Introduction
The lifetime prevalence of eating disorders (EDs) in adults is about 0.6% for anorexia nervosa (AN), 1% for bulimia nervosa (BN) and 3% for binge-eating disorder (BED) (Treasure et al. Reference Treasure, Claudino and Zucker2010). These disorders are often chronic, relapsing and devastating. The pathogenesis is poorly understood, and robust aetiological models to guide treatment are therefore lacking. Behavioural abnormalities driven by underlying cognitive processes are a prominent feature, and neuropsychological assessment thus seems to be an obvious approach to gaining greater insight into the information-processing alterations that lead to these behaviours. In adults with AN, recent evidence from meta-analyses has indicated specific neurocognitive traits such as a poor set-shifting (Roberts et al. Reference Roberts, Tchanturia, Stahl, Southgate and Treasure2007) and weak central coherence (Lang et al. Reference Lang, Lopez, Stahl, Tchanturia and Treasure2014a ). The exploration of neurocognitive pathways has both enhanced our understanding of the vulnerability to developing an ED and suggested directions for developing innovative therapeutic programmes (Dingemans et al. Reference Dingemans, Danner, Donker, Aardoom, van Meer, Tobias, van Elburg and van Furth2014; Tchanturia et al. Reference Tchanturia, Doris and Fleming2014).
Impaired decision-making may be a factor of vulnerability and an endophenotype of EDs. The ability to make adequate decisions about possible courses of action is a core cognitive function in daily living, and altered decision-making – for example, following damage to the orbitofrontal cortex – can lead to disadvantageous and sometimes disastrous consequences in life (Damasio, Reference Damasio1994). Of considerable clinical interest, therefore, is the hypothesis that decision-making deficits may be involved in the pathophysiology and/or the chronicity of psychiatric disorders (Goschke, Reference Goschke2014). Impaired decision-making may also be a heritable marker for the identification of vulnerable patients (Courtet et al. Reference Courtet, Gottesman, Jollant and Gould2011) and, should this be so, it would be a relevant target for prevention and treatment. Although many tasks simulate the various aspects of decision-making (such as uncertainty, risk-taking and temporal discounting), the most widely used is the Iowa gambling task (IGT), which measures the preference for risky and disadvantageous choices in a context of uncertainty (the participant is unable to assess the long-term risk associated with each option). The IGT shows reproducible results in a range of experimental conditions (e.g. real money or not, presence or absence of delay before feedback) (Bowman et al. Reference Bowman, Evans and Turnbull2005). Moreover, performance does not reflect intelligence or other cognitive functions (Bowman et al. Reference Bowman, Evans and Turnbull2005). The decision-making deficits revealed by the IGT have been identified in mental illnesses like bipolar disorder (Adida et al. Reference Adida, Jollant, Clark, Besnier, Guillaume, Kaladjian, Mazzola-Pomietto, Jeanningros, Goodwin, Azorin and Courtet2011) and in addictive (Brevers et al. Reference Brevers, Bechara, Cleeremans, Kornreich, Verbanck and Noel2014) or suicidal (Richard-Devantoy et al. Reference Richard-Devantoy, Berlim and Jollant2013) behaviours.
Over the last 10 years, decision-making in EDs has become a focus for research because of the clinical observation that these patients tend to show a preference for immediate reward despite the long-term adverse consequences (e.g. maintaining behaviour restriction and starvation despite the negative physical and psychosocial consequences). In addition, evidence has accumulated suggesting that AN is linked to a dysfunction in reward mechanisms (Wagner et al. Reference Wagner, Aizenstein, Venkatraman, Fudge, May, Mazurkewicz, Frank, Bailer, Fischer, Nguyen, Carter, Putnam and Kaye2007; Zink & Weinberger, Reference Zink and Weinberger2010; Kaye et al. Reference Kaye, Wierenga, Bailer, Simmons, Wagner and Bischoff-Grethe2013), which are key motivational processes in decision-making. These motivational processes engage various frontostriatal structures (e.g. orbitofrontal prefrontal cortex, dorsolateral prefrontal cortex and striatum) and neurotransmitter systems (serotonergic and dopaminergic) that are implicated in the aetiology of EDs (Kaye et al. Reference Kaye, Wierenga, Bailer, Simmons, Wagner and Bischoff-Grethe2013) and have been found to be differentially activated in patients with AN during decision-making tasks (Bischoff-Grethe et al. Reference Bischoff-Grethe, McCurdy, Grenesko-Stevens, Irvine, Wagner, Yau, Fennema-Notestine, Wierenga, Fudge, Delgado and Kaye2013; Bodell et al. Reference Bodell, Keel, Brumm, Akubuiro, Caballero, Tranel, Hodis and McCormick2014; Decker et al. Reference Decker, Figner and Steinglass2014). In some of the studies that have used the IGT, impaired decision-making was observed in patients with a history of AN and/or BN compared with healthy controls (HCs), whereas other studies found no impairment. These discrepant findings point to some of the unresolved questions. For example, malnutrition causes cognitive impairment independently of the presence of an ED (Keys et al. Reference Keys, Brožek, Henschel, Mickelsen and Taylor1950), and therefore the poor decision-making of actively symptomatic AN patients may be a consequence of the illness and/or starvation, rather than a pre-morbid or underlying impairment that contributes to vulnerability. Even in the case of state markers, there are often difficulties in discriminating clinical changes secondary to starvation that will improve with weight regain from those related to the disease and at least partially independent of nutritional state (obsessional preoccupation with body image, distorted body image, interoception). Also, given the frequent psychiatric co-morbidity in EDs, impaired decision-making could be the consequence of a co-occurring psychiatric condition (such as mood disorder, suicidal vulnerability or addiction) rather than a marker of EDs in itself. It is also possible that the decision-making process differs in AN and BN (Chan et al. Reference Chan, Ahn, Bates, Busemeyer, Guillaume, Redgrave, Danner and Courtet2014).
It seems clear that the reports in the literature now need to be cumulated and analysed in order to determine whether decision-making is actually impaired in EDs. Moreover, if this is indeed the case, it might also be important to disentangle the potential decision-making deficits associated with AN from those more closely related to BN. In the current paper, we systematically reviewed the literature on decision-making in EDs and conducted a meta-analysis to explore the putative decision-making markers of EDs.
Method
Data sources
The English and French literature in the Medline, EMBASE and PsycINFO databases was systematically searched for human studies published up to 15 April 2015. The medical subject heading (MeSH) term ‘Eating Disorders’ was combined with the MeSH term ‘Decision Making’ and with the title and abstract (TIAB) terms ‘Iowa Gambling Task’ and ‘Orbitofrontal Cortex’. An iterative process was used to ensure that all relevant articles were obtained. A further manual search of the bibliographical references in the selected papers and existing reviews was conducted to identify additional potential studies.
Study selection
Abstract selection was based on the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist (Von Elm et al. Reference Von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke2008), which describes items that should be included in reports of cohort studies. The abstracts identified through the literature search were independently evaluated by two reviewers (S.R.-D. and S.G.) and selected by consensus from all authors.
Studies that met the following inclusion criteria were included in this meta-analysis: (1) published in an English- or French-language peer-reviewed journal; (2) including at least one decision-making task; and (3) comparing at least two groups, one of which comprised patients with a diagnosis of eating disorder [defined as per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria; American Psychiatric Association, 2000]. Full articles were then obtained for the final analyses.
Of the 371 originally identified abstracts, 35 studies met the inclusion criteria. Of these, nine studies did not use the IGT to assess decision-making: four used the Game of dice task (Brand et al. Reference Brand, Franke-Sievert, Jacoby, Markowitsch and Tuschen-Caffier2007; Svaldi et al. Reference Svaldi, Brand and Tuschen-Caffier2010; Van den Eynde et al. Reference Van den Eynde, Samarawickrema, Kenyon, DeJong, Lavender, Startup and Schmidt2012; Wu et al. Reference Wu, Giel, Skunde, Schag, Rudofsky, de Zwaan, Zipfel, Herzog and Friederich2013) and five used a delay-discounting task (Steinglass et al. Reference Steinglass, Figner, Berkowitz, Simpson, Weber and Walsh2012; Decker et al. Reference Decker, Figner and Steinglass2014; Mole et al. Reference Mole, Irvine, Worbe, Collins, Mitchell, Bolton, Harrison, Robbins and Voon2015; Ritschel et al. Reference Ritschel, King, Geisler, Flohr, Neidel, Boehm, Seidel, Zwipp, Ripke, Smolka, Roessner and Ehrlich2015; Wierenga et al. Reference Wierenga, Bischoff-Grethe, Melrose, Irvine, Torres, Bailer, Simmons, Fudge, McClure, Ely and Kaye2015) with distinct methodology of analyses across the studies. Although eligible, three studies were not included for the following reasons: the means and standard deviations were not given and could not be obtained after contacting the authors (Davis et al. Reference Davis, Patte, Curtis and Reid2010), the study explored the IGT in obese patients only (Muller et al. Reference Muller, Brandl, Kiunke, Georgiadou, Horbach, Kohler and de Zwaan2014), and the sample was composed only of males (Tchanturia et al. Reference Tchanturia, Liao, Forcano, Fernandez-Aranda, Uher, Treasure, Schmidt, Penelo, Granero, Jimenez-Murcia, Sanchez and Campbell2012). Finally, 20 studies with AN and BN patients and three with BED patients were definitively included in the meta-analysis. HCs were most often defined as groups of subjects ‘free of any psychopathology’ (see Table 1). Also, patients suffering from BED, defined as per the DSM-IV-TR criteria, were currently ill.
Table 1. Studies included in the meta-analysis

AN, Anorexia nervosa; BN, bulimia nervosa; BED, binge-eating disorder; HCs, healthy controls; s.d., standard deviation; BMI, body mass index; ANR, restrictive anorexia nervosa; ANP, purging anorexia nervosa; ED, eating disorder; PD, Axis I psychiatric disorders (DSM-IV).
a In all studies HCs were matched on gender.
b Data not available.
The quality of each study was assessed independently by two reviewers (S.R.-D. and S.G.) using the Crombie criteria adapted by Petticrew & Roberts (Reference Petticrew and Roberts2006). The study selection procedure is shown on a flow diagram (online Supplementary Fig. S1).
Data extraction and analyses
A standardized form was used to extract the data, which included authors, publication date, study design, setting, study population, decision-making tests used, definition of ED, and decision-making scores (mean and s.d.).
When the IGT was used in at least three separate studies, we ran meta-analyses. Overall, we used the IGT net score (IGT net score = number of advantageous minus disadvantageous choices). Analyses were performed using Comprehensive Meta-Analysis version 3.0 (Biostat, USA) and IBM SPSS version 20 (USA).
We compared four groups: AN patients, BN patients, BED patients and HCs. When two groups of AN were reported in one study (e.g. restrictive and purging types) (Cavedini et al. Reference Cavedini, Bassi, Ubbiali, Casolari, Giordani, Zorzi and Bellodi2004, Reference Cavedini, Zorzi, Bassi, Gorini, Baraldi, Ubbiali and Bellodi2006; Tchanturia et al. Reference Tchanturia, Liao, Forcano, Fernandez-Aranda, Uher, Treasure, Schmidt, Penelo, Granero, Jimenez-Murcia, Sanchez and Campbell2007; Guillaume et al. Reference Guillaume, Sang, Jaussent, Raingeard, Bringer, Jollant and Courtet2010; Garrido & Subirá, Reference Garrido and Subirá2013; Aloi et al. Reference Aloi, Rania, Caroleo, Bruni, Palmieri, Cauteruccio, De Fazio and Segura-Garcia2015), or symptomatic and recovered patients (Bosanac et al. Reference Bosanac, Kurlender, Stojanovska, Hallam, Norman, McGrath, Burrows, Wesnes, Manktelow and Olver2007; Tchanturia et al. Reference Tchanturia, Liao, Forcano, Fernandez-Aranda, Uher, Treasure, Schmidt, Penelo, Granero, Jimenez-Murcia, Sanchez and Campbell2007; Danner et al. Reference Danner, Sanders, Smeets, van Meer, Adan, Hoek and van Elburg2012b ), the combined means and standard deviations were calculated to obtain a global group, using the following formula:
 $$\eqalign{& \mu _{X \cup Y} = \displaystyle{{N_{X\mu X} + N_{Y\mu Y}} \over {N_X + N_Y}} \cr & \sigma _{X \cup Y} = \sqrt {\displaystyle{{N_X \sigma ^2 _X + N_Y \sigma ^2 _Y} \over {N_X + N_Y}} + \displaystyle{{N_X N_Y} \over {(N_X + N_Y )^2}} (\mu _X - \mu _Y )^2}} $$
$$\eqalign{& \mu _{X \cup Y} = \displaystyle{{N_{X\mu X} + N_{Y\mu Y}} \over {N_X + N_Y}} \cr & \sigma _{X \cup Y} = \sqrt {\displaystyle{{N_X \sigma ^2 _X + N_Y \sigma ^2 _Y} \over {N_X + N_Y}} + \displaystyle{{N_X N_Y} \over {(N_X + N_Y )^2}} (\mu _X - \mu _Y )^2}} $$
Separate analyses were conducted for each AN subtype: restrictive v. purging (Cavedini et al. Reference Cavedini, Bassi, Ubbiali, Casolari, Giordani, Zorzi and Bellodi2004, Reference Cavedini, Zorzi, Bassi, Gorini, Baraldi, Ubbiali and Bellodi2006; Tchanturia et al. Reference Tchanturia, Liao, Forcano, Fernandez-Aranda, Uher, Treasure, Schmidt, Penelo, Granero, Jimenez-Murcia, Sanchez and Campbell2007; Guillaume et al. Reference Guillaume, Sang, Jaussent, Raingeard, Bringer, Jollant and Courtet2010; Garrido & Subirá, Reference Garrido and Subirá2013), as well as for symptomatic v. recovered status, and then each AN subtype was compared with HCs. All BN patients were currently ill, and the BN and BED groups were separately compared with HCs.
We used a random-effects model as we assumed that the true effect sizes probably varied between the included studies (Riley et al. Reference Riley, Higgins and Deeks2011). Pooled Hedges’ g effect sizes for subjects’ IGT net scores, age, body mass index (BMI) and depression ratings were computed (Hedges & Olkin, Reference Hedges and Olkin1985). Qualitative descriptors of the obtained effect sizes are usually considered small if <0.3, moderate if between 0.4 and 0.8, and large if >0.8 (Egger et al. Reference Egger, Davey Smith and Altman2001).
Heterogeneity was assessed using the Q statistic and the I 2 index (Cooper et al. Reference Cooper, Hedges and Valentine2009). Values of p < 0.10 for the former and >35% for the latter were deemed as indicative of study heterogeneity. We used funnel plots and Egger's regression intercept (Egger et al. Reference Egger, Davey Smith, Schneider and Minder1997) to test for the presence of publication bias (Cooper et al. Reference Cooper, Hedges and Valentine2009), which is defined as the omission of unpublished articles from a meta-analysis (Sedgwick, Reference Sedgwick2015). Rosenthal's fail-safe N (Rosenthal, Reference Rosenthal1979) is designed to assess whether it is likely that the overall effect is entirely due to this bias.
Lastly, age, BMI and depression were taken into account for each comparison (i.e. the mean age in AN v. the mean age in HCs, etc.). When 10 or more studies were included in the meta-analysis, a meta-regression analysis was performed using Comprehensive Meta-Analysis Version 3.0 (Biostat, USA).
Results
In all, 23 studies were included (Table 1), for a total of 2044 participants: 693 AN [34% of the total; mean age = 25.9 (s.d. = 6.5) years; 100% females; mean BMI = 16.3 (s.d. = 2.2) kg/m2]; 221 BN [10.8% of the total; 25.4 (s.d. = 5.2) years; 100% females; mean BMI = 23.2 (s.d. = 4.4) kg/m2]; 1037 HCs [50.7% of the total; 26.4 (s.d. = 7.0) years; 100% females; mean BMI = 21.3 (s.d. = 2.3) kg/m2]; and 93 BED [4.5% of the total; 38.3 (s.d. = 10.8) years; 100% females; mean BMI = 37.1 (s.d. = 5.9) kg/m2]. In the AN group, 566 (79.3% of AN) were currently symptomatic [mean age = 25.7 (s.d. = 6.5) years; mean BMI = 15.6 (s.d. = 2.2) kg/m2], whereas 141 (20.3% of AN) were in remission from restrictive-type AN [mean age = 29.2 (s.d. = 6.7) years; mean BMI = 20.7 (s.d. = 1.8) kg/m2]. A total of 284 had restrictive AN [mean age = 25.3 (s.d. = 6.2) years; mean BMI = 14.6 (s.d. = 2.2) kg/m2], and 109 had purging AN [mean age = 24.3 (s.d. = 5.8) years; mean BMI = 16.6 (s.d. = 2.1) kg/m2].
Table 2 presents the IGT net scores of the four main groups and the AN subtypes. Detailed information on heterogeneity and publication bias can also be found in online Supplementary Table S1.
Table 2. Effect sizes for the contrasts between AN patients, BN patients and HCs for the IGT

AN, Anorexia nervosa; BN, bulimia nervosa; HCs, healthy controls; IGT, Iowa gambling task; CI, confidence interval; ANR, restrictive anorexia nervosa; ANP, purging anorexia nervosa; BED, binge-eating disorder.
a Test for the significance of the effect size.
b Results became significant after excluding the study responsible for the heterogeneity.
*p < 0.05, **p < 0.01, ***p < 0.001.
Decision-making in AN (Fig. 1)
Symptomatic AN patients v. HCs
Compared with HCs, the symptomatic AN patients had significantly lower IGT net scores, indicating a moderate effect size. The fail-safe N was 488. Mean age did not differ between groups, ruling out the hypothesis that this variable acts as a confounding factor. Patients with symptomatic AN had lower BMI and a higher depression score compared with HCs, but the effect size was not explained by BMI [β = −0.02; 95% confidence interval (CI) −0.05 to 0.01, p = 0.2] or depression (β = 0.001, 95% CI −0.03 to 0.03, p = 0.9). Heterogeneity exceeded that expected by chance at p < 0.05 (online Supplementary Table S1). The studies by Brogan et al. (Reference Brogan, Hevey, O'Callaghan, Yoder and O'Shea2011) and Galimberti et al. (Reference Galimberti, Fadda, Cavallini, Martoni, Erzegovesi and Bellodi2013) most probably explained the heterogeneity between HCs and symptomatic AN patients. When these studies were excluded, the heterogeneity disappeared and the main results remained significant.

Fig. 1. Comparison of the net Iowa gambling task scores in anorexia nervosa (AN). (a) Symptomatic AN patients v. healthy controls (HC): impaired decision-making (symptomatic AN patients made significantly more risky than safe choices relative to HC). (b) Recovered AN patients v. HC: no between-group differences. (c) Restrictive AN patients v. purging AN: impaired decision-making. (d) Restrictive AN patients v. HC: impaired decision-making. (e) Purging AN patients v. HC: no between-group differences. CI, Confidence interval.
Recovered AN patients v. HCs
The recovered AN patients did not have significantly lower IGT net scores than HCs. Mean age did not differ between groups. A significantly lower mean BMI and significantly higher mean depression level were found in the recovered AN patients compared with HCs.
Heterogeneity exceeded that expected by chance at p < 0.05 (online Supplementary Table S1), and the study by Danner et al. (Reference Danner, Sanders, Smeets, van Meer, Adan, Hoek and van Elburg2012b ) was responsible. When this study was excluded, the heterogeneity disappeared and the main results remained non significant.
Decision-making according to the subtype of AN (Fig. 1)
The restrictive AN patients had significantly lower IGT net scores than the AN patients with the purging subtype, indicating a moderate effect size. The fail-safe N was 9. Mean age did not differ between groups, but the restrictive AN patients had significantly lower mean BMI than the purging AN patients.
Heterogeneity did not exceed that expected by chance at p < 0.05 (online Supplementary Table S1). The funnel plots were reasonably symmetrical, and the more conservative Egger's regression intercept suggested no publication bias.
The restrictive AN patients also had significantly lower IGT net scores compared with HCs, indicating a moderate effect size. The fail-safe N was 107. Mean age did not differ between groups, but BMI and depression were significantly different. Heterogeneity exceeded that expected by chance at p < 0.05 (online Supplementary Table S1). The study by Guillaume et al. (Reference Guillaume, Sang, Jaussent, Raingeard, Bringer, Jollant and Courtet2010) was probably responsible for this heterogeneity. After this study was excluded, the heterogeneity disappeared and the main result remained significant. The funnel plots were reasonably symmetrical, and Egger's regression intercept suggested no publication bias.
Last, there was no difference between patients with the purging subtype of AN and HCs. Mean age did not differ between groups, and significantly lower mean BMI and a higher mean depression level were found in the purging subtype compared with HCs. Heterogeneity exceeded that expected by chance at p < 0.05 (online Supplementary Table S1), and the study by Adoue et al. (Reference Adoue, Jaussent, Olie, Beziat, Van den Eynde, Courtet and Guillaume2015) was responsible. When this study was excluded, the heterogeneity disappeared and the contrast between HCs and purging AN patients was significant. The purging subtype patients had significantly lower IGT net scores than HCs.
Decision-making in BN (Fig. 2)
BN patients v. HCs
The BN patients had significantly lower IGT net scores than HCs, indicating a moderate effect size. The fail-safe N was 91. Mean age did not differ between the two groups. BMI and depression were different between the two groups, but only nine studies were included in this meta-analysis, so meta-regression was not performed.

Fig. 2. Comparison of the Iowa gambling task net scores in bulimia nervosa (BN). (a) BN patients v. healthy controls (HC): impaired decision-making. (b) Symptomatic AN patients v. BN patients: no between-group differences. (c) Binge eating disorder (BED) patients v. HC: impaired decision-making. CI, Confidence interval.
Heterogeneity exceeded that expected by chance at p < 0.05 (online Supplementary Table S1), and the study by Brogan et al. (Reference Brogan, Hevey, O'Callaghan, Yoder and O'Shea2011) was probably responsible: When this study was excluded, the heterogeneity disappeared and the results remained significant.
Symptomatic AN patients v. BN patients
The symptomatic AN patients did not have significantly lower IGT net scores than the BN patients. Mean age and depression level did not differ between the two groups. The symptomatic AN patients had lower mean BMI compared with the BN patients. Heterogeneity did not exceed that expected by chance at p < 0.05 (online Supplementary Table S1).
Decision-making in BED (Fig. 2)
BED patients v. HCs
The BED patients had significantly lower IGT net scores than HCs, although mean age, depression level and mean BMI were higher in these patients compared with HCs.
Heterogeneity exceeded that expected by chance at p < 0.05 (online Supplementary Table S1), and the study by Danner et al. (Reference Danner, Evers, Sternheim, van Meer, van Elburg, Geerets, Breteler and de Ridder2013) was probably responsible. Once this study was excluded, the heterogeneity disappeared and the results remained significant.
For all contrast in AN or BN, and BED, the funnel plots were reasonably symmetrical, suggesting a low risk of publication bias, and the more conservative Egger's regression intercept suggested no publication bias.
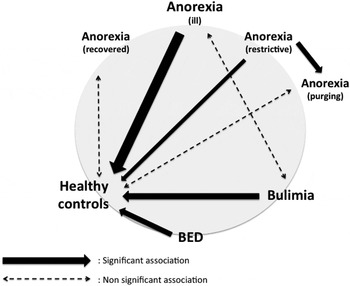
Decision-making in different EDs (Fig. 3)
To be able to compare the different types of EDs regarding the IGT specificities analysed in the present meta-analyses, a figure was drawn showing significant differences, if any (Fig. 3).
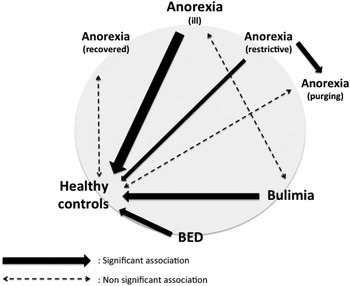
Fig. 3. Decision-making in different eating disorders. This figure sums up the different meta-analyses, which compared anorexia nervosa (anorexia), bulimia nervosa (bulimia), binge eating disorder (BED) and healthy controls. Solid arrows represent significant associations (their thickness reflecting Hedges’ g value) whereas dashed arrows represent non-significant associations.
Discussion
To our knowledge, this is the first meta-analysis of the literature on decision-making in the context of EDs. We report here that performance on the IGT was significantly altered in patients with an ED diagnosis (AN, BN and BED) compared with HCs. Hedges’ g effect sizes were moderate (0.72 and 0.62, respectively), which is particularly striking given that we were comparing two patient groups. The IGT score was significantly different between symptomatic AN patients and HCs, but not between recovered AN patients and HCs, suggesting that the measured deficit is more altered during symptomatic AN than in the remission period (Table 3). Neither starvation (assessed by current BMI) nor depression explained the results. The AN patients with the restrictive subtype had significantly lower IGT net scores than those with the purging subtype, and both subtypes had worse performances than HCs.
Table 3. Summary of findings

AN, Anorexia nervosa; HCs, healthy controls; IGT, Iowa gambling task; ANR, restrictive anorexia nervosa; ANP, purging anorexia nervosa; BN, bulimia nervosa; BED, binge-eating disorder.
a No statistically significant difference.
b Contrast became significant after excluding studies responsible for heterogeneity.
*p < 0.05, **p < 0.01, ***p < 0.001.
Overall, these results show that decision-making is altered in AN, especially in the acute phase of the illness. Mean age did not differ between groups for any comparison, thus ruling out a confounding role for this variable. Moreover, both recovered and symptomatic AN had significantly lower mean BMI and a higher mean depression level compared with HCs. Last, the effect size of the meta-analysis comparing symptomatic AN patients and HCs was not explained by BMI (β = −0.02, 95% CI −0.05 to 0.01, p = 0.2) or depression (β = 0.001, 95% CI −0.03 to 0.03, p = 0.9).
The potential impact of starvation on decision-making abilities in AN is a crucial point for consideration because of the clinical ramifications: refeeding in this case might ‘mechanically’ improve the decision-making process, whereas another type of support would be more appropriate in another situation. The observation that the current BMI did not make an impact on IGT performance confirms the reports of two longitudinal studies that found no improved performance after v. before refeeding (Cavedini et al. Reference Cavedini, Zorzi, Bassi, Gorini, Baraldi, Ubbiali and Bellodi2006; Bodell et al. Reference Bodell, Keel, Brumm, Akubuiro, Caballero, Tranel, Hodis and McCormick2014). This suggests that decision-making abilities under conditions of uncertainty do not change in AN participants with weight restoration. Nevertheless, it is important to bear in mind that IGT only assesses one aspect of decision-making, and other aspects may be more sensitive to starvation. Thus, in a study using a delay discounting paradigm, symptomatic AN patients showed a preference for delayed as opposed to earlier rewards compared with HCs, but AN patients after weight restoration did not differ from HCs (Decker et al. Reference Decker, Figner and Steinglass2014). It appears that differences in the decision-making paradigm, the types of patients included, and the sensitivity of the behavioural task may all contribute to explain such discrepancies.
We also found that the decision-making abilities of recovered patients and HCs are somewhat similar, suggesting a state rather than a trait impairment. This might seems contradictory, with our data suggesting that BMI in the acute phase of the disease does not predict decision-making. Among the possible explanations of this contradiction we can speculate on:
-
(1) A lack of statistical power may have played a role as only four studies included recovered patients; also, all studies except for that of Lindner et al. (Reference Lindner, Fichter and Quadflieg2012) had fewer than 15 participants per group.
-
(2) A state impairment (related to the disease and not to starvation). The recovered subjects in the studies included in the meta-analyses were defined by both normal weight and the reversal of other criteria of AN. This strongly underlines the need to discriminate in further studies two distinct phases: the weight-restoration phase in patients with normal weight but still ill and the recovery phase.
-
(3) No studies have to date prospectively assessed patients before and after recovery. It might be possible than patients who recover are patients who did not have decision-making impairment in the acute stage of the disease. It should further be noted that impaired decision-making was observed in the unaffected relatives of patients with AN (Galimberti et al. Reference Galimberti, Fadda, Cavallini, Martoni, Erzegovesi and Bellodi2013). Thus, more studies are needed to draw definitive conclusions.
Our results also seem to suggest impaired decision-making in the acute phase of BN and BED. This is an important result because, in these disorders in particular, the evidence for specific neurocognitive profiles has generally been inconclusive (Van den Eynde et al. Reference Van den Eynde, Guillaume, Broadbent, Stahl, Campbell, Schmidt and Tchanturia2011). From a state/trait perspective on decision-making impairment in EDs, the next step will be to assess decision-making in people remitted from BN. Furthermore, given the high percentage of patients with BN who were formerly AN patients, it will be important to determine whether this impairment is related to BN or to the former AN. Future studies targeting BN patients should therefore take into account any past history of AN. Also, three subtypes of bulimic disorders have been described (Steiger & Bruce, Reference Steiger and Bruce2007): the psychologically intact although perfectionistic type, the overregulated or compulsive type, and the dysregulated or impulsive type. The latter is associated with higher rates of co-morbidity, developmental disturbances and possibly also poorer treatment outcome. Comparisons among the bulimic subtypes would be helpful to determine which types of bulimic patients are more likely to show impaired decision-making.
Other questions and issues should also be addressed in future studies. A recent study using cognitive modelling analysis suggested differential impairments underlying IGT performances in AN and BN (Chan et al. Reference Chan, Ahn, Bates, Busemeyer, Guillaume, Redgrave, Danner and Courtet2014). Impaired decision-making in AN might involve impaired learning/memory functions, whereas in BN it might involve altered reward and punishment sensitivity (Chan et al. Reference Chan, Ahn, Bates, Busemeyer, Guillaume, Redgrave, Danner and Courtet2014). In the present meta-analysis, we found different impairments according to the subtype of AN. Garrido & Subirá (Reference Garrido and Subirá2013) found a correlation between decision-making and impulsivity only in purging EDs. A number of studies have found that restrictive EDs are different from the purging subtype in clinical presentation (Peat et al. Reference Peat, Mitchell, Hoek and Wonderlich2009), impulsivity level (Waxman, Reference Waxman2009), emotion regulation and self-regulatory behaviour (Danner et al. Reference Danner, Sternheim and Evers2014), and brain activation (Lock et al. Reference Lock, Garrett, Beenhakker and Reiss2011). Taken together, these findings suggest that the neural pathways that underlie decision-making differ, depending on the clinical presentation.
Our results showed that decision-making was altered in AN and BN, especially in the symptomatic phase of the illness. This raises the question: how exactly does poor decision-making contribute to cognitive functioning in the context of an ED? As stated earlier, the IGT simulates real-life decision-making in situations that involve uncertainty, reward and punishment. Decisions that involve uncertainty, options with multiple features, and changes over time place particularly high demands on cognitive control (Walton et al. Reference Walton, Behrens, Buckley, Rudebeck and Rushworth2010). A recent meta-analysis found a deficit in cognitive control in BN (Wu et al. Reference Wu, Giel, Skunde, Schag, Rudofsky, de Zwaan, Zipfel, Herzog and Friederich2013), whereas patients with AN were prone to excessive self-control. Cognitive control abilities in general depend on the associative cortices comprising the lateral frontoparietal and cingulo-opercular networks (Milham et al. Reference Milham, Erickson, Banich, Kramer, Webb, Wszalek and Cohen2002). Studies have indicated that the dorsolateral prefrontal cortex is crucial in working memory processes and in the ability to inhibit responses (McDowd et al. Reference McDowd, Oseas-Kreger, Filion, Dempster and Brainerd1995; Kane & Engle, Reference Kane and Engle2002). The deficits in cognitive control and the putative frontoparietal and cingulo-opercular alterations in EDs appear distinct from impairments in value-based decision-making paralleled by paralimbic and particularly ventromedial prefrontal cortex (Noonan et al. Reference Noonan, Walton, Behrens, Sallet, Buckley and Rushworth2010; Glascher et al. Reference Glascher, Adolphs, Damasio, Bechara, Rudrauf, Calamia, Paul and Tranel2012) and anterior cingulate cortex dysfunctions (Glascher et al. Reference Glascher, Adolphs, Damasio, Bechara, Rudrauf, Calamia, Paul and Tranel2012). Thus, we suspect two independent vulnerability pathways, one characterized by cognitive control/frontoparietal dysfunction and the other by value/paralimbic dysfunction. The first pathway may involve an inability to find and implement alternative solutions – that is, cognitive inflexibility – and this would seem to fit better with the types of impairment observed in AN. The second, ‘value/paralimbic’ pathway, illustrated by the current behavioural findings, may involve impulsivity, a low threshold for ED behaviour, and a disregard of deterrents – an explanation that may better describe the decision-making in BN.
Limitations
First, the studies included in this review examined various populations. For example, some enrolled only AN patients with the restrictive subtype, whereas others did not distinguish subtypes. Also, some of the studies were conducted with acutely symptomatic patients, whereas others focused on those in remission. Some studies excluded patients with an acute depressive disorder, and some included them. In still other studies, the participants were on medication, while in others they were not. In contrast, the HC groups are very homogeneous across studies, with most subjects having no lifetime history of psychiatric disorder. Potential biases due to gender, age, education level or intelligence quotient were taken into account through initial matching or multivariate analysis. Meta-analyses have often been criticized for combining heterogeneous studies, their potential for publication bias, and their inclusion of poor-quality trials. In the present study, however, these concerns were addressed by the use of stringent inclusion criteria and the objective examination of both publication bias and heterogeneity.
Second, most studies have almost exclusively used the IGT to measure the complex concept of decision-making, although a single test is unlikely to be adequate. Moreover, the IGT does not reveal the mechanisms of impairment, since the typically reported ‘net score’ does not provide access to the underlying components. The task would require a deconstruction of the task into component parts – that is, the cognitive, motivational and response processes – to identify which components show impairment in the context of a given ED subtype (Chan et al. Reference Chan, Ahn, Bates, Busemeyer, Guillaume, Redgrave, Danner and Courtet2014). Futures studies could also consider assessment with a combination of tasks measuring different and potentially non-overlapping aspects of decision-making impairment, such as temporal discounting, decision-making under risk or reversal learning.
Third, most of the currently available decision-making tasks are based on monetary rewards and punishments. Developing tasks based on more salient stimuli such as food would probably provide insight into the decision-making processes in EDs. Also, people with EDs may be particularly susceptible to hunger states. A recent study using a delay discounting paradigm showed that hunger raised the valuation response to financial cues, suggesting that the metabolic state plays an important role in modulating the brain's response to reward during a decision-making task in participants remitted from AN (Wierenga et al. Reference Wierenga, Bischoff-Grethe, Melrose, Irvine, Torres, Bailer, Simmons, Fudge, McClure, Ely and Kaye2015). Almost none of the studies reviewed here reported the hunger/satiety state. This may have affected the results presented, and we suggest that future studies record this information.
Last, the studies in this review included mainly adult patients, yet it would be interesting to determine whether decision-making is impaired in adolescents. Other cognitive dysfunctions that are reported in the literature on the adult AN population, such as impaired set-shifting, have not been found to be as pronounced in younger AN patients (Lang et al. Reference Lang, Stahl, Espie, Treasure and Tchanturia2014b ).
Conclusion
Our results suggest a decision-making impairment in all acute EDs. In AN, this may be related to subtypes and not to malnutrition, but further study is needed to conclude a state or trait cognitive vulnerability. The results emphasize the need to discern in AN studies the clinical changes resulting from weight regain from those resulting from improvement of the disease. Future studies should also assess the prognostic value of decision-making, as one study suggested that decision-making ability might be associated with treatment outcome in AN (Cavedini et al. Reference Cavedini, Zorzi, Bassi, Gorini, Baraldi, Ubbiali and Bellodi2006). This cognitive process indeed seems to have a powerful impact on daily-life functioning and treatment refractoriness. Overall, an interesting goal would be to determine whether the decision-making process is a potential target for treatment in the context of EDs.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017//S003329171500152X
Acknowledgements
S.R.-D. received a fellow grant from the Canadian Institutes for Health Research (CIHR). Part of this study received financial support from CHU Montpellier (AOI UF 8854).
Declaration of Interest
None.