Since the 1900s, there has been a rapid evolution of medical technologies. However, in the face of constrained health budgets, health systems worldwide need to ensure efficiency and demonstrate value for investment. Hence there is an urgent need to make informed decisions about technologies which (a) are ineffective, (b) are no longer cost-effective, and (c) have been superseded by more effective innovations.
Technology assessment as a general principle emerged in the mid-1960s to evaluate unintended harmful consequences of a technology (Reference Goodman1). It gave rise to a comprehensive paradigm, the Health Technology Assessment (HTA), an approach which specifically addresses the health sector. HTA has developed over the past decades to strengthen evidence-based prioritization, selection, and rational use of health interventions and technologies. An effective HTA process includes scoping, assessment, appraisal and implementation, and monitoring (Reference Kristensen, Husereau, Huić, Drummond, Berger and Bond2). It also provides governance and structure (Reference Kristensen, Husereau, Huić, Drummond, Berger and Bond2). The results obtained from HTAs include both context-free and context-dependent factors. These are utilized in varying degrees to shape sustainable financing benefit packages and to formulate clinical practice guidelines and protocols for public health programs (3). They are also applied to reorganize service provisions and plan capacities. There are geographical and political variations in the development, legislation, and institutionalization of HTA. Furthermore, there are different kinds of HTA organizations—some holding national or regional mandates and others that support decisions at the hospital level. Their outcomes may be required by legislative mandates or can have an advisory character.
The national governments of the BRICS countries—Brazil, the Russian Federation, India, China, and South Africa—are committed to ensuring that universal health coverage of its population is achieved (4) even if the countries are at different stages of HTA introduction and implementation (4–Reference MacQuilkan, Baker, Downey, Ruiz, Chalkidou and Prinja6). Brazil has an effective HTA program that has gone through a stepwise evolution, pooling together various stakeholders involved with HTA and, making a conscientious effort to include societal and public input into the process. The assessment and decision-making processes are separated, and have included the involvement of universities, various relevant committees and departments, and the Ministry of Health (Reference Lessa and Ferraz7). Russia, on the other hand, is at the beginning of introducing HTA; HTA centers have been created, and pharmacoeconomics studies conducted (Reference Marsh8). Despite India's complex, fragmented health system, the Indian government has demonstrated a keen interest in HTA introduction by forming various boards and committees to support and undertake HTA activities (Reference MacQuilkan, Baker, Downey, Ruiz, Chalkidou and Prinja6). The Chinese government is committed to the introduction of HTA. However, the activities among different actors are still fragmented and need a concerted effort to integrate the principles, processes, and governance fully into the healthcare system (Reference Chen, He, Chi, Wei and Shi9).
In South Africa, there is no structured and formal utilization of HTA (Reference Govender, Letshokgohla and Basu10). However, some decision-making bodies (e.g., private insurance companies, National Essential Medicines List Committee (Reference Perumal-Pillay and Suleman11), public entity (Reference Croce, Mueller and Tivey12)) use HTA-like methods and tools (e.g., domains such as safety and efficacy). It should be recalled that South Africa is a democracy with a three-tier system of government and an independent judiciary. It is divided into nine provinces. The national, provincial, and local (including district) levels of government have legislative and executive authority in their spheres. Health services fall under national and provincial legislatures.
South Africa's health system consists of a large public sector and a smaller, faster growing private sector. Health care in South Africa varies from the most basic primary health care to highly advanced specialized health services. While primary health care is offered by the state to the uninsured population free of charge, specialized health services are available mainly in the private sector. Total health expenditure was reported at 8.6 percent (percent of GDP) in 2016 (13). While the public health sector accounts for about 40 percent of all expenditure on health, it is under pressure to deliver services to about 80 percent of the population. The private sector caters to mostly middle- and high-income earners who are also members of various medical schemes. This two-tiered system is not only inequitable, but also results in an underfunded and deteriorating infrastructure and poor management in the public sector institutions (4;14). Public health challenges, including the burden of diseases such as HIV/AIDS, tuberculosis and noncommunicable diseases, high maternal and child mortality, and high levels of violence and injuries in addition to a shortage of key medical personnel has compounded the situation.
This study sought to investigate the challenges faced in the structured implementation and utilization of HTA which impacts how the systematic utilization and consistent practice of HTA in the public health sector in South Africa will be adopted.
Methods
To fulfill the aim of the study, data from a comprehensive HTA-related document review, and a self-administered questionnaire (provided to key stakeholders) were collected and analyzed. The details of the questionnaire can be found in Supplementary File 2. The study took place between February 2016 and September 2017.
Document Review
The document review examined existing regulations, policies, guidelines, and legislation on health technology introduced in the country since 1965. This grey literature was identified and sourced from stakeholders within national and provincial departments of health, the WHO Web site (https://www.who.int/medical_devices/countries/regulations/zaf.pdf?ua=1), and hand-searched from references of cited documents. All documents found were included. Each document was reviewed and summarized in a data collection sheet that included the document title and type, the date, its purpose, key features, and its relevancy to HTA. The list of documents can be found in Supplementary File 1.
Walt and Gilson's policy triangle framework (Reference Walt, Shiffman, Schneider, Murray, Brugha and Gilson15) and Kingdon's multiple streams theory (Reference Kingdon16) were determined as suitable models to understand and evaluate the current Health Technology policies in the country. Supplementary File 1 methods section has the details.
Self-Completion Questionnaire
A survey conducted between February 2016 and September 2017 on the current practices in decision making concerning health technologies at all levels of the public healthcare system supplemented the review from the grey literature. Respondents were identified through direct contact with ministries of health at both national and provincial levels, key officials from private sectors, and professional societies. They were also recruited from workshops conducted at two HTA capacity-building workshops on 31 October 2016 and 24 July 2017 in Durban and Pretoria, respectively. The first workshop held at the International Hospital Federation World Hospital Congress brought together forty-one representatives from hospitals, provincial governments, industry, and nongovernmental organizations. This workshop on “Health Technology Assessments: the essentials” had only twenty-two participants responding to the survey. Out of the twenty-nine participants, twenty-eight representing the national and provincial governments, hospitals, and academics attending the second workshop hosted by the Department of Health filled out the questionnaire. Additionally, five others who were identified directly also responded to the survey questionnaire. The questionnaire addressed the issues on the current state of HTA in the country and requested information on the challenges of incorporating formal HTA mechanisms into all levels of health care. The questions posed were:
• What is your current job function (e.g., researcher, clinician, hospital-manager, health economist, etc.)?
• In what kind of setting do you work?
• How are new health services approved currently in your setting?
• Do you see a role for health technology assessment in your setting?
• HTA can determine the clinical effectiveness, cost-effectiveness, and safety—would you decide to fund a project/program involving HTA and how would you decide whether to fund or not?
• Should HTA be centralized or devolved?
Results
The results of the thematic analysis of the grey literature provide an insight into the country's current HTA policy and are presented below. A schematic representation of the results is also shown in Figure 1. Findings include (i) content of the policy, (ii) actors who formulate and implement the policy, (iii) context of the development, defining the reason, and (iv) the process of the implementation (how).
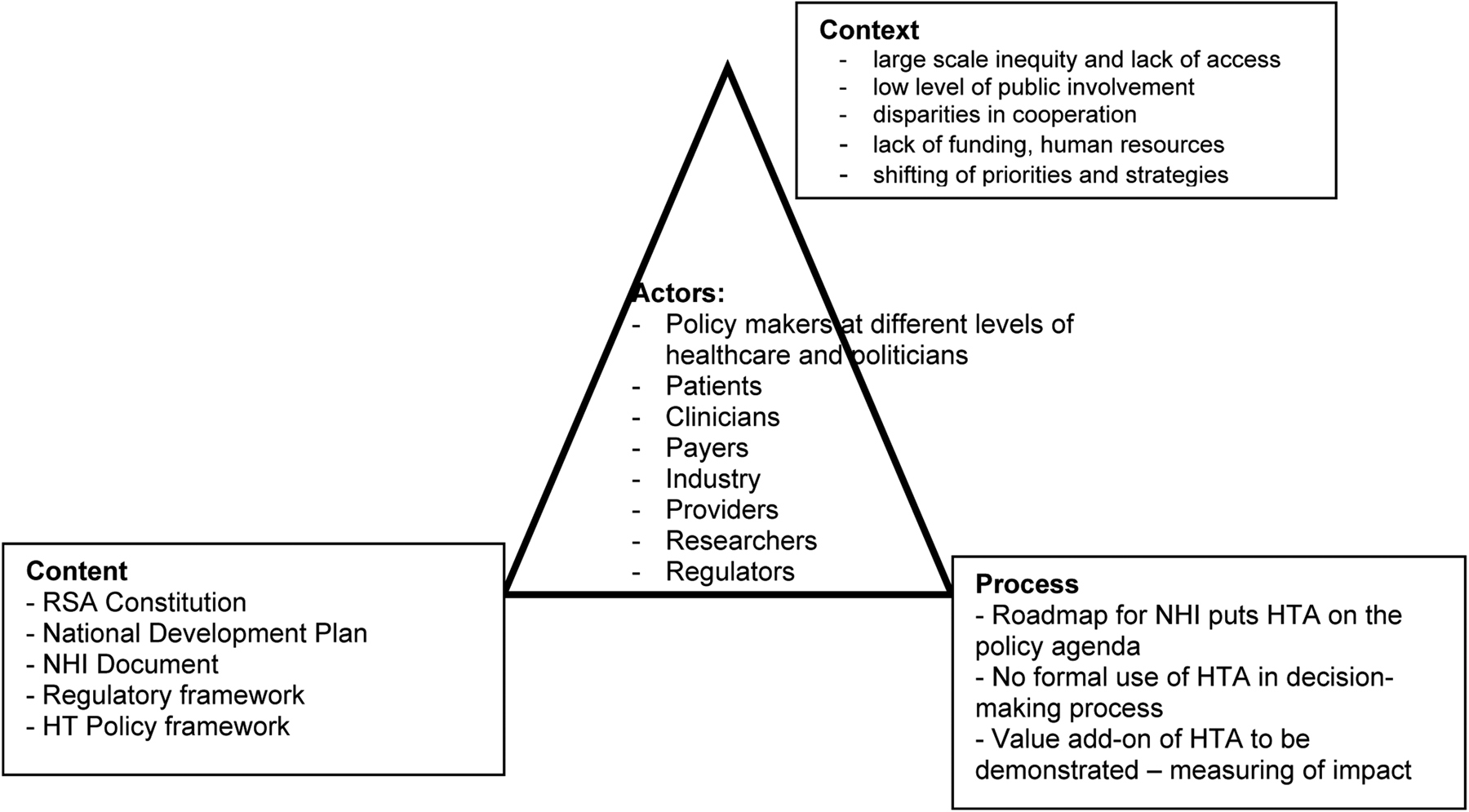
Figure 1. Policy triangle analysis pertaining to Health Technology Policy in South Africa (adapted from Walt and Gilson 1994).
Content
The identified content of the government policy documents obtained through the review is organized chronologically and listed with references in Supplementary File 1.
Section 27 of the Constitution of South Africa guarantees its citizens the right to healthcare, asserting access to healthcare services for all. In 2001, to address HTA, the Health Technology Policy Framework (HTPF) was drafted with the vision to create a consolidated health technology system to ensure that access is equitable and limited resources are optimally used. The HTPF contains information on the integration of HTA into routine operational planning of services in public and private institutions with recommendations for implementation. Hence this illustrates the government's recognition of its added value to achieve efficiency for healthcare spending and to maintain healthcare sustainability.
The formulation of policies lies within the mandate of the National Department of Health while the parliament approves regulations and legislation leading to policy. In 2010, a strategic program to achieve universal health coverage through “the 10-point plan” was laid out. Its focus was on improving the infrastructure, human resource planning, management, and the quality of the healthcare system.
Besides, the National Health Technology Strategy, which focuses on medical devices, was devised to operationalize the HTPF.
The National Development Plan stresses a revitalized and integrated healthcare system—that is, an evidence-based approach to public and private healthcare delivery systems calling for clear separation of policy making from oversight and operations. Consequently, this ensures unbiased decision making, devolvement of authority and administration to lowest levels addressing greater use of information technologies. Additionally, this safeguards the rationalization of clinical processes and systematic use of data on community health, prevention, and environmental concerns.
Passed in 2015, the Medicines and Related Substances Amendment Act 14 makes provision for the establishment of a new regulatory authority with a mandate that extended to medical devices and in vitro diagnostics, cosmetics, and food. The South African Health Products Regulatory Agency (SAHPRA) replaced the Medicine Control Council and was established with a mandate broader than medicines alone. Currently, the regulation of medical devices primarily encompasses proof of efficacy of the product and ensures public safety.
A National Health Insurance (NHI) Bill gazette was drafted in 2019 that specifies HTA as an integral part of the NHI program and that it should be used to review various health interventions and technologies to determine the benefits to be covered.
In summary, the different policies are well-intentioned at their core, laying down the foundation to achieve coverage of healthcare to its entire population by taking an evidence-based approach.
Actors Involved
Figures 2 and 3 display the distribution of respondents according to their job description and their work setting. Further details are found in Supplementary File 2. Of the seventy-five people identified and approached, fifty-five responded to the survey, including directors of health technology at the provincial and national level, directors of hospitals, senior pharmacists and nurses from academic hospitals and representatives from the medical device industry and medical schemes. The findings of the survey are represented schematically in Figure 4, illustrating the influence of the respondents in decision making.

Figure 2. Percentage of respondents working in different health sectors (HCA: healthcare administrators denotes various health professionals working in the ministry of health).

Figure 3. Respondents' work setting.
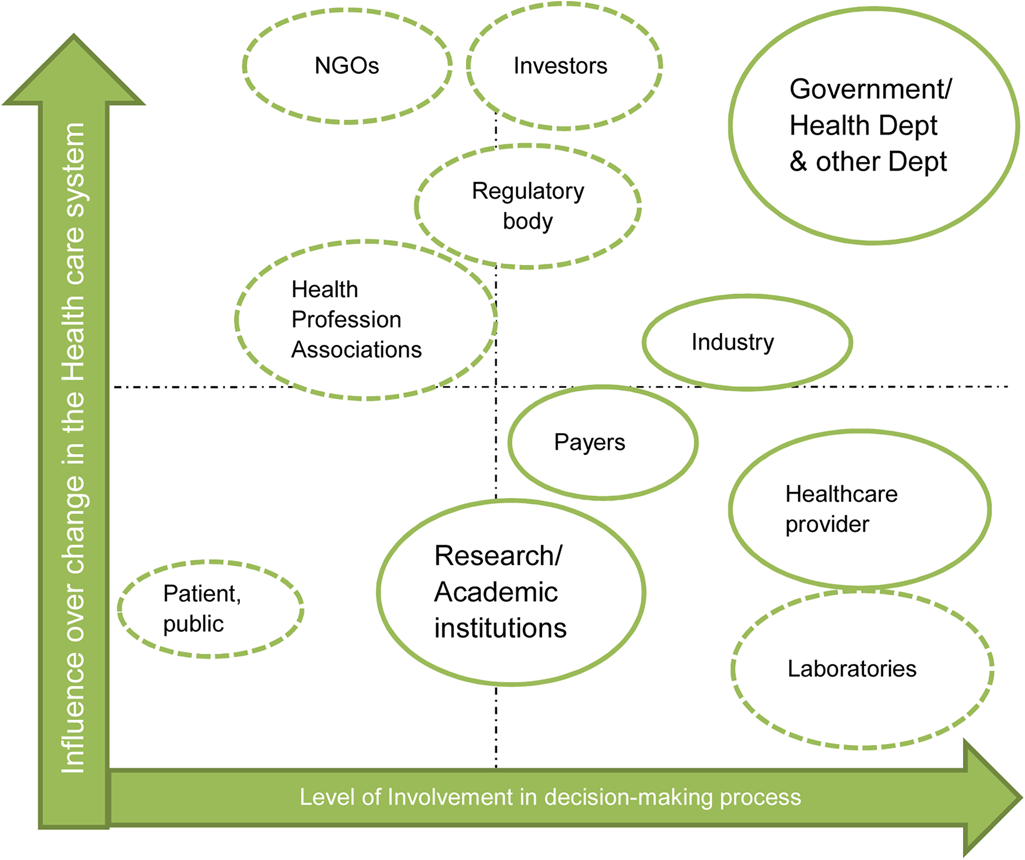
Figure 4. Different stakeholders influencing HTA in South Africa.
This demonstrates the wide variety of stakeholders, with different and essential influences in healthcare decision making. They play an important role not only in formulation, but also in the implementation of the changes in the healthcare system and in the overall policy process.
Contextual Factors
In 2014, the importance of health intervention and technology assessment was emphasized at the World Health Assembly (3), which recognized the necessity of evidence-based policy and decision making in health systems and encouraged member countries to utilize HTA systematically to inform policy makers on health interventions and technologies.
The government has prioritized the provision of universal health coverage to its citizens, providing an optimal basis for formalizing and institutionalizing HTA into policy and decision making. Contextual factors affecting policy development and implementation are social, cultural, political, ethical, economic, organizational, and institutional (Reference Walt, Shiffman, Schneider, Murray, Brugha and Gilson15).
Social and cultural factors affecting HTPF implementation include large-scale inequity and lack of access to quality health care (Reference Maphumulo and Bhengu14), as mentioned by some of the respondents. The economic and financial factors include a lack of funding necessary for optimal use of the program, and the slow implementation of the national health insurance coverage program (Reference Maphumulo and Bhengu14). The political factors that influence the use of HTA are shifting of priorities and strategies with changing governments or ministries and weak political commitment.
The difficulties encountered in moving ahead with the implementation of HTA can be traced to:
(a) limited availability of financial resources
(b) limited provision of systems to support HTA processes
(c) lack of adequate number of trained professionals and the insufficient provision of local education and hands-on training opportunities
(d) lack of collaboration and commitment of actors in public and private systems
(e) differences in service delivery within the public system across different provinces.
According to the respondents, funding for an HTA program at the national or at the provincial level is dependent on the anticipated benefit of the program. They affirmed an unawareness of the concept of HTA at the senior level, and in general, a need for building capacity in HTA. Consequently, developing local champions for HTA concepts and benefits is essential, and has been recommended in other emerging settings (Reference Wild, Stricka and Patera17).
Process
The aim of the HTA strategy stems from the need for efficient utilization of health technologies for improving healthcare service. Based on the survey responses, the framework for HT policy, including the strategy for HTA, was observed to be inconsistently implemented. Most of the respondents (25 percent) indicated that standard treatment guidelines and evidence are used for the approval of new medicines and technologies. Others (12.5 percent) found that conducting policy analysis, pharmacoeconomic evaluations, feasibility studies, and studies showing an impact on clinical outcome played a role in the approval process (see Supplementary File 2).
The current policy process does not ensure that state-of-the-art and novel health technologies reach all segments of the population. There was ambiguity among participants about the relative roles of different levels (governmental or provincial actors) in the policy decision-making process. For participants working at the national level, the importance of HTA lies in the assessment of medical schemes, reviewing current benefit packages, and cost containment. Hence, this may increase access to cost-effective and clinically effective technologies.
Participants working at the provincial and local levels found the potential of HTA in systematic and transparent procurement processes. The participants presumed that the clinicians determine the need for new technologies within the boundaries of the available budget. Using HTA, which involves various decision makers, would contribute toward fairness and transparency. It would also enable managing public expectation and patient demand.
The role of HTA at the local level differs from that at the national level. There was a degree of skepticism at the local level; the survey participants need further demonstration of the anticipated added value of HTA to healthcare services and its relevancy in improving the health status of individuals. According to them, the type of HTA service will depend on the size and complexity of the service.
Thus, a coordinated effort and a transparent systematic mechanism are required for integrating all levels of the healthcare system.
Discussion
Current Challenges
After a relatively early promising start in South Africa, the expected progress toward a high-quality healthcare system supported by HTA failed to materialize. Based on Kingdon's (Reference Kingdon16) multiple streams theory, the convergence of the problem, policy, and politics streams in this work is assessed to understand this. The problem stems from the nonuse of a robust, systematic, transparent, and unbiased scientific process to evaluate healthcare technologies to inform policies (such as setting priorities and to define health benefit policies). HTA is identified as a potential solution to this problem, the development of which requires a stable policy. The NHI Bill stipulating the use of HTA in the decision-making process would constitute the policy stream. Lastly, the political stream includes the various stakeholders: governmental agencies, the medical device and pharmaceutical industries, hospitals, insurers, regulatory bodies, and research institutions. This stream is responsible for the formal introduction of HTA. Political factors may be a crucial determinant of HTA implementation and sustainability as observed in other low- and middle-income countries (LMICs) (Reference Mueller, Tivey and Croce18–Reference Oliver, Innvar, Lorenc, Woodman and Thomas20). Political will is required for HTA introduction, as observed in countries like India, Argentina, or Russia (5;6;19). Raising awareness of the benefits of HTA utilization may lead to political support and commitment (10;20;21). Different policies and regulations referencing HTA, such as the NHI paper, demonstrate that the stakeholders at various levels of the healthcare system are aware of the usefulness of HTA.
Government officials and politicians have a powerful influence on the policy-making processes when determining the implementation of healthcare technology assessment. Payers can exercise power on the decision-making process by providing access to services and products. The healthcare industry and professional associations can influence the implementation of HTA via their technical expertise and field analysis. The research institutions, while having a low involvement in the decision-making process itself, contribute to the development of novel technologies based on feedback from industry and healthcare professionals. No matter what the degree of influence the stakeholders have, their contribution at each stage of the HTA process is valuable. However, stakeholder involvement in a public and transparent manner varies from country to country; few LMICs have explicit mechanisms in place (Reference Oortwijn, Broos, Vondeling, Banta and Todorova5). Even though it is not yet formalized and transparent to the public, there are instances of stakeholder engagement in the preparation of HTA or HTA-like reports in South Africa.
Potential Solutions
The lack of action in HTA implementation may be a consequence of its top-down approach resulting in limited buy-in at different levels. The national government needs to be aware of the benefits of using HTA and to involve the provincial governments, hospitals, and other key stakeholders (including the public) in the healthcare system in providing quality and equitable access to healthcare for the population. As has also been pointed out by the participants of the survey and reinforced in Mueller et al. (Reference Mueller, Tivey and Croce18), evidence-based research activities on health projects and publications on HTA, health-economic evaluations, teaching, and training in HTA are crucial to the practice of HTA. These can mitigate challenges such as a lack of context-specific evidence (clinical and cost), knowledge about evidence-based medicine, and HTA utilization. Inadequate availability of relevant education and training programs in HTA catering to the needs of LMICs and covering multi-disciplinary skills and competencies in HTA have been observed globally (22;23) and is not unique to South Africa. Another solution to limited resources would be to adapt and adopt HTAs from other jurisdictions. For instance, the joint assessments resulting from the European collaboration in the field of relative effectiveness assessments are an example; contextualization of these assessments though remains the remit of the national entities (Reference Kristensen, Lampe, Wild, Cerbo, Goettsch and Becla24).
These actions can also reduce resistance to change in existing decision-making culture and practice routines. For the HTA system to be effective, good governance, adequate and expert staffing, funding, and sustainable collaboration with various actors were identified as the key factors. The inadequacy of funding is perceived as a hurdle in establishing HTA (22;25). The respondents also expressed concern about the low number of trained professionals. In their opinion, continued emphasis on education and training in HTA should be consolidated with attractive career opportunities. Poor routine data, low-quality patient databases, and weak or nonexistent linkages between different databases were mentioned and have been observed to be a common issue in many developing countries (19;22).
Many countries have HTA organizations, the so-called agency established to support the different HTA activities. These organizations respond to requests to inform transparent and legitimate decisions incorporating participation of public, patient, and society (18;22). The respondents' opinions varied on the structure of these agencies and range from a centralized model to a decentralized system. The public model is prevalent in OECD countries; the organizations are either included in the public healthcare systems' organization at national or at regional levels or independent stand-alone agencies connected to the public healthcare system. The need for application of HTA at the regional and hospital levels has further led to the drive for decentralization.
Strengths and Limitations
Conducting the self-completion questionnaire led to certain challenges. First, there was the issue of identifying key persons familiar with HTA and the state of implementation in the country. This is due to the limited expertise in HTA in the public sector. The need for capacity building has been uniformly highlighted by the respondents. Second, the response rate of the survey was initially relatively low. Efforts were then made to obtain a higher response rate by involving participants of HTA workshops held in the country. While this may lead to a biased participant sample, the participants were more informed about HTA and their feedback, therefore, more relevant. Findings convey that sometimes HTA is interpreted in different ways. For example, a participant believed that HTA is a “software program.” The relatively low response rate may be due to people considering themselves “not qualified enough” on the subject to express their opinion.
Conclusion
This study offers a snapshot of current opportunities and challenges of HTA institutionalization in the public sector in South Africa. Despite the attention given to the HTA issue and the discussions on HTA implementation and its integration into the health system, the structured implementation and transparent utilization in the public sector is still limited. Systematic use of HTA requires methodological capacity, legislations, and clear lines of accountability in addition to strong coordination and adequate financial support. Accountability lies not only with the national and regional (provinces and districts) government, but also with other key stakeholders such as healthcare professionals and providers, patients, and public, as well as with industry representatives. Education and hands-on training opportunities at local universities during various workshops at conferences (regional, local, and international) or short courses through private institutions can increase capacity. This also includes raising awareness of HTA through different activities such as providing in-house training at the Department of Health or academic hospitals. Taking a bottom-up approach at the meso- and micro-level by creating positive interest among stakeholders, such as healthcare professionals, clinicians, patients, patient advocates, and patient organizations, can be a driving force for uptake of HTA. Principally fostering and creating sustainable collaboration between policymakers, HTA professionals, and all relevant stakeholders can lead to general acceptance of HTA. Local champions and advocacy in HTA would further solidify and sustain these partnerships.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266462320000562.






