Introduction
Schizophrenia is among the world's leading causes of disability (Murray et al. Reference Murray, Vos, Lozano, Naghavi, Flaxman, Michaud, Ezzati, Shibuya, Salomon, Abdalla, Aboyans, Abraham, Ackerman, Aggarwal, Ahn, Ali, Alvarado, Anderson, Anderson, Andrews, Atkinson, Baddour, Bahalim, Barker-Collo, Barrero, Bartels, Basáñez, Baxter, Bell, Benjamin, Bennett, Bernabé, Bhalla, Bhandari, Bikbov, Bin Abdulhak, Birbeck, Black, Blencowe, Blore, Blyth, Bolliger, Bonaventure, Boufous, Bourne, Boussinesq, Braithwaite, Brayne, Bridgett, Brooker, Brooks, Brugha, Bryan-Hancock, Bucello, Buchbinder, Buckle, Budke, Burch, Burney, Burstein, Calabria, Campbell, Canter, Carabin, Carapetis, Carmona, Cella, Charlson, Chen, Cheng, Chou, Chugh, Coffeng, Colan, Colquhoun, Colson, Condon, Connor, Cooper, Corriere, Cortinovis, de Vaccaro, Couser, Cowie, Criqui, Cross, Dabhadkar, Dahiya, Dahodwala, Damsere-Derry, Danaei, Davis, De Leo, Degenhardt, Dellavalle, Delossantos, Denenberg, Derrett, Des Jarlais, Dharmaratne, Dherani, Diaz-Torne, Dolk, Dorsey, Driscoll, Duber, Ebel, Edmond, Elbaz, Ali, Erskine, Erwin, Espindola, Ewoigbokhan, Farzadfar, Feigin, Felson, Ferrari, Ferri, Fèvre, Finucane, Flaxman, Flood, Foreman, Forouzanfar, Fowkes, Fransen, Freeman, Gabbe, Gabriel, Gakidou, Ganatra, Garcia, Gaspari, Gillum, Gmel, Gonzalez-Medina, Gosselin, Grainger, Grant, Groeger, Guillemin, Gunnell, Gupta, Haagsma, Hagan, Halasa, Hall, Haring, Haro, Harrison, Havmoeller, Hay, Higashi, Hill, Hoen, Hoffman, Hotez, Hoy, Huang, Ibeanusi, Jacobsen, James, Jarvis, Jasrasaria, Jayaraman, Johns, Jonas, Karthikeyan, Kassebaum, Kawakami, Keren, Khoo, King, Knowlton, Kobusingye, Koranteng, Krishnamurthi, Laden, Lalloo, Laslett, Lathlean, Leasher, Lee, Leigh, Levinson, Lim, Limb, Lin, Lipnick, Lipshultz, Liu, Loane, Ohno, Lyons, Mabweijano, MacIntyre, Malekzadeh, Mallinger, Manivannan, Marcenes, March, Margolis, Marks, Marks, Matsumori, Matzopoulos, Mayosi, McAnulty, McDermott, McGill, McGrath, Medina-Mora, Meltzer, Mensah, Merriman, Meyer, Miglioli, Miller, Miller, Mitchell, Mock, Mocumbi, Moffitt, Mokdad, Monasta, Montico, Moradi-Lakeh, Moran, Morawska, Mori, Murdoch, Mwaniki, Naidoo, Nair, Naldi, Narayan, Nelson, Nelson, Nevitt, Newton, Nolte, Norman, Norman, O'Donnell, O'Hanlon, Olives, Omer, Ortblad, Osborne, Ozgediz, Page, Pahari, Pandian, Rivero, Patten, Pearce, Padilla, Perez-Ruiz, Perico, Pesudovs, Phillips, Phillips, Pierce, Pion, Polanczyk, Polinder, Pope, Popova, Porrini, Pourmalek, Prince, Pullan, Ramaiah, Ranganathan, Razavi, Regan, Rehm, Rein, Remuzzi, Richardson, Rivara, Roberts, Robinson, De Leòn, Ronfani, Room, Rosenfeld, Rushton, Sacco, Saha, Sampson, Sanchez-Riera, Sanman, Schwebel, Scott, Segui-Gomez, Shahraz, Shepard, Shin, Shivakoti, Singh, Singh, Singh, Singleton, Sleet, Sliwa, Smith, Smith, Stapelberg, Steer, Steiner, Stolk, Stovner, Sudfeld, Syed, Tamburlini, Tavakkoli, Taylor, Taylor, Taylor, Thomas, Thomson, Thurston, Tleyjeh, Tonelli, Towbin, Truelsen, Tsilimbaris, Ubeda, Undurraga, van der Werf, van Os, Vavilala, Venketasubramanian, Wang, Wang, Watt, Weatherall, Weinstock, Weintraub, Weisskopf, Weissman, White, Whiteford, Wiebe, Wiersma, Wilkinson, Williams, Williams, Witt, Wolfe, Woolf, Wulf, Yeh, Zaidi, Zheng, Zonies, Lopez, AlMazroa and Memish2012; Neil et al. Reference Neil, Carr, Mihalopoulos, Mackinnon and Morgan2014b ) and is associated with substantial health-related and economic costs (Neil et al. Reference Neil, Carr, Mihalopoulos, Mackinnon, Lewin and Morgan2014a ). The main driver of treatment costs is hospitalization. Although hospitalization costs vary from setting to setting, these have been reported to be 77% of total treatment costs, whereas unemployment is the main driver of indirect costs (Carr et al. Reference Carr, Neil, Halpin, Holmes and Lewin2003).
In most patients, a first episode of psychosis is preceded by a prodromal period. In the last decades this so-called ultra-high-risk (UHR) state can be detected. The UHR state is characterized by subclinical psychotic symptoms and/or a genetic predisposition and, most importantly, by functional decline and social withdrawal (Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005, Reference Yung, Nelson, Stanford, Simmons, Cosgrave, Killackey, Phillips, Bechdolf, Buckby and McGorry2008). Because 31.5% [95% confidence interval (CI) 23.8–35.0%] of people at UHR have been found to develop first-episode psychosis within 3 years (Fusar-Poli et al. Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton, Valmaggia, Barale, Caverzasi and McGuire2012), this allows us to apply targeted prevention of a first episode of psychosis. Prevention may help to maintain quality of life, reduce the risk of onset and reduce the downstream costs of intensive treatment and productivity losses. In line with recent studies (Fusar-Poli et al. Reference Fusar-Poli, Borgwardt, Bechdolf, Addington, Riecher-Rössler, Schultze-Lutter, Keshavan, Wood, Ruhrmann, Seidman, Valmaggia, Cannon, Velthorst, De Haan, Cornblatt, Bonoldi, Birchwood, McGlashan, Carpenter, McGorry, Klosterkötter, McGuire and Yung2013; Hutton & Taylor, Reference Hutton and Taylor2013; Stafford et al. Reference Stafford, Jackson, Mayo-Wilson, Morrison and Kendall2013; van der Gaag et al. Reference Van der Gaag, Smit, Bechdolf, French, Linszen, Yung, McGorry and Cuijpers2013b ), we demonstrated the feasibility of such a preventive approach showing that cognitive–behavioural therapy (CBT) had a favourable effect on reducing the risk of a transition from UHR to frank psychosis by about 50% (van der Gaag et al. Reference Van der Gaag, Nieman, Rietdijk, Dragt, Ising, Klaassen, Koeter, Cuijpers, Wunderink and Linszen2012).
Until now, health economic evaluations in this field were either based on decision modelling (Valmaggia et al. Reference Valmaggia, McCrone, Knapp, Woolley, Broome, Tabraham, Johns, Prescott, Bramon, Lappin, Power and McGuire2009, Reference Valmaggia, McGuire, Fusar-Poli, Howes and McCrone2012; Mihalopoulos et al. Reference Mihalopoulos, Vos, Pirkis and Carter2011) or were based on a small sample (Phillips et al. Reference Phillips, Cotton, Mihalopoulos, Shih, Yung, Carter and McGorry2009).
The current study was designed to evaluate whether adding CBT to routine care (RC) for UHR patients is effective in reducing the risk of first-episode psychosis and improving quality of life in the population in a cost-effective way. To that end, a trial-based (van der Gaag et al. Reference Van der Gaag, Nieman, Rietdijk, Dragt, Ising, Klaassen, Koeter, Cuijpers, Wunderink and Linszen2012) cost-effectiveness analysis (CEA; with prevented onset of psychosis as outcome) and cost–utility analysis [CUA; with quality-adjusted life years (QALYs) as outcome] were conducted in the out-patient mental healthcare setting in the Netherlands, comparing add-on CBT with RC alone. QALYs are a generic and standardized metric that capture improvements in quality of life and play an important role in health economic evaluations, because QALYs can be compared across various illnesses and disorders. This adds to the generalizability of CUA.
Method
Design and participants
The Dutch Early Detection and Intervention Evaluation (EDIE-NL; Rietdijk et al. Reference Rietdijk, Dragt, Klaassen, Ising, Nieman, Wunderink, Delespaul, Cuijpers, Linszen and van der Gaag2010) study is a multi-centre trial, in which add-on CBT was compared with RC alone. The study was approved by the Medical Ethics Committee for mental health service research and registered at Current Controlled Trials (ISRCTN21353122). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Participants were recruited by four treatment centres offering services at 12 different locations in the Netherlands between February 2008 and February 2010. To be included, participants had to meet the following criteria: (1) age 14–35 years; (2) UHR status according to the Comprehensive Assessment of At-Risk Mental States (CAARMS; Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005, Reference Yung, Nelson, Stanford, Simmons, Cosgrave, Killackey, Phillips, Bechdolf, Buckby and McGorry2008); and (3) a decline in social functioning by a score of 50 or less on the Social and Occupational Functioning Assessment Scale (SOFAS; Goldman et al. Reference Goldman, Skodol and Lave1992), and/or a reduction by 30% on the SOFAS for at least 1 month in the past year. The exclusion criteria were: (1) current or previous use of antipsychotic medication with a cumulative dose of ≥15 mg haloperidol equivalents; (2) severe learning impairment; (3) problems due to a somatic condition; (4) insufficient competence in the Dutch language; (5) history of psychosis; and (6) aggression towards healthcare professionals.
After providing informed consent, 201 participants agreed to participate (Fig. 1). Five participants were removed from the analyses because two were already psychotic at baseline and three disclosed a history of psychosis. Thus, the final sample consisted of 196 participants: 101 in the control condition and 95 in the experimental condition.
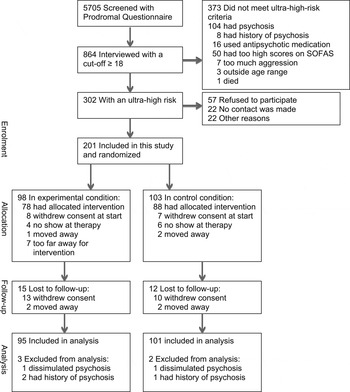
Fig. 1. Flowchart of the participants in the Dutch Early Detection and Intervention Evaluation (EDIE-NL) study. SOFAS, Social and Occupational Functioning Assessment Scale.
Interventions
Patients in both conditions were treated with RC provided for the non-psychotic Axis 1 or Axis 2 disorders that they were seeking treatment for. RC was given according to the evidence-based clinical Dutch (Landelijke Stuurgroep Multidisciplinaire Richtlijnontwikkeling in de GGZ, 2014) and the NICE (National Institute for Health and Care Excellence, 2013) guidelines for non-psychotic Axis 1 or Axis 2 disorders. Guidelines for anxiety disorders, depression, post-traumatic stress disorder, eating disorders, attention-deficit/hyperactive disorder, personality disorders, autism spectrum disorders, somatoform disorders, etc. were applied if indicated.
The experimental group received RC plus add-on individual CBT aiming at the prevention of a first psychosis (van der Gaag et al. Reference Van der Gaag, Nieman, Rietdijk, Dragt, Ising, Klaassen, Koeter, Cuijpers, Wunderink and Linszen2012). The CBT intervention consisted of the more generic protocol by French & Morrison (Reference French and Morrison2004) enriched with education on dopamine supersensitivity, the effects of dopaminergic supersensitivity on perception and reasoning, and a metacognitive awareness training (van der Gaag et al. Reference Van der Gaag, Nieman and van den Berg2013a ). CBT consisted of a maximum of 26 sessions; the mean (and median) number of sessions was 10 (95% CI 8–12), partly caused by early completers, dropouts or early transitions. The therapists were all experienced in CBT for psychosis and had work experience between 1 and 26 years.
Outcome measures
Transitions to psychosis
We conducted a CEA with the primary clinical outcome of interest, i.e. the costs per averted transition to psychosis, calculated as the proportion of participants that did not develop first-episode psychosis within 18 months as assessed with the CAARMS (Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005, Reference Yung, Nelson, Stanford, Simmons, Cosgrave, Killackey, Phillips, Bechdolf, Buckby and McGorry2008). The CAARMS is a semi-structured interview used to rate the intensity and frequency of subclinical symptoms and discriminate between psychosis, UHR or neither. The CAARMS was repeatedly administered at 0, 2, 4, 6, 9, 12, 15 and 18 months to detect transition to psychosis in a precise way. In case of a CAARMS transition to psychosis the Dutch version of the Schedules for Clinical Assessment in Neuropsychiatry (SCAN-2.1; Rijnders et al. Reference Rijnders, van den Berg, Hodiamont, Nienhuis, Furer, Mulder and Giel2000) was used to diagnose participants.
Health-related quality of life
As indicated, the CEA was conducted with the costs per averted psychosis. In addition, we conducted a CUA with effectiveness expressed as QALYs, based on the EuroQol, three-level version (EQ-5D-3L; Brooks, Reference Brooks1996). The EQ-5D-3L is a self-report questionnaire consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) each with three levels: no problems, some problems and extreme problems. The digits for the five dimensions can be combined in a five-digit number describing the respondent's health state. A state ‘11111’, for example, indicates no problems on any of the five dimensions. A total of 35 = 243 possible health states are defined in this way. For each of these health states a utility score can be obtained, which is anchored between 0 (death) and 1 (full health). This study used the UK tariff to value health states, which was estimated using the time trade-off method in a sample of 3395 respondents from the general UK population (Dolan, Reference Dolan1997). QALYs were computed as the amount of time spent in a health state weighted by the corresponding utility ‘U’. In the present study we had three time intervals (0–6 months, 6–12 months, and 12–18 months) between the measurements and applied the mean area under the curve method (Matthews et al. Reference Matthews, Altman, Campbell and Royston1990) as: QALY = (t 2 − t 1) × (U 1 + U 2 )/2 + (t 3 − t 2) × (U 2 + U 3)/2 + (t 4 − t 3) × (U 3 + U 4)/2, where the time interval, between the measurement points t, is 6 months (i.e. 0.5 of a year). The cumulative QALY gains thus computed for all participants allowed comparison across conditions in terms of average changes in health-related quality of life.
Other measures
The SOFAS (Goldman et al. Reference Goldman, Skodol and Lave1992) was used to assess overall functioning in a single score (0–100). Depression was assessed with the Beck Depression Inventory-II (BDI-II; Beck et al. Reference Beck, Steer and Brown1996) and with the Calgary Depression Scale (CDS; Addington et al. Reference Addington, Addington, Maticka-Tyndale and Joyce1992).
To assess the participants’ subjective appraisal of their illness, the revised Personal Beliefs About Illness Questionnaire (PBIQ-R; Birchwood et al. Reference Birchwood, Jackson, Brunet, Holden and Barton2012) was used. Social anxiety was measured with the Social Interaction Anxiety Scale (Mattick & Clarke, Reference Mattick and Clarke1998) and quality of life with the Manchester Short Assessment of Quality of Life (Priebe et al. Reference Priebe, Huxley, Knight and Evans1999).
Resource use and costing
The present study included: (1) intervention costs, (2) direct medical costs (other than the intervention), (3) participants’ travel costs, and (4) costs stemming from lower productivity over 18 months.
The sample included adolescents and young adults who made their first appearance in the healthcare system, i.e. without a significant history of healthcare use; therefore, no baseline cost data were collected. Research assistants collected cost data at both 6 and 18 months, using the Trimbos Institute and Institute of Medical Technology Assessment Questionnaire for Costs associated with Psychiatric Illness (Hakkaart-van Rooijen, Reference Hakkaart-van Rooijen2002), which is the most widely used health service receipt interview in the Netherlands. The interview consisted of questions on the number of contacts with healthcare providers. A retrospective cost interview is a valid and reliable method for quantifying costs in trial-based economic evaluations in healthcare (van den Brink et al. Reference Van den Brink, van den Hout, Stiggelbout, Putter, van de Velde and Kievit2005), but we cross-checked outcomes using patient files in about 90% of the cases.
Direct medical costs
Direct medical costs were calculated by multiplying health service units (e.g. sessions, visits, hospital days) with their standard full economic cost price (online Supplementary Table S1). To these we added the costs of antipsychotic medication according to the Dutch Health Care Insurance Board (Zorginstituut Nederland, 2014a ), calculated as the cost price per standard daily dose as reported in the Pharmaceutical Compass (Zorginstituut Nederland, 2014b ), plus 6% value added tax (not deductible by patients), multiplied by the number of prescription days, plus the pharmacist's dispensing costs of US$7.67 per monthly prescription (Hakkaart-van Rooijen et al. Reference Hakkaart-van Rooijen, Tan and Bouwmans2010).
The therapists recorded the number of CBT sessions that were provided in the experimental period. The costs of the intervention were calculated by multiplying the number of sessions by the standard full economic cost prices for a session with a psychologist or psychiatrist (online Supplementary Table S1).
Travel costs
Travel costs arose when participants travelled to health services. Travel costs were computed as the average distance to a health service (7 km) multiplied by the costs per km (US$0.25) (Hakkaart-van Rooijen et al. Reference Hakkaart-van Rooijen, Tan and Bouwmans2010), as most participants used public transport.
Productivity costs
Research assistants monitored changes in the participants’ work status at baseline, and at 6, 12 and 18 months, using the SOFAS (Goldman et al. Reference Goldman, Skodol and Lave1992). Productivity losses in paid work were calculated according to the human capital approach (Rice & Cooper, Reference Rice and Cooper1967b ) reflecting changes in the (contractual) number of hours worked per week and adjusting these for work loss days arising from sick leave over the full period of 18 months. Thus, economic losses (or gains, i.e. increase in working hours after, for example, sick leave) owing to changes in working hours were calculated for the three different time periods, i.e. from baseline to 6 months follow-up, from 6 to 12 months, and from 12 to 18 months follow-up, and the cumulative productivity costs were again calculated using the area under the curve method (Matthews et al. Reference Matthews, Altman, Campbell and Royston1990) using age- and gender-specific hourly productivity costs (Hakkaart-van Rooijen et al. Reference Hakkaart-van Rooijen, Tan and Bouwmans2010) (online Supplementary Table S2).
Costs were originally expressed in Euro for the reference year 2009 on a per-participant basis for the period of 18 months. The costs were then converted to US dollars using the purchasing power parities (PPP) from the Organization for Economic Co-operation and Development (2014), thus taking into account both the exchange rate and the differential buying power across countries. For the reference year 2009, US$1.00 was equated to NL€0.841026. Key outcomes are also reported in pound sterling, £, where US$1.00 is equated with £0.653432 for the year 2009.
Analysis
Statistical analysis
The data were analysed in agreement with the intention-to-treat principle. Missing data were imputed using the expectation maximization (EM) algorithm as implemented in SPSS 20.0.0 (USA).
Missing cost data were imputed by condition, referral strategy, age, CDS, gender, and transition to psychosis as predictor variables.
In the main analysis, missing clinical outcome on the CAARMS at 18 months (n = 27) was imputed using EM with baseline age, gender, ethnicity (Dutch or non-Dutch), baseline CAARMS distress positive symptoms and BDI scores as predictors. Missing QALYs (at 18 months, CBT 21.1% v. RC 14.9%) were estimated using EM with condition and baseline SOFAS, PBIQ-R, BDI-II and QALYs as predictor variables. All EM predictor variables were identified as significant predictors of the imputed variable by multivariate regression analyses.
Testing differential effectiveness
Differences between the conditions in terms of the transition rates to psychosis were estimated under a linear probability model. Incremental effects with regard to QALY health gains were tested in a regression model. These analyses were conducted with STATA 12.1 (USA) using robust standard errors that were based on the first-order Taylor-series linearization method, because the trial was conducted as a multi-site trial.
Analysis of cost–utility and cost effectiveness
In the present study, two incremental cost-effectiveness ratios (ICERs) were determined: the cost per averted psychosis, and the cost per QALY gained. These were calculated as (C 1 − C 0)/(E 1 − E 0), where C is the average per-participant cost, E is the effect and subscripts 1 and 0 refer to the CBT and RC condition, respectively.
To handle stochastic uncertainty in the cost and effect data, non-parametric bootstraps were conducted in Microsoft Excel to simulate 2500 ICERs (Briggs, Reference Briggs2000; Drummond et al. Reference Drummond, Sculpher, Torrance, O'Brien and Stoddart2005; Petrou & Gray, Reference Petrou and Gray2011). The simulated ICERs can be presented as a scatter over a cost-effectiveness plane, with differences in costs on the vertical axis and differences in effects on the horizontal axis. If the ICER appears in the northwest (NW) quadrant of the plane, less effect is obtained for additional costs; this is the worst possible outcome and CBT is then unacceptable from a cost-effectiveness perspective while RC remains the treatment of choice. If the ICER appears in the southeast (SE) quadrant, more health gains are obtained for fewer costs; the intervention is then preferred over standard care. In the other two quadrants, greater (or lesser) effectiveness has to be weighted against higher (or lower) costs.
Sensitivity analyses
The robustness of the results was assessed in sensitivity analyses. The main CEA was based on EM imputation and was reanalysed under last observation carried forward (LOCF) imputation. Both analyses were conducted from the healthcare perspective to which productivity losses were alternately included or excluded. In addition, both EM and LOCF imputation were used for the CUA. Furthermore, because the time-frame of this study exceeded 1 year, discount rates were used to calculate the net present value of all costs and effects in the EM-based cost-effectiveness and cost–utility analyses. In accordance with the pertinent Dutch guideline, effects were discounted by 1.5% while costs were discounted by 2.25% (Hakkaart-van Rooijen et al. Reference Hakkaart-van Rooijen, Tan and Bouwmans2010). In addition, we conducted sensitivity analyses to vary the add-on CBT intervention costs by 10, 15 and 20% higher or lower costs.
Results
Sample characteristics
Table 1 shows that there were no statistically significant differences between the two conditions, indicating that randomization had resulted in comparable groups.
Table 1. Baseline demographic and clinical characteristics of the study participants for both conditions (n = 196)
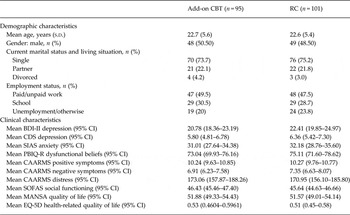
CBT, Cognitive–behavioural therapy; RC, routine care; s.d., standard deviation; BDI-II, Beck Depression Inventory II; CI, confidence interval; CDS, Calgary Depression Scale; SIAS, Social Interaction Anxiety Scale; PBIQ-R, Personal Beliefs About Illness Questionnaire Revised; CAARMS, Comprehensive Assessment of At-Risk Mental States; SOFAS, Social and Occupational Functioning Assessment Scale; MANSA, Manchester Short Assessment of Quality of Life; EQ-5D, five-dimensions EuroQol.
Resource use
We compared 90% of the self-reports with the electronic patient files and found an overestimation of service use in 5% of the cases, which was then corrected; 10% of the electronic patient dossiers were not available for inspection.
Incremental costs
Table 2 presents the mean costs after EM imputation in both conditions over the 18-month period. The larger share of the total costs was attributable to direct medical costs. Overall, the CBT condition generated lower costs than the RC condition: i.e. the difference between US$8851 and 8007 = a cost reduction of US$844 (or a cost saving of £551) per prevented psychosis.
Table 2. Estimated per-participant costs (in 2009 US$) by condition (after expectation maximization imputation)

Data are given as mean (standard deviation).
CBT, Cognitive–behavioural therapy; n.a., not applicable.
a Travel costs in the CBT condition included travel costs for the add-on intervention, i.e. US$33.11 (s.d. = 25.49).
b A gain in productivity is indicated with a negative value; loss of productivity is indicated with a positive value.
Thus the intervention costs were more than compensated for by cost savings elsewhere.
Incremental effects
Averted transitions to psychosis
In the add-on CBT condition, 10.5% of the participants made the transition from UHR status to psychosis during 18 months (5.3% at 6 months, at the end of experimental period). In the condition that received RC alone, 23.8% of the participants made the transition during 18 months (13.9% made the transition at 6 months). The risk difference was 0.238 − 0.105 = 0.133, favouring the intervention over RC and was statistically significant (b = 0.132, robust s.e. b = 0.045, z = 2.92, p = 0.004). This is equivalent to a number needed to be treated of 1/0.133 = 7.52, or eight people (rounded).
QALYs
The QALY score was 0.60 (95% CI 0.55–0.65) for CBT compared with 0.57 (95% CI 0.52–0.63) over 18 months in the condition that received RC alone. The difference in QALY gains between the conditions was therefore 0.60 – 0.57 = 0.03, favouring the CBT condition, but only very slightly. The QALYs at each measurement moment are presented in Supplementary Table S3.
Incremental cost per prevented psychosis
The incremental costs were –US$844 (savings) and the incremental effect was 0.13 (a larger fraction of averted psychoses in the CBT condition). This represents a situation that health economists refer to as ‘dominant’ (i.e. the new intervention dominates the RC condition from a cost-effectiveness perspective).
The ICER is subject to stochastic uncertainty. Including all costs, and while basing the analysis on EM imputation, the intervention is associated with 63.7% of the 2500 simulated ICERs appearing in the superior SE quadrant (Table 3), indicating a likelihood of about 64% that more psychoses are averted for fewer costs by the CBT intervention relative to RC alone. The northeast (NE) quadrant contained 34.9% of the simulated ICERs, the NW quadrant 0.5%, and the final 0.9% was located in the southwest (SW) quadrant. In the add-on CBT group, 9.3% of the simulated ICERs fell below the threshold of US$20 000. Fig. 2 shows the scatterplot of 2500 simulated ICERs on the ICER plane.
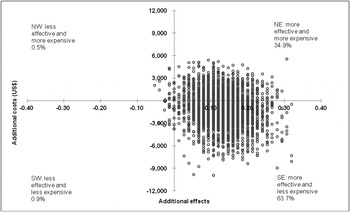
Fig. 2. Scatterplot of simulated incremental cost-effectiveness ratios (n = 2500) on the cost-effectiveness plane: add-on cognitive–behavioural therapy v. routine care alone (under expectation maximization imputation). NW, Northwest quadrant; NE, northeast quadrant; SW, southwest quadrant; SE, southeast quadrant.
Table 3. Results of the main and sensitivity analyses
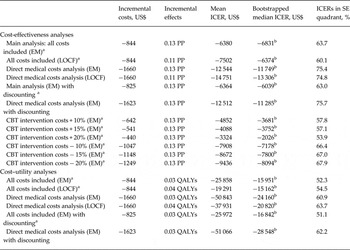
ICER, Incremental cost-effectiveness ratio; SE, southeast; EM, expectation maximization; PP, prevented psychosis; LOCF, last observation carried forward; CBT, cognitive–behavioural therapy; QALYs, quality-adjusted life years.
a All costs included analysis. The main analysis included the following costs: intervention costs, direct medical costs (other than the intervention), participants’ travel costs, and costs stemming from lower productivity over 18 months.
b Dominant, i.e. falling in the SE quadrant of the ICER plane.
Incremental cost per QALY
As noted, the incremental costs were −US$844 (savings) and the QALY difference was 0.03. Therefore, the intervention must be regarded as the better choice (or ‘dominant’) relative to RC, because the intervention generated similar or more QALY health gains for fewer costs.
From this point on, we take a probabilistic medical decision-making approach to decide if add-on CBT offers good value for money. Of the 2500 simulated ICERs, 52.3% fell into the SE quadrant, indicating a likelihood of 52% that more QALYs were generated for fewer costs by the intervention relative to RC. The NE quadrant contained 26.2% of the simulated ICERs, the inferior NW quadrant 10.3%, with the final 11.2% located in the SW quadrant. These data suggest a higher than 50% probability that the intervention is dominant. In the add-on CBT group, 26.2% of the simulated ICERs fell below the threshold of US$20 000.
Sensitivity analyses
Costs per prevented psychosis
Sensitivity analyses were conducted by repeating the main CEA analysis under LOCF imputation, and with only direct medical costs included under (1) EM and (2) LOCF imputation. For costs per prevented psychosis, the results were robust and consistent with the main analyses, the discounted costs and effects, and for varied CBT intervention costs, implying that CBT dominated RC in all these scenarios (Table 3).
Costs per QALY
Sensitivity analyses were also conducted by repeating the main CUA analysis under LOCF imputation, and with only direct medical costs included under (1) EM and (2) LOCF imputation and, finally, both EM-based analyses were repeated with discounting. Again, sensitivity analyses attested to the robustness of our findings: CBT remained cost saving (Table 3).
Discussion
Main findings
To our knowledge, this is the first trial-based CEA of a preventive psychological intervention in psychosis. Previous studies were modelling studies (Valmaggia et al. Reference Valmaggia, McCrone, Knapp, Woolley, Broome, Tabraham, Johns, Prescott, Bramon, Lappin, Power and McGuire2009, Reference Valmaggia, McGuire, Fusar-Poli, Howes and McCrone2012) suggesting that early intervention may be cost saving, but were less firmly rooted in empirical data. In line with recent meta-analytic evidence showing that preventing psychosis in UHR groups is a feasible option (Fusar-Poli et al. Reference Fusar-Poli, Borgwardt, Bechdolf, Addington, Riecher-Rössler, Schultze-Lutter, Keshavan, Wood, Ruhrmann, Seidman, Valmaggia, Cannon, Velthorst, De Haan, Cornblatt, Bonoldi, Birchwood, McGlashan, Carpenter, McGorry, Klosterkötter, McGuire and Yung2013; Hutton & Taylor, Reference Hutton and Taylor2013; Stafford et al. Reference Stafford, Jackson, Mayo-Wilson, Morrison and Kendall2013; van der Gaag et al. Reference Van der Gaag, Smit, Bechdolf, French, Linszen, Yung, McGorry and Cuijpers2013b ), the present study demonstrates that add-on CBT for UHR is clinically effective.
In the CBT condition only 10.5% made the transition from UHR status to psychosis over 18 months, compared with 23.8% in the RC condition. In addition, the CEA showed that the intervention had a 63.7% likelihood of being the superior option, because it was associated with cost savings of US$844 (£551) per averted psychosis while much reducing the risk of a first onset of psychosis. Furthermore, the CUA demonstrated that the add-on CBT intervention had a 52.3% probability of being the superior option, and the costs of the intervention were US$844 (£551) lower than those of RC. Various sensitivity analyses attested to the robustness of these findings.
Costs of identifying UHR patients
In the present study, patients at UHR of psychosis were already involved with mental healthcare services; therefore the additional costs are mainly screening costs. The Prodromal Questionnaire (PQ-16; Ising et al. Reference Ising, Veling, Loewy, Rietveld, Rietdijk, Dragt, Klaassen, Nieman, Wunderink, Linszen and van der Gaag2012) is an online screener used to preselect patients for the CAARMS interview in mental healthcare. The costs of identifying UHR patients were not factored into the operating costs of offering the intervention because these costs are the same in both conditions. Nevertheless, these costs are interesting in their own right. For example, in a catchment area of 600 000 inhabitants, one part-time assessor could identify about 100 UHR patients (aged 16–35 years) per year. Finding one UHR patient costs about US$724 (£473) (additional calculations are available from the first author H.K.I.).
Importance of these findings for clinical practice
Many Western countries are confronted with declining resources in healthcare, and economic evaluations are becoming increasingly important to inform decision makers about the cost effectiveness of an intervention. The present study suggests that CBT for people at UHR is effective and cost saving and could therefore become a recommended practice for treating UHR patients. Furthermore, because the intervention was developed in clinical practice, it is ready for widespread implementation in RC.
An important issue is whether an averted psychosis is a prevented psychosis or merely a delay in transition. A recent meta-analysis showed that the 2- to 4-year follow-up still had a risk reduction of 37%. In some cases an averted psychosis was a delay, whereas in others psychosis may have been prevented (van der Gaag et al. Reference Van der Gaag, Smit, Bechdolf, French, Linszen, Yung, McGorry and Cuijpers2013b ). In addition, in a recent long-term follow-up study, no transitions from UHR status to psychosis were found at 10-year follow-up, while 86% of the transitions to psychosis took place in the first 5 years (Nelson et al. Reference Nelson, Yuen, Wood, Lin, Spiliotacopoulos, Bruxner, Broussard, Simmons, Foley, Brewer, Francey, Amminger, Thompson, McGorry and Yung2013), suggesting that the risk of developing a psychosis is confined to a critical period and is not a lifelong threat. This may imply that 5-year monitoring, and offering booster sessions when subclinical symptoms worsen, may help reshape the landscape of psychotic disorders, with fewer patients who progress into a chronic and disabling condition. This is unlikely to put additional pressure on the already stretched healthcare budgets, as the need and economic costs for hospital-based services will be reduced.
The results of this study are in accordance with other studies, whether based on trials or simulations. All conclude that preventing a first episode of psychosis in people at UHR saves more than the costs of the intervention (Phillips et al. Reference Phillips, Cotton, Mihalopoulos, Shih, Yung, Carter and McGorry2009; Valmaggia et al. Reference Valmaggia, McCrone, Knapp, Woolley, Broome, Tabraham, Johns, Prescott, Bramon, Lappin, Power and McGuire2009, Reference Valmaggia, McGuire, Fusar-Poli, Howes and McCrone2012; Mihalopoulos et al. Reference Mihalopoulos, Vos, Pirkis and Carter2011).
Limitations
Some limitations of our study need to be considered when interpreting the findings. First, healthcare uptake was identified by a retrospective health service receipt interview, which could have introduced recall bias. However, we compared 90% of the self-reports with the electronic patient files and found an overestimation of service use in 5% of the cases, which was then corrected. Because 10% of the electronic patient dossiers were not available for inspection, we had to rely on the reported healthcare uptake.
Second, some dropout occurred and missing endpoints had to be replaced by their most likely value. This may have contributed to uncertainty in our outcomes. Therefore, we used two different imputation strategies and conducted various sensitivity analyses.
Third, because the trial was conducted in the Netherlands the results may not be generalizable to other settings or countries with a different healthcare system. Therefore, our cost-effectiveness study is perhaps best seen as a ‘proof of principle’ in need of replication in other demographic and epidemiological settings.
Finally, it is unknown how the cost effectiveness of the two interventions will be affected after a follow-up period exceeding 18 months. However, such data will become available when we have finished our 4-year follow-up study.
Implications
The present study not only shows clinical effectiveness but also reduced healthcare costs of a preventive intervention for people at UHR of developing first-episode psychosis, as compared with RC. Both the favourable clinical outcomes (corroborated meta-analysis) and the present economic case suggest that preventive CBT in help-seeking UHR patients could be considered for future clinical guidelines. Psychotic disorders are extremely costly because (in many patients) they last a lifetime. Therefore, the screening and preventive treatment of those at UHR not only save costs in the short and medium term, but may also save healthcare costs in the long term.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291714002530
Acknowledgements
The Netherlands Organization for Health Research and Development (ZonMw) supported this study (grant number 120510001). ZonMw had no further role in the study design, collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors gratefully acknowledge the contribution of all participants, research assistants, therapists and all others who took part or contributed to the EDIE-NL study. We also thank Ms Marion Bruns for preparation and organization related to the study.
Declaration of Interest
None.







