Supraventricular tachycardia is a common rhythm disorder in childhood. Its incidence in children (0–18 years) is estimated at 0.1–0.4%.Reference Ludomirksy, Garson, Garson, Bricker and McNamara1,Reference Wu, Chen, Kao and Huang2 In children with structurally normal hearts, the most common mechanisms of supraventricular tachycardia are atrioventricular re-entrant tachycardia, atrioventricular nodal re-entrant tachycardia, and focal atrial tachycardia.Reference Abrams3 Therapeutic options for clinically relevant tachycardias include administration of antiarrhythmic drugs and curative radiofrequency ablation.
The anatomical and electrical characteristics of the myocardium change with age and determine the incidence, course, and type of paediatric supraventricular tachycardia. Two age segments have a frequency peak: infancy, including the neonatal period, and school age, including adolescence.Reference Ko, Deal, Strasburger and Benson4 During the first year of life, there is a high rate of spontaneous resolution in both atrioventricular re-entrant tachycardia and focal atrial tachycardia.Reference Deal, Keane, Gillette and Garson5,Reference Cannon and Snyder6 The tachycardia mechanism is also age dependent. Accessory pathway-mediated tachycardia, that is atrioventricular re-entrant tachycardia, is relatively more common in young children, whereas atrioventricular nodal re-entrant tachycardia tends to occur more often in older children. The incidence of focal atrial tachycardia, in contrast, shows no significant childhood age-related dependence.Reference Ko, Deal, Strasburger and Benson4
The correct diagnosis of supraventricular tachycardia can be derived in most patients from a 12-lead surface electrocardiography at the time of tachycardia. However, the exact mechanism of tachycardia often cannot be determined with certainty at that time. Distinguishing between atrioventricular re-entrant tachycardia and atrioventricular nodal re-entrant tachycardia is often impossible from a surface electrocardiography. Invasive electrophysiological examination is the gold standard and remains the only method by which the exact mechanism of tachycardia and location of the accessory pathway can be identified. To the best of our knowledge, the age-dependent arrhythmia substrates, including accurate location and conduction properties of accessory pathways, if present, have not yet been analysed recently in a larger paediatric population.
We analysed a cohort of paediatric patients with supraventricular tachycardia who underwent cardiac catheterization for an invasive electrophysiological study. The aim of this study is to describe the individual arrhythmia substrates and the exact tachycardia mechanisms in a contemporary paediatric cohort, taking into account the clinical findings and the therapeutic outcome. We focussed on the age difference at the first manifestation of supraventricular tachycardia.
Material and methods
Retrospective analysis of all consecutive patients with supraventricular tachycardia who underwent cardiac catheterization below the age of 18 years for electrophysiological study with or without radiofrequency ablation between January 2005 and December 2015 at two tertiary referral centres: the Children’s University Hospital Zurich, Switzerland and the Lake Constance Heart Centre, Germany. Informed consent was obtained from the patients and caregivers.
Both centres use the same approach and technique using electrophysiological study and radiofrequency ablation. The rate of success and failure were defined at the end of the intervention. The recurrence rate was defined as the need for reintervention through a second electrophysiological study and radiofrequency ablation. The study population was divided into two groups based on the age when the diagnosis of tachycardia was first established. The first group, early-onset, contained patients who were diagnosed as having a tachycardia within the first year of life. This group was compared with all other children and adolescents who were included in the study as the late-onset group.
To analyse the mechanism of tachycardia, patients with structural heart defects were excluded from this cohort as they are likely to bias the analysis in several ways including different diagnostic pathways, association of pathway location with specific structural defects and the likelihood of earlier catheter intervention.
The following data were retrieved from the patient charts and electronic cardiology database file: age, gender, weight, antiarrhythmic drugs, presence of structural heart disease, symptoms present before ablation, comorbidities, documented tachycardia, and preexcitation at rest. Intra- and post-procedural information included energy type of ablation, mechanism of arrhythmia, location and number of accessory pathways, refractory time of the accessory pathway and AV-node, fluoroscopy time, duration of the procedure, heart rate at tachycardia, numbers of success, complication, and reintervention. The antegrade and retrograde effective refractory periods of the accessory pathways were always assessed at baseline without administration of isoproterenol. The location of the accessory pathway was classified according to the North American Society of Pacing and Electrophysiology consensus paperReference Cosío Francisco, Anderson Robert and Kuck7 (Fig 1).

Figure 1. Schematic representation of the atrioventricular junction in the left anterior oblique projection showing the anatomic (bold) and conventional nomenclature (italic) for the location of accessory pathways. Tricuspid valve (TV), mitral valve (MV), atrioventricular node (AVN), coronary sinus (CS).Reference Cosío Francisco, Anderson Robert and Kuck7
Results are presented both as absolute numbers and relative frequencies. Categorical parameters between groups were compared using χ2 or Fisher’s exact test, as appropriate. The Mann-Whitney U test was used to compare continuous variables. p-values < 0.01 were considered to be statistically significant. The study was approved by the local ethical boards.
Results
During the 10-year period 2005–2015, 531 patients met the inclusion criteria. The proportion of patients with the first detection of tachycardia in infancy was 10.7% (57/531 patients = early); 15 of these 57 patients were diagnosed during the foetal period (26.3%).
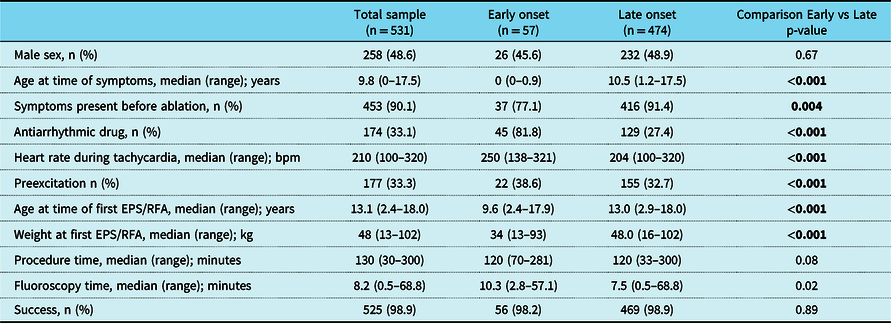
Demographic data, procedural data, and clinical characteristics of the patients are shown in Table 1. The early-onset group is not different from the late-onset group regarding sex, or comorbidities (allergic bronchial asthma, hyperactivity attention deficit syndrome, and neurological symptoms). More patients of the early-onset group than of the late-onset group received antiarrhythmic drugs. In the weeks before the catheter ablation, there was a trend that more patients of the late-onset group than of the early-onset group were symptomatic.
Table 1. Clinical characteristics and procedural data of the first electrophysiological study and radiofrequency ablation (EPS/RFA).
Clinical findings at presentation
More patients showed a pre-excited QRS complex at the pre-procedural baseline 12-lead electrocardiography in the early-onset than in the late-onset group (Table 1). Documentation of the tachycardia at the time of diagnosis was available in 311 of 531 patients (58.6%). A wide-complex tachycardia due to rate-dependent aberrancy or bundle branch block was documented in 29 of 266 (10.9%) available tachycardia electrocardiograms with no difference between the two age groups. Antidromic tachycardia was only documented in patients with Mahaim fibres (n = 2). Patients in the early-onset group also had faster tachycardia heart rates than patients in the late-onset group (Table 1).
Procedural details
Patient characteristics and procedural details of the catheter intervention are shown in Table 1. Patients in the early-onset group were younger at the time of the catheter intervention than patients in the late-onset group. In the whole study population, 461 of 531 (86.8%) patients underwent an intervention at the electrophysiological study, whereas 70 patients (n = 70/531, 13.1%) only received an electrophysiological study. The ablation was performed with radiofrequency energy in 457 patients (non-irrigated tip n = 415/457; 90.8%, irrigated tip n = 42/457; 9.2%) and with cryoenergy in 8 patients (n = 8/461; 1.7%). The proportion of techniques used for ablation was similar in both age groups. The duration of the ablation procedure and fluoroscopy time was almost equal in both age groups (Table 1).
The overall success rate of the first catheter ablation was 98.9% and was similar in both early-onset and late-onset groups (Table 1), p = 0.89. The success rate depended on the tachycardia substrate. In patients with accessory pathways, the success rate was 97.8% in the early-onset group and 98.3% in the late-onset group (p = 0.87). In patients with focal atrial tachycardia, the success rate was 100% in both groups (p = 1.0). In patients with atrioventricular nodal re-entrant tachycardia, the success rate was 100% for both groups (p = 0.93). The overall complication rate was 1.5% (8/531), with no significant difference among either – early-onset and late-onset – groups (p = 0.21). The following complications were seen: first-degree atrioventricular block (n = 3), second-degree atrioventricular block (n = 1), intermittent (n = 1) and permanent complete atrioventricular block (n = 1), pericardial tamponade (n = 1), and paresis of the phrenic nerve (n = 1). A re-intervention for recurrent tachycardia had to be performed in 70/531 patients (13.1%); early-onset group: 9/57; 15.8%, late-onset group 61/474; 12.9%, (p = 0.54).
Mechanisms of tachycardia
Table 2 summarises the occurrence of the arrhythmia substrates as seen during electrophysiological study. In this paediatric study cohort, accessory pathway-mediated tachycardia was the most common type, accounting for more than half (53.3%) of all tachycardia. The second most common type of tachycardia was atrioventricular nodal re-entrant tachycardia, followed by focal atrial tachycardia in 33.5 and 4.5%, respectively. Atrioventricular re-entrant tachycardia with pre-excitation was relatively more common in the early-onset group whereas atrioventricular nodal re-entrant tachycardia was more common in the late-onset group (Table 2). In 33 patients, it was impossible to induce tachycardia at the time of electrophysiological study; all these patients were in the late-onset group. Although tachycardia was not inducible, the presence of an accessory pathway in these patients could be ruled out by detailed electrophysiological study. In patients with focal atrial tachycardia and structurally normal heart (n = 33), the focus was located as follows: early-onset group (n = 4): 2 in the right and 2 in the left atrium; late-onset group (n = 29): 18 in the right atrium, 10 in the left atrium, and in 1 patient in both atria.
Table 2. Type of tachycardia.1

1 15 patients had more than 1 type of arrhythmia
2 4 patients had typical and atypical atrioventricular nodal re-entrant tachycardia (AVNRT) at the same time
3 Others = atrial fibrillation (n = 6), atrial flutter (n = 5), junctional ectopic tachycardia (n = 1), tachycardia with Mahaim fibre (n = 2). AVRT in table stands for atrioventricular re-entrant tachycardia
Number of accessory pathways
280 patients had an accessory pathway-mediated tachycardia, 47 in the early-onset group, and 233 in the late-onset group. A single accessory pathway was present in 262 of 280 (93.6%): 45 of 47 patients (95.7%) in the early group and 217 of 233 (93.1%) in the late group. Two accessory pathways were seen in 13 of 280 patients (4.6%): 2 of 47 patients in the early-onset group (4.3%) and 11 of 233 patients (4.7%) in the late-onset group. Three accessory pathways were present in 3 of 280 patients, none in the early-onset group, and 3 of 233 (1.3%) in the late-onset group.
Location of accessory pathways
The location of the accessory pathways is shown in Figure 2. There was a trend towards more left-sided than right-sided pathways (159 versus 127) in the whole study population. This finding was more prominent in the early-onset group (35/47: 74.5%) than in the late-onset group (124/231; 53.7%); p = 0.01. The posterior left location of the accessory pathway was the most common, irrespective of age group.

Figure 2. Location of accessory pathways in 280 patients. 16 patients with multiple pathways. The ablation sites are shown as percentages of the groups. Black = early-onset group, White = late-onset group. One patient with Mahaim fibre and ablation site in the right ventricular apex is not represented in this figure.
Electrophysiological properties of accessory pathways
The median antegrade effective refractory period of the accessory pathway of all patients (n = 126) was 310 (with a range from 200 to 750 ms). Of these patients, 9 (7.1%) had an effective refractory period which was below 250 ms (early group: 3; late group 6; p = 0.71). The median retrograde effective refractory period of the accessory pathway was 280 (with a range from 210 to 470 ms) in n = 136 patients. The effective refractory periods in the early-onset group were statistically not different compared to the effective refractory periods in the late-onset group (median effective refractory period antegrade 305 versus 310 ms; retrograde 265 versus 290 ms).
In patients with left-sided accessory pathways, the median antegrade effective refractory period (n = 58) was 310 ms (with a range from 220 to 400 ms) in the early group and 300 ms (with a range from 200 to 750 ms) in the late group respectively without significant difference (p = 0.54). The median retrograde effective refractory period in the same cohort (n = 80) was 290 ms (with a range from 210 to 350 ms) in the early group and 280 ms (with a range from 210 to 440 ms) in the late group (p = 0.65).
In summary, the effective refractory periods of the accessory pathways were similar in patients of the early-onset group and patients in the late-onset group. Moreover, neither the patient’s age at the time of electrophysiological study nor the location of the accessory pathways was statistically associated with the effective refractory period of the accessory pathway.
Antiarrhythmic drug therapy
In the weeks before the catheter ablation, 174 of 531 (33.1%) patients were on medication with antiarrhythmic drugs. Patients in the early-onset group were significantly more often treated with antiarrhythmic drugs (n = 45/57, 81.8%) than patients in the late-onset group (n = 129/474, 27.4%, p < 0.001). Beta-receptor blocking agents were overall the most commonly used drugs. Class 1C antiarrhythmic drugs and amiodarone were used significantly more often in patients in the early-onset group. Digoxin was exclusively used in patients in the early-onset group. Patients in the early-onset group had significantly more multiple drugs than patients in the late-onset group (p < 0.001).
Discussion
As expected, atrioventricular re-entrant tachycardia was the most common mechanism of tachycardia, accounting for 53.3% of the tachycardia in our whole study cohort. It was significantly more prevalent in the early-onset group than in the late-onset group. Atrioventricular re-entrant tachycardia accounts for 82.5 % of the supraventricular tachycardia in the early-onset group and 50.0% in the late-onset group (Table 2), which is consistent with previous reports.Reference Moak8,Reference Saul, Shoei and Huang9 The higher incidence of accessory pathways in patients with early-onset tachycardia could be explained by the pathogenesis of accessory pathways and by postnatal changes of the myocardium with increasing age. In the normal development of the heart, progressive anatomical and electrical separation develops between the initially undivided muscle masses of the atria and the ventricles until finally, at the gestational age of 20 weeks, only one muscular connection exists, the bundle of His.Reference Hahurij, Gittenberger-De Groot and Kolditz10 An accessory pathway is thought to result from a lack of regression of one of these earlier muscular connections and therefore is often present at birth. Postnatally, separation can progress further.Reference Jongbloed, Vicente Steijn and Hahurij11 These phenomena are well documented in pre-excitation in patients with Wolff-Parkinson-White syndrome. The delta wave on the surface electrocardiograms disappeared in as many as 62% of the patients during childhood.Reference Perry and Garson12 After the age of 5 years, a spontaneous regression is less likely.Reference Perry and Garson12
Left-sided accessory pathways are more common than right-sided pathways in patients with structurally normal hearts. This applies to all age groups,Reference Huang, Wood and Miller13 and it could also be shown in this cohort (Fig 2). There was a trend towards even more left-sided pathways in the early-onset group (74.5%) than in the late-onset group (53.7%) and – interestingly three locations – posterosuperior left, posterior left, and posteroinferior left – together accounted for two-thirds (66.3%) of all accessory pathway locations in the early-onset group (Fig 2). Thus, the two groups – early onset and late onset – do not differ statistically significantly with respect to the localisation of the accessory pathway when using a significance threshold of 0.01 for the p-value. The same result could already be shown in earlier paediatric cohorts.Reference Perry and Garson12 In summary, the age difference in the clinical manifestation of tachycardia cannot be attributed to the localisation of the accessory pathway.
Drug treatment was more often necessary in patients of the early-onset group than in patients of the late-onset group. We assume that this is simply a result of our current treatment guidelines. In clinical practice, the invasive electrophysiologic study and radiofrequency ablation is postponed in the “early- onset” group, if possible, until the patient reaches a body weight of approximatively 20 kg. During this time, the patients may need antiarrhythmic medication for some months or even years. The “late-onset” group patients however may be scheduled for catheter intervention very soon after the onset of symptoms and during this time, some of these patients may not even need any prophylactic antiarrhytmic medication at all.
The fact that patients of the early-onset group underwent earlier electrophysiologic study and radiofrequency ablation than patients of the late-onset group may best be explained by the fact that in this group, the patient suffers from tachycardia or its treatment earlier than in the late-onset group. Recurrent tachycardia can be annoying and frightening and the wish of patients and families to be freed from the daily prophylactic antiarrhythmic medication may also occur much earlier than in the late-onset group.
In our cohort, the median antegrade effective refractory period of the accessory pathway was 310 ms. Similar values for median antegrade effective refractory period, 320 ms, have recently been published for another paediatric cohort.Reference Kubuš, Vit, Gebauer, Materna and Janousek14 However, the early-onset group did not differ statistically from the late-onset group. The age dependency of the effective refractory period of the accessory pathway (i.e. the older the patient, the longer the refractory period), which was described in earlier studies,Reference Michelucci, Padeletti and Mezzani15-Reference Jung, Ju, Hyun, Lee and Kim17 could not be demonstrated in our cohort. On the one hand, this may be due to the relatively small age difference within the cohort and the relatively broad inter-individual variation of the measured values of the refractory periods and, on the other hand, it can also be explained by other external factors, for example the impact of anaesthesia, which has already led to inconsistent results in earlier studies.Reference Chang, Wetzel, Shannon, Stevenson and Klitzner16,Reference Gillette, Garson and Kugler18 Patients with an antegrade effective refractory period of the accessory pathway of less than 250 ms are thought to be at increased risk for sudden death due to rapid antegrade conduction of atrial fibrillation over the accessory pathway resulting in ventricular fibrillation.Reference Huang, Wood and Miller13 In our cohort, this criterion applied to nine patients of the whole cohort: three in the early-onset group and six in the late-onset group. Again, this difference was not statistically significant. Few data have been published on the retrograde effective refractory period of accessory pathways in children. In adults, it is known that the retrograde effective refractory period of the accessory pathway is generally shorter than the antegrade effective refractory period.Reference Tonkin, Miller, Svenson, Wallace and Gallagher19 The same was true in our cohort. However, the retrograde effective refractory period of the accessory pathway in the early-onset group was not different from that of the late-onset group. In summary, there was no statistically significant difference between the early-onset group and the late-onset group in the refractory times of the accessory pathways during either antegrade or retrograde conduction. Therefore, the persistence of the symptomatic accessory pathway beyond infancy cannot be explained by the conductive properties of these pathways alone.
A high success rate of 98.9% and a low complication rate of 1.5% were observed in our study population with radiofrequency catheter ablation. These figures are within the range expected from previous reports of the therapy in paediatric supraventricular tachycardia.Reference Kubuš, Vít and Gebauer20,Reference Saul and Dale21 However, there is no difference in outcome between patients with early-onset tachycardia and patients with late-onset tachycardia.
This study’s inclusion of 531 paediatric patients who underwent uniform diagnosis and therapy means that it provides a relatively large quantity of relevant data. However, the findings of this study cannot be generalised without restriction as considerable selection bias remains. Patients who did not undergo invasive electrophysiological study, such as neonates with spontaneously resolving tachycardia and individuals with asymptomatic accessory pathways, are not represented in this cohort. Furthermore, there are episodes of non-sustained tachycardia in infants which go unnoticed because the patients are asymptomatic. These patients could then later be incorrectly assigned to the “late onset” group. Although electrophysiological study is the gold standard for the assessment of supraventricular tachycardia, relatively large diagnostic uncertainty remains in the group with early-onset tachycardia. The time between the first appearance of tachycardia and the exact identification of the tachycardia mechanism by invasive electrophysiological study is several years, so the diagnosis may not correspond with the initial tachycardia. The power of this analysis is further limited by its retrospective cross-sectional study design. A true assessment of the development of intracardiac electrophysiological properties in paediatric patients with supraventricular tachycardia ideally requires a prospective longitudinal cohort study with repeated invasive electrophysiological study in every individual. However, such a study design would not be ethically justifiable. The induction of tachycardia in children does not depend only on the anatomical and electrophysiological substrates. Other factors that have not yet been more precisely identified, such as changes in the autonomic nervous system and hormonal changes with growth,Reference Hahurij, Gittenberger-De Groot and Kolditz10,Reference Cohen, Wieand, Rhodes and Vetter22 could not be taken into account in this cohort.
Conclusion
The underlying arrhythmia substrate of supraventricular tachycardia in children differs between patients whose first episode of tachycardia occurs within the first year of life and those with later onset. Congruent with previous reports, this study with a relatively large contemporary paediatric cohort found that accessory pathway-mediated re-entrant tachycardia is the most common mechanism of tachycardia within the first year of life, whereas the incidence of supraventricular tachycardia due to atrioventricular nodal re-entrant tachycardia increases with greater age. Left-sided accessory pathways are relatively often found to be the cause of supraventricular tachycardia in patients with structurally normal hearts who experienced their first episode of tachycardia in infancy. To our knowledge, this is the first study showing that the refractory periods of the accessory pathway in paediatric patients with accessory pathway-mediated tachycardia do not differ between patients with early-onset tachycardia and those with late-onset tachycardia. Radiofrequency catheter ablation can be applied in childhood with a high rate of success and a low rate of complications, regardless of age at the first occurrence of the tachycardia.
Acknowledgement
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Humanforschungsgesetz 2014) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Kantonale Ethikkommission Zürich 2015-00204).






