3) Å and M2Cr–O = 2.014(3) Å (Table 6). These values are slightly higher, than the Cr–O distance of 1.98 Å, calculated for ideal octahedron (Shannon, Reference Shannon1976), and are caused by substitutions of other elements in M1, M2 crystallographic sites.

Fig. 5. (a) Ellinaite post-spinel structure, projection perpendicular to the tunnel direction (different octahedral sites are CrM1 = light-green and CrM2 = dark-green, Ca sites drawn in orange). (b) Ca sites (orange balls) in tunnel coordinated by O (red). Rutile-like chains of two types of octahedra: CrM1 (light-green) and CrM2 (dark-green) are shown.
Mcconnellite from Jordan, CuCrO2 (R $\bar{3}$![]() m, a = b = 2.9756(1) and c = 17.124(1) Å) is a structural analogue of delafossite, CuFeO2 (R $\bar{3}$
m, a = b = 2.9756(1) and c = 17.124(1) Å) is a structural analogue of delafossite, CuFeO2 (R $\bar{3}$![]() m, a = b = 3.03Å and c = 17.09 Å, Pabst, Reference Pabst1946). Dannhauser and Vaughan (Reference Dannhauser and Vaughan1955) described synthetic cuprous chromite to the same space group after Stroupe (Reference Stroupe1949) who identified identical unit cell parameters though with a hexagonal primitive cell instead of rhombohedral. The mcconnellite crystal structure consists of distorted (Cr3+O6) octahedra with a Cr–O distance of 1.991(1) Å. Each octahedron shares six edges with six neighbours, forming (CrO2)– infinite layers. Every two adjacent layers are linked by linear two-fold coordinated Cu+ ions, forming (O–Cu–O)3– groups. Copper (I) forms a hexagonal plane where each ion is 2.9756(1) Å away from the six neighbours. The Cu+–Cu+ distance determines the length of the unit cell parameter a. The (CrO2)– layers and Cu+ planes are perpendicular to the z axis and alternate along it (Fig. 6).
m, a = b = 3.03Å and c = 17.09 Å, Pabst, Reference Pabst1946). Dannhauser and Vaughan (Reference Dannhauser and Vaughan1955) described synthetic cuprous chromite to the same space group after Stroupe (Reference Stroupe1949) who identified identical unit cell parameters though with a hexagonal primitive cell instead of rhombohedral. The mcconnellite crystal structure consists of distorted (Cr3+O6) octahedra with a Cr–O distance of 1.991(1) Å. Each octahedron shares six edges with six neighbours, forming (CrO2)– infinite layers. Every two adjacent layers are linked by linear two-fold coordinated Cu+ ions, forming (O–Cu–O)3– groups. Copper (I) forms a hexagonal plane where each ion is 2.9756(1) Å away from the six neighbours. The Cu+–Cu+ distance determines the length of the unit cell parameter a. The (CrO2)– layers and Cu+ planes are perpendicular to the z axis and alternate along it (Fig. 6).

Fig. 6. Mcconnellite structure formed by octahedral layers (CrO2)–, linked together by Cu+ cations. Cu – blue balls, CrO6 – green octahedra and oxygen – red balls.
Discussion
Recent investigations have shown that Fe3+-analogues of ellinaite and mcconnellite: harmunite, CaFe2O4 (Galuskina et al., Reference Galuskina, Vapnik, Lazic, Armbruster, Murashko and Galuskin2014) and delafossite, CuFeO2 (Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Włodyka, Dzierżanowski and Murashko2015; Krzątała et al., Reference Krzątała, Panikorovskii, Galuskina and Galuskin2018), respectively, are relatively widely distributed in pyrometamorphic rocks of the Hatrurim Complex. Areas of high chromium occur locally in the Complex, and Cr is found in the composition of the primary high-temperature pyrometamorphic oxide minerals such as spinel, oxy-deficient perovskite of the brownmillerite–srebrodolskite and shulamitite–sharyginite series and other ferrites. Rarer high chromium contents are noted in Ti-bearing andradite and pyrrhotite (Sharygin et al., Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013; Galuskina et al., Reference Galuskina, Vapnik, Lazic, Armbruster, Murashko and Galuskin2014, Reference Galuskina, Galuskin, Pakhomova, Widmer, Armbruster, Krüger, Grew, Vapnik, Dzierażanowski and Murashko2017; Juroszek et al., Reference Juroszek, Krüger, Galuskina, Krüger, Jeżak, Ternes, Wojdyla, Krzykawski, Pautov and Galuskin2018a). Low-temperature hydrothermally altered products of chromium-bearing pyrometamorphic rocks contain minerals of the hashemite–baryte and bentorite–ettringite–siwaqaite series (Seryotkin et al., Reference Seryotkin, Sokol, Kokh and Sharygin2019; Juroszek et al., Reference Juroszek, Krüger, Banasik, Vapnik and Galuskina2018b, Reference Juroszek, Krüger, Galuskina, Krüger, Vapnik and Galuskin2020a, Reference Juroszek, Krüger, Galuskina, Krüger, Tribus and Kürsten2020b), and also chromatite (Sokol et al., Reference Sokol, Gaskova, Kokh, Kozmenko, Seryotkin, Vapnik and Murashko2011).
Varicoloured apatite–spurrite marbles of the pyrometamorphic complex are characterised by a very high chemical inhomogeneity. This is reflected not only in mineral composition and the colour of the rocks inherited in the primary layering of the protolith (Fig. 1a), but also in enrichment of separate micro-layers by various rare and accessory minerals (Khoury et al., Reference Khoury, Sokol, Kokh, Seryotkin, Nigmatulina, Goryainov, Belogub and Clark2016; Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Zeliński and Prusik2021). In the sample studied (Fig. 1a), in the green apatite zone, a new Zr–Y(+REE) garnet – priscillagrewite-(Y) was found (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Zeliński and Prusik2021); whereas in the spurrite zone, Cr and Cu oxides were noted (Figs 1b, 2). A source of Zr and Y is proposed as detrital minerals of primary sedimentary rocks (zircon), and Cr is connected with organic matter in the oil shales in the Siwaqa area (Sokol et al., Reference Sokol, Kozmenko, Khoury, Kokh, Novikova, Nefedov, Sokol and Zaikin2017), whereas Cu, probably, comes from primary sulfides of the pyrometamorphic rocks.
There is a discrepancy with the temperature of varicoloured marble formation. On the basis of mineral paragenesis it was determined to be 800–850°C (Khoury et al., Reference Khoury, Sokol, Kokh, Seryotkin, Nigmatulina, Goryainov, Belogub and Clark2016); however temperatures of 1000–1300°C are proposed for the holotype ellinaite formation in gehlenite pyrometamorphic rocks (Sharygin, Reference Sharygin2019). This question has been discussed, considering conditions of priscillagrewite-(Y) formation, for which a temperature of crystallisation ~1000°C was proposed (Galuskina et al., Reference Galuskina, Galuskin, Vapnik, Zeliński and Prusik2021).
Natural fires are non-equilibrium processes, and it is expected that a local increase of temperature for a short duration near gaseous channels penetrating the rock have taken place. These fluctuations of temperature are not reflected in alteration of the whole-rock composition, but can be decisive in the formation of μm-sized crystals of rare minerals. We consider that ellinaite and mcconnellite of varicoloured marble are formed at temperatures ~1000°C as a result of transformations of earlier minerals in the pyrometamorphic rocks.
There are no doubts that the structural data obtained for the holotype ellinaite from Israel needs further verification, although it is necessary to underline that experimental data on the composition and Raman spectroscopy (Sharygin, Reference Sharygin2019; Sharygin et al., Reference Sharygin, Britvin, Kaminsky, Wirth, Nigmatulina, Yakovlev, Novoselov and Murashko2020) point out that this is a phase analogous to the synthetic post-spinel β-CaCr2O4.
We interpret that the holotype mcconnellite described by Milton et al. (Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976) from the hydrated ore of Guyana should be re-investigated using modern methods. Mcconnellite belongs to the 3R polytype of CuCrO2 and a synthetic polytype 2Н is also known (hexagonal, P63/mmc (No. 194), a = 2.9740(3) Å, c = 11.400(1) Å, V = 87.3 Å3 and Z = 2, Grottaz and Kubel, Reference Grottaz and Kubel1996, Fig. 7). Mcconnellite was described as a Cu-enriched zone in grimaldiite, СrOOH (R $\bar{3}$![]() m, a = b = 2.973(2) and c = 13.39(1) Å) (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). Taking into consideration the similarity of octahedral layers in grimaldiite, two polytypes of CuCrO2 that are defined by their oriented intergrowths, and also the conformity of powder X-ray diffraction patterns, accurate determination of the holotype mcconnellite structure is needed. Experimental data indicate that the polytype CuCrO2-2Н is a lower temperature phase compared to CuCrO2-3R (Miclau et al., Reference Miclau, Ursu, Kumar and Grozescu2012). There is a possibility that the holotype mcconnellite is not a separate mineral species but corresponds to grimaldiite enriched in copper.
m, a = b = 2.973(2) and c = 13.39(1) Å) (Milton et al., Reference Milton, Appleman, Appleman, Chao, Cuttitta, Dinnin, Dwornik, Ingram and Rose1976). Taking into consideration the similarity of octahedral layers in grimaldiite, two polytypes of CuCrO2 that are defined by their oriented intergrowths, and also the conformity of powder X-ray diffraction patterns, accurate determination of the holotype mcconnellite structure is needed. Experimental data indicate that the polytype CuCrO2-2Н is a lower temperature phase compared to CuCrO2-3R (Miclau et al., Reference Miclau, Ursu, Kumar and Grozescu2012). There is a possibility that the holotype mcconnellite is not a separate mineral species but corresponds to grimaldiite enriched in copper.
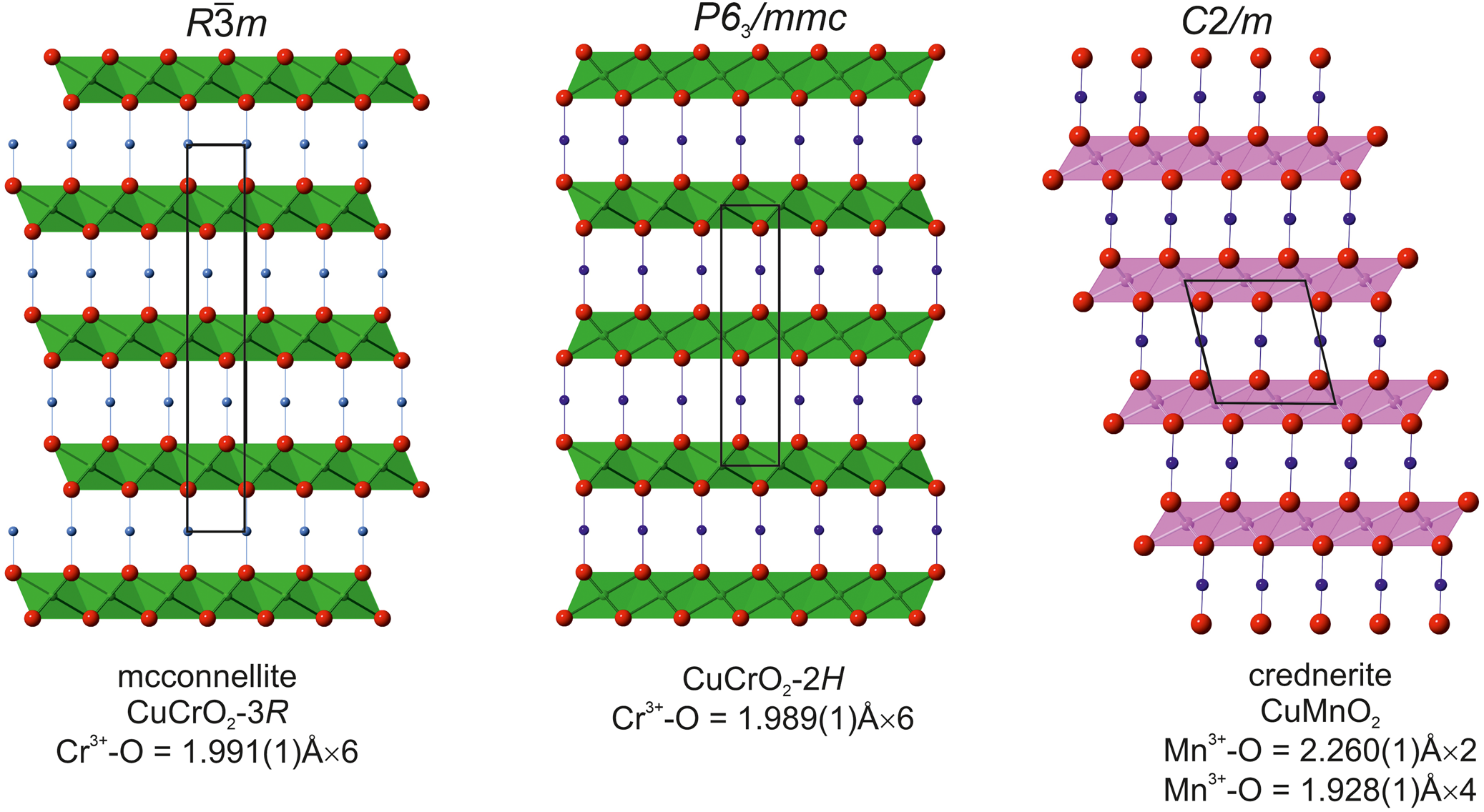
Fig. 7. Comparison of two structures of the CuCrO2 polytypes – 3R (our data) and 2H (Grottaz and Kubel, Reference Grottaz and Kubel1996) with the crednerite structure (Töpfer et al., Reference Töpfer, Trari, Gravereau, Chaminade and Doumerc1995), in which trioctahedral layers are formed by strongly deformed octahedra (Mn3+O6). All projections on (010). Cr3+O6 – green, Mn3+O6 – pink, Cu – blue balls and O – red balls.
Except delafossite (R $\bar{3}$![]() m, a = 3.02–3.04 and c = 17.10–17.12; Pabst, Reference Pabst1946) which is isostructural with mcconnellite, only one structurally related mineral – crednerite, CuMnO2 (C2/m, a = 5.578(6), b = 2.881(2) and c = 5.886(7) Å; Töpfer et al., Reference Töpfer, Trari, Gravereau, Chaminade and Doumerc1995), is known in Nature. Crednerite does not have three-fold symmetry as a result of the Jahn–Teller effect of Mn3+ (Fig. 7).
m, a = 3.02–3.04 and c = 17.10–17.12; Pabst, Reference Pabst1946) which is isostructural with mcconnellite, only one structurally related mineral – crednerite, CuMnO2 (C2/m, a = 5.578(6), b = 2.881(2) and c = 5.886(7) Å; Töpfer et al., Reference Töpfer, Trari, Gravereau, Chaminade and Doumerc1995), is known in Nature. Crednerite does not have three-fold symmetry as a result of the Jahn–Teller effect of Mn3+ (Fig. 7).
In conclusion, we would like to dwell on a problem related to mcconnellite. In the Raman spectra of mcconnellite from Jordan, there is a strong band at 537–538 cm–1, and a shoulder at 556–558 cm–1 (Fig. 4), which also appears in the Raman spectra of synthetic analogues (Aktas et al., Reference Aktas, Truong, Otani, Balakrishnan, Clouter, Kimura and Quirion2012; Monteiro et al., Reference Monteiro, Jurelo and Siqueira2017). Additionally, if the other strong bands at 458 cm–1 (Eg) and 709 cm–1 (Ag1) are related to the Raman selection rule, then the band at 537–538 cm–1 (and the shoulder at 556–558 cm–1) breaks this rule and they are connected with structural defects (Majee and Bhobe, Reference Majee and Bhobe2020; Han et al., Reference Han, Lu, Liu, Hu, Chen, Jiang, Zhang and Li2020). In eskolaite, Cr2O3, the main band at ~548 cm–1 is related to the symmetric stretching vibrations of Cr–O in (CrO6) octahedra (Shim et al., Reference Shim, Duffy, Jeanloz, Yoo and Iota2004), i.e. only 10 cm–1 lower than in mcconnellite (Fig. 4). In the mcconnellite structure, the layers are trioctahedral, whereas in the eskolaite structure they are dioctahedral (Fig. 8). Most likely, the strong band at 537–538 cm–1 in mcconnellite results from the Cr–O stretching symmetric vibrations, and the shoulder at 556-558 cm–1 – from the Cr–O asymmetric vibrations in (CrO6) octahedra connected with defects of eskolaite type in the (CrO2)– octahedral layer.

Fig. 8. (a) Trioctahedral layer in mcconnellite, CuCrO2; and (b) dioctahedral layer in eskolaite, Cr2O3.
Acknowledgements
The investigations were partially supported by the National Science Centre (NCN) of Poland, grant no. 2016/23/B/ST10/00869 (EG, IG). KW acknowledges a financial support within the Polish National Science Centre (NCN) OPUS17 no. 2019/33/ B/ST10/02671. We are grateful to Viktor Sharygin for providing additional data on the holotype ellinaite. We thank two anonymous referees for constructive comments that help to improve the first version of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.27.