Introduction
Spermatozoa released from the seminiferous tubules are morphologically mature cells, but must undergo two additional processes of functional maturation that allow them to fertilize. The first of such processes occurs during epididymal transit and ejaculation. As spermatozoa migrate from the proximal to the distal region of the epididymis, they experience a series of morphological, biochemical and physiological changes, including modifications in size and appearance of the acrosome and nucleus, migration of the cytoplasmic droplet along the tail, as well as structural changes in several intracellular organelles, all of which result in spermatozoa with progressive motility (Cornwall, Reference Cornwall2009). After leaving the epididymis, spermatozoa are transported to the vas deferens towards the base of the prostate and the excretory duct of the seminal vesicle. Secretions from the seminal vesicle, prostate, and bulbourethral glands constitute the seminal plasma in which spermatozoa are suspended during delivery to the female reproductive tract and are responsible for the changes that occur in the spermatozoa plasma membrane at ejaculation (Juyena and Stelletta, Reference Juyena and Stelletta2012).
After semen deposition in the female reproductive tract, the second sperm maturation process takes place. Ejaculated semen contains spermatozoa that need to experience changes to acquire the capacity to fertilize oocytes, a process known as capacitation (Austin, Reference Austin1952; Chang, Reference Chang1951). This process begins soon after the removal of membrane stabilizing factors originated in the seminal plasma by the cervical mucus, proceeds throughout sperm transit along the female reproductive tract and is considered to be complete when spermatozoa are able to undergo the acrosome reaction (De Jonge, Reference De Jonge2005). Such membrane stabilizing factors comprise several epididymal and accessory glands secreted proteins associated to sperm membrane surface denoted as decapacitation factors (Nixon et al., Reference Nixon, MacIntyre, Mitchell, Gibbs, O’Bryan and Aitken2006; Aitken et al., Reference Aitken, Nixon, Lin, Koppers, Lee and Baker2007). The coordinated loss of decapacitation factors is associated with cholesterol efflux from the sperm plasma membrane (Davis, Reference Davis1979) that increases its fluidity and permeability, decreases the membrane potential (Abou-haila and Tulsiani, Reference Abou-haila and Tulsiani2009) and initiates signal transduction mechanisms that allow spermatozoa to become capacitated. In addition, an increase in the intracellular calcium concentration from the extracellular medium takes place (Costello et al., Reference Costello, Michelangeli, Nash, Lefievre, Morris, Machado-Oliveira, Barratt, Kirkman-Brown and Publicover2009). This calcium increase is accompanied by changes in motility, from a progressive and rapid pattern to a non-progressive movement known as hyperactivation (Suarez, Reference Suarez2008), and the increase in tyrosine phosphorylation of various spermatozoa proteins (Visconti et al., Reference Visconti, Bailey, Moore, Pan, Olds-Clarke and Kopf1995a, Reference Visconti, Moore, Bailey, Leclerc, Connors, Pan, Olds-Clarke and Kopf1995b).
Decapacitation factors may be provided via the secretory pathway or by extracellular membrane vesicles, such as the epididymosomes (Martin-DeLeon, Reference Martin-DeLeon2015) and the prostasomes (Ronquist, Reference Ronquist2015) which transport proteins (Thimon et al., Reference Thimon, Frenette, Saez, Thabet and Sullivan2008; Akintayo et al., Reference Akintayo, Legare and Sullivan2015) as well as lipids, DNA, microRNAs and mRNA (Ronquist et al., Reference Ronquist, Ronquist, Carlsson and Larsson2009; Belleannee, Reference Belleannee2015; Zijlstra and Stoorvogel, Reference Zijlstra and Stoorvogel2016). Similarly, the sperm capacitation progress is modulated by its interaction with molecules from the female reproductive tract that are either secreted into the lumen (Chirinos et al., Reference Chirinos, Durand, Gonzalez-Gonzalez, Hernandez-Silva, Maldonado-Rosas, Lopez and Larrea2017), delivered by extracellular vesicles (Al-Dossary et al., Reference Al-Dossary, Bathala, Caplan and Martin-DeLeon2015; Martin-DeLeon, Reference Martin-DeLeon2016), or in the surface of oviductal epithelial cells (Ghersevich et al., Reference Ghersevich, Massa and Zumoffen2015). Therefore, the regulation of capacitation appears to involve dynamic interactions between the decapacitation factors from the male reproductive tract and the stimulatory/regulatory factors released by the female reproductive tract (Nixon et al., Reference Nixon, MacIntyre, Mitchell, Gibbs, O’Bryan and Aitken2006). The aim of the present work was to review the role of mammalian proteins secreted from male and female reproductive tracts that have been characterized by their ability to interact with spermatozoa and modulate their competence to fertilize.
Proteins derived from male reproductive tract that interact with the sperm
Decapacitation factors on sperm plasma membrane surface keep the gamete protected from premature capacitation. Such factors associate with the sperm plasma membrane surface during epididymal transit and ejaculation (see Fig. 1A, B ). Early investigations in mouse have shown that removal of surface-associated proteins from uncapacitated spermatozoa plasma membrane lead to an immediate increase in fertilizing ability, as determined by a rise in the proportion of fertilized eggs (Fraser, Reference Fraser1984). In the following pages, evidence is presented of some male reproductive tract proteins that have been identified as decapacitation factors.
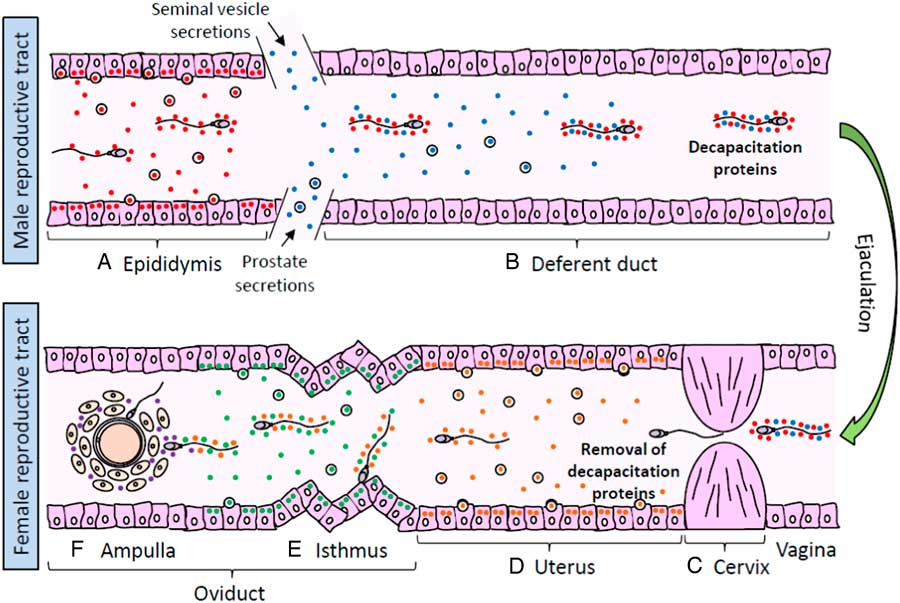
Figure 1 Sperm interaction with proteins from male and female reproductive tracts. (A) Spermatozoa released from the seminiferous tubules interact with epididymal proteins (red circles) that promote post-testicular maturation. (B) During ejaculation, spermatozoa are transported to the vas deferens and mixed up with seminal vesicle and prostate secretions, containing proteins that interact with the sperm surface and act as decapacitation factors (blue circles). (C) When semen is deposited in the female reproductive tract, decapacitation proteins are removed from spermatozoa during their passage through the cervical mucus in the cervix. (D) Spermatozoa migrating towards the uterus are exposed local secreted proteins (orange circles) that regulate the progress of sperm capacitation. (E) Once in the oviduct, spermatozoa may either move forward to the ampulla stimulated by secreted proteins (green circles) or held on the epithelium surface of mucosal folds in the isthmus to create a sperm reservoir. When ovulation draws near, local secretions stimulate the sperm release from the reservoir and the resumption of capacitation. (F) Spermatozoa that successfully reach the cumulus–oocyte complex are exposed to follicular fluid proteins (purple circles) which promote sperm penetration of the oocyte vestments and fertilization.
Epididymal proteins
One of the first decapacitation factors described is a 40 kDa glycoprotein acquired during mouse sperm epididymal transit known as DF (Fraser et al., Reference Fraser, Harrison and Herod1990). This protein binds to spermatozoa via a glycophosphatidylinositol (GPI)-anchored membrane receptor called the DF receptor, mainly located in the post-acrosomal region (Fraser, Reference Fraser1998). Its elimination from the uncapacitated spermatozoa surface resulted in highly fertile gametes and, when added back to capacitated spermatozoa produced poorly fertile cells in which the acrosome reaction was blocked (Fraser et al., Reference Fraser, Harrison and Herod1990). DF stimulates calmodulin-sensitive calcium ATPase activity, thereby ensuring the maintenance of low intracellular calcium concentrations. As capacitation proceeds, DF is lost and calcium ATPase activity declines, allowing intracellular calcium to rise and hence promoting capacitation-related changes (Adeoya-Osiguwa and Fraser, Reference Adeoya-Osiguwa and Fraser1996). In human, the addition of mouse DF to capacitated sperm suspensions causes a significant reversal in the capacitation state of the cells, suggesting that a similar mechanism could take place in both species (DasGupta et al., Reference DasGupta, Mills and Fraser1994). The DF receptor on sperm is the phosphatidylethanolamine-binding protein 1 (PEBP1), a 23 kDa protein located extracellularly on the acrosome, the post-acrosomal region and the flagellum of mouse and human spermatozoa (Gibbons et al., Reference Gibbons, Adeoya-Osiguwa and Fraser2005). Investigations carried out in the mouse model showed that PEBP1 along with other three proteins, identified as the plasma membrane fatty acid binding protein, the cysteine-rich secretory protein 1 (CRISP1), and a decapacitation factor named DF10, were able to inhibit sperm–zona pellucida (ZP) interaction as well as the sperm ability to acrosome react in response to progesterone (Nixon et al., Reference Nixon, MacIntyre, Mitchell, Gibbs, O’Bryan and Aitken2006). CRISPs are a family of proteins closely involved in the process of fertilization. In human and rat, there are three CRISPs (CRISP1, CRISP2 and CRISP3 for human; CRISP1, CRISP2 and CRISP4 for rat), while mice produce four CRISPs named CRISP1, CRISP2, CRISP3 and CRISP4 (Koppers et al., Reference Koppers, Reddy and O’Bryan2011). CRISP2 (originally known as Tpx-1) is expressed exclusively in the mammalian testicle and is incorporated into the developing sperm acrosome and flagellum (O’Bryan et al., Reference O’Bryan, Sebire, Meinhardt, Edgar, Keah, Hearn and De Kretser2001), while CRISP1 and CRISP4 are secreted by the epididymis and incorporated onto the spermatozoa during epididymal storage (Reddy et al., Reference Reddy, Gibbs, Merriner, Kerr and O’Bryan2008). Experiments performed in the rat model have shown that the addition of CRISP1 to the sperm inhibits tyrosine phosphorylation in a dose-dependent manner, therefore inhibiting capacitation and ultimately the acrosome reaction and that such inhibition occurs upstream the production of cAMP by the sperm (Roberts et al., Reference Roberts, Wamstad, Ensrud and Hamilton2003). However, there are two populations of CRISP1 in spermatozoa: a loosely bound that is released during capacitation and the form strongly associated with the sperm that remains on cells after capacitation (Cohen et al., Reference Cohen, Rochwerger, Ellerman, Morgenfeld, Busso and Cuasnicu2000b). It has been shown that CRISP1 that remain associated with the sperm head after capacitation, migrates to the equatorial segment during the acrosome reaction and is involved in sperm–ZP interaction and subsequent gamete fusion (Rochwerger et al., Reference Rochwerger, Cohen and Cuasnicu1992; Cohen et al., Reference Cohen, Ellerman and Cuasnicu2000a, Reference Cohen, Maldera, Vasen, Ernesto, Munoz, Battistone and Cuasnicu2011). Similarly, human CRISP1 also participates in sperm binding to the ZP and in gamete fusion (Maldera et al., Reference Maldera, Weigel Muñoz, Chirinos, Busso, Raffo, Battistone, Blaquier, Larrea and Cuasnicu2014; Da Ros et al., Reference Da Ros, Munoz, Battistone, Brukman, Carvajal, Curci, Gomez-ElIas, Cohen and Cuasnicu2015). Recent investigations with Crisp1 and Crisp4 knockout mice indicated that these proteins may act redundantly or autonomously on sperm function. CRISP1 is essential for the establishment of normally motile sperm, but CRISP4 enhances capacitation-associated tyrosine phosphorylation, and both are required for normal acrosome function and sperm–egg interaction (Hu et al., Reference Hu, Merriner, O’Connor, Houston, Furic, Hedger and O’Bryan2018). In contrast, CRISP3 shows a wider tissue distribution and is secreted by the prostate and seminal vesicles into the seminal plasma (Koppers et al., Reference Koppers, Reddy and O’Bryan2011), but its participation in sperm function regulation is still unknown.
Another protein expressed and secreted in the adult rat epididymis that binds to sperm head plasma membrane is HongrES1, a member of the serine proteinase inhibitor (SERPIN) protein family (Hu et al., Reference Hu, Liu, Shang, Zheng, Yang and Zhang2002). The co-culture of caudal spermatozoa with anti-HongrES1 antibodies resulted in an increased proportion of capacitated spermatozoa. Moreover, the percentage of capacitated spermatozoa increased when HongrES1 was downregulated by RNAi in vivo (Zhou et al., Reference Zhou, Zheng, Shi, Zhang, Zhen, Chen and Zhang2008). This protein has also been found in the cauda epididymis of guinea pig, where it is gradually exfoliated during capacitation and redistributed to patches on the sperm head and tail, and disappears after acrosome reaction. Removal of HongrES1 from the sperm surface facilitates extracellular calcium influx, thereby allowing the progress of capacitation and hyperactivation (Ni et al., Reference Ni, Zhou, Chen, Zheng, Yu, Li, Zhang and Shi2009). Moreover, it has been demonstrated that human and equine epididymal secretomes contain lactotransferrin (LTF) (Dacheux et al., Reference Dacheux, Belghazi, Lanson and Dacheux2006, Reference Dacheux, Belleannee, Jones, Labas, Belghazi, Guyonnet, Druart, Gatti and Dacheux2009), a protein that has been shown to decrease sperm ability to interact with ZP (Zumoffen et al., Reference Zumoffen, Gil, Caille, Morente, Munuce and Ghersevich2013) and to have dose-dependent effects on sperm proteins tyrosine phosphorylation (Zumoffen et al., Reference Zumoffen, Massa, Caille, Munuce and Ghersevich2015; Hernández-Silva et al., Reference Hernández-Silva, Durand, Larrea and Chirinos2018).
Seminal vesicle proteins
Seminal vesicles secretions contribute with most of the proteins existing in the seminal fluid and semenogelin-1 (Sg1) is the most abundant protein secreted by seminal vesicles and the main component of semen coagulum. After ejaculation, it is degraded by the serine protease prostate-specific antigen (PSA) to generate peptides of various biological activities and allow coagulum liquefaction (de Lamirande et al., Reference de Lamirande, Yoshida, Yoshiike, Iwamoto and Gagnon2001). Previous studies have shown that Sg1 inhibits human sperm motility (Robert and Gagnon, Reference Robert and Gagnon1996), protein tyrosine phosphorylation and the amount of superoxide anion generated during capacitation (de Lamirande et al., Reference de Lamirande, Yoshida, Yoshiike, Iwamoto and Gagnon2001), which is related to the progress of hyperactivation (de Lamirande and Gagnon, Reference de Lamirande and Gagnon1995). Interestingly, during ejaculation, Sg1 binds to the Eppin protein complex in the surface of human spermatozoa (Wang et al., Reference Wang, Widgren, Sivashanmugam, O’Rand and Richardson2005), a heteromultimer containing the epididymal protease inhibitor (Eppin), LTF and clusterin (Wang et al., Reference Wang, Widgren, Richardson and O’Rand2007), to inhibit the progressive motility of ejaculated spermatozoa (Mitra et al., Reference Mitra, Richardson and O’Rand2010) by promoting the loss of intracellular calcium (O’Rand and Widgren, Reference O’Rand and Widgren2012). However, during Sg1 digestion after ejaculation, Eppin modulates PSA hydrolytic activity on Sg1 resulting in spermatozoa with forward motility (O’Rand et al., Reference O’Rand, Widgren, Wang and Richardson2006).
Another protein from seminal vesicles that interacts with sperm plasma membrane is the mouse seminal vesicle autoantigen (SVA), which binds to sperm membrane phospholipids and suppresses sperm motility, BSA-stimulated sperm hyperactivation (Huang et al., Reference Huang, Chu and Chen1999) and capacitation-related protein tyrosine phosphorylation (Huang et al., Reference Huang, Chu and Chen2000). SVA has two orthologues genes in rat (Yoshida et al., Reference Yoshida, Kaneko, Kurachi and Osawa2001) and the human homologue is prolactin induced protein (PIP), a protein highly expressed on the surface of spermatozoa from donors with asthenozoospermia and oligoasthenoteratozoospermia (Capkova et al., Reference Capkova, Elzeinova and Novak2007). Interestingly, PIP interacts with serum albumin, zinc-α-2 glycoprotein and Sg1 (Tomar et al., Reference Tomar, Sooch, Raj, Singh and Yadav2013), suggesting that it may participate in human sperm capacitation regulation. In addition, it seems that the protein family known as seminal vesicle secretory proteins (SVS) may also regulate sperm capacitation. Murine SVS II, the major component of the copulatory plug (Lundwall, Reference Lundwall1996), binds to ganglioside GM1 on the post-acrosomal region of the head sperm (Kawano et al., Reference Kawano, Yoshida, Iwamoto and Yoshida2008) and decreases sperm protein tyrosine phosphorylation and the progesterone-induced acrosome reaction (Kawano and Yoshida, Reference Kawano and Yoshida2007).
Glycodelin (Gd) is a glycoprotein that has four well defined isoforms named S, A, F and C, according to their origin (semen, amniotic fluid, follicular fluid and cumulus oophorus, respectively) (Seppala et al., Reference Seppala, Koistinen, Koistinen, Chiu and Yeung2007). GdS is one of the most abundant glycoproteins in the seminal plasma (Julkunen et al., Reference Julkunen, Wahlstrom, Seppala, Koistinen, Koskimies, Stenman and Bohn1984) and is able to reduce the cyclodextrin-induced cholesterol efflux and down-regulate the adenylyl cyclase/protein kinase A/tyrosine kinase signalling pathway, resulting in suppression of human sperm capacitation (Chiu et al., Reference Chiu, Chung, Tsang, Koistinen, Koistinen, Seppala, Lee and Yeung2005). A different group of seminal vesicle proteins that binds to sperm are spermadhesins, a protein family comprised by five polypeptides called AQN-1, AQN-3, AWN (isoforms 1 and 2), PSP-I, and PSP-II. These proteins have been identified in pig, in which a large subpopulation of spermadhesins is loosely associated with the sperm surface and functions as decapacitation factors (Dostalova et al., Reference Dostalova, Calvete, Sanz and Topfer-Petersen1994). Nonetheless, it seems that each spermadhesin plays specific roles in sperm function regulation, given that a PSP-I/PSP-II heterodimer exerts a decapacitation effect by decreasing sperm intracellular calcium (Caballero et al., Reference Caballero, Vazquez, Mayor, Alminana, Calvete, Sanz, Roca and Martinez2009) while AQN-3 has been described as a ZP-binding protein (Calvete et al., Reference Calvete, Carrera, Sanz and Topfer-Petersen1996).
Protease inhibitors are widely distributed in nearly all species and are crucial in balancing protease activities. In mouse, the serine protease inhibitor Kazal-type 3 (SPINK3), also called P12, has been found in the seminal vesicle, coagulating gland and prostate of adults. The P12-binding sites are on the anterior region of the acrosome and the protein inhibits sperm binding to the ZP and the ZP-induced acrosome reaction (Boettger-Tong et al., Reference Boettger-Tong, Aarons, Biegler, Lee and Poirier1992). Furthermore, the protein suppresses calcium uptake by spermatozoa during capacitation (Chen et al., Reference Chen, Lin, Lai and Chen1998). Additionally, a related serine protease inhibitor known as Kazal-type-like (SPINKL) is secreted by mouse seminal vesicle and has been found to bind to sperm, enhance sperm progressive motility, suppress BSA-stimulated sperm capacitation and block sperm–oocyte interactions in vitro (Lin et al., Reference Lin, Lee, Hwu, Lu, Chu, Chen, Chang and Li2008).
Binder of sperm proteins (BSP, previously known as bovine seminal plasma proteins) are part of a family of structurally related proteins characterized by the presence of tandem fibronectin type II domains. They are highly expressed by seminal vesicles, but BSP-related genes have been found to be also expressed in the epididymis (Fan et al., Reference Fan, Lefebvre and Manjunath2006; Plante and Manjunath, Reference Plante and Manjunath2015a). BSPs are involved in sperm binding to oviductal epithelium assisting in the formation of the sperm storage reservoir (Gwathmey et al., Reference Gwathmey, Ignotz and Suarez2003). Bovine seminal vesicles secrete three BSP (BSP1, BSP3 and BSP5) that are adsorbed onto sperm (Manjunath et al., Reference Manjunath, Chandonnet, Leblond and Desnoyers1994), but after in vitro capacitation BSP5 is almost completely released from sperm surface while BSP1 and a small molecular mass isoform of BSP3 remain associated to sperm, suggesting that BSP3 undergoes proteolytic modifications on the sperm surface that may regulate sperm release from the storage reservoir (Hung and Suarez, Reference Hung and Suarez2012). Moreover, when BSPs interact with the sperm surface remove cholesterol from the plasma membrane (Therien et al., Reference Therien, Moreau and Manjunath1999) and bind to choline phospholipids (Desnoyers and Manjunath, Reference Desnoyers and Manjunath1992), preventing free movement of phospholipids and therefore stabilizing sperm membrane. However, they have also been found to promote sperm capacitation in bulls, boars, humans and mice and such effects on capacitation are mediated by stimulation of sperm membrane cholesterol and phospholipid efflux in the presence of heparin or HDL, both components of follicular and oviductal fluids (for review see Manjunath and Thérien, Reference Manjunath and Thérien2002). Recently, it has been shown that antibodies against murine BSPH1 could block capacitation induced by HDLs, suggesting a specific interaction between HDL and BSPH1 (Plante and Manjunath, Reference Plante and Manjunath2015b). Nevertheless, as the capacitation promoting effect is observed under conditions resembling the milieu of the female reproductive tract while in seminal fluid BSPs stabilize sperm membrane and prevent premature capacitation, it has been proposed that they are multifunctional proteins that employ different mechanisms to exert such effects (Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016).
Prostate proteins
The major protein secreted by the prostate is the serine protease known as PSA (or human kallikrein) that plays a well known role in seminal coagulum liquefaction that allows the release of motile spermatozoa (Malm et al., Reference Malm, Hellman, Hogg and Lilja2000), but there is no evidence that it may directly interact with the sperm membrane. Instead, the boar prostate-derived seminal plasma protein identified as WGA16 (wheat germ agglutinin) has been proven to bind to the sperm surface during ejaculation and is later removed during capacitation. It has two functional sites that allow sperm surface association through interaction between its N-glycans and the surface galactosyltransferase, whereas the heparin-binding domain may be involved in binding to sulfated glycosaminoglycans from the female tract, enabling removal of WGA16 from the sperm surface during capacitation (Garenaux et al., Reference Garenaux, Kanagawa, Tsuchiyama, Hori, Kanazawa, Goshima, Chiba, Yasue, Ikeda, Yamaguchi, Sato and Kitajima2015), but its involvement in sperm function regulation has not been examined.
Prostasomes are small vesicles secreted by prostatic epithelial cells that fuse with sperm plasma membranes and are the main source of cholesterol in seminal fluid. Previous studies have shown that incubation of sperm with prostasomes decreases the tyrosine phosphorylation intensity and induces motility changes (Bechoua et al., Reference Bechoua, Rieu, Sion and Grizard2011; Pons-Rejraji et al., Reference Pons-Rejraji, Artonne, Sion, Brugnon, Canis, Janny and Grizard2011). The prostasome molecular entities responsible for those effects are unknown, but among other proteins human prostasomes enclose LTF (Utleg et al., Reference Utleg, Yi, Xie, Shannon, White, Goodlett, Hood and Lin2003), supporting the idea that proteins transferred by prostasomes to sperm may regulate its capability to fertilize.
Proteins derived from female reproductive tract that interact with the sperm
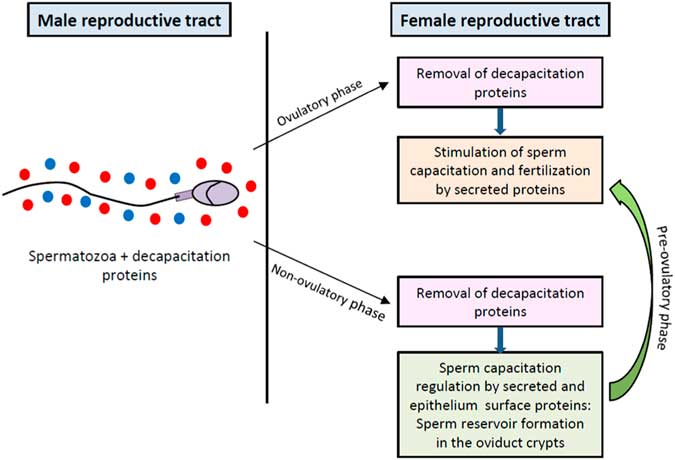
Sperm plasma membranes from ejaculated spermatozoa are scrubbed by the ultrastructural elements in the vagina, uterus and oviduct (De Jonge, Reference De Jonge2005). The cervical mucus is the first selective fluid encountered by sperm after entering the female genital tract and modulates sperm capacitation in ruminants (Wergin, Reference Wergin1985) and human (Gould et al., Reference Gould, Overstreet and Hanson1984; Perry et al., Reference Perry, Barratt, Warren and Cooke1996) (see Fig. 1C ). It has been shown that the sperm cholesterol content is markedly decreased after migration into the mucus (Feki et al., Reference Feki, Therond, Couturier, Limea, Legrand, Jouannet and Auger2004). For this purpose, albumin in the cervical mucus plays a key role during the capacitation, acting as a cholesterol acceptor and contributing to the dispersion of the released proteins (De Jonge, Reference De Jonge2005), but no other specific protein from this fluid has been characterized as sperm function modulator. After the cervix, spermatozoa must across the uterus towards the fallopian tubes, a journey in which the sperm plasma membrane also undergoes modifications by interacting with molecules secreted or exposed on the surface of utero-tubal epithelium that are relevant for their ability to fertilize (see Fig. 1D, E ). Finally, when spermatozoa meet the cumulus–oocyte complex in the ampulla of the oviduct, proteins contained in the follicular fluid promote the ultimate changes in the male gamete that allow ZP penetration and fusion with the oocyte (see Fig. 1F ). Contrasting with proteins secreted by the male reproductive tract, those from the female reproductive tract are supposed to stimulate capacitation and sperm–ZP interaction. However, the progress of capacitation should be controlled to synchronize capacitation with ovulation, and therefore spermatozoa placed in the female reproductive tract during the pre-ovulatory phase are compelled to move towards the ampulla, but when placed out of this period of the cycle they may be held back in the oviduct sperm reservoir waiting for ovulation signals that should reinitiate the capacitation process (see Fig. 2). Consequently, the secretome of the female reproductive fluids must change according to the different phases of the menstrual/estrous cycle.
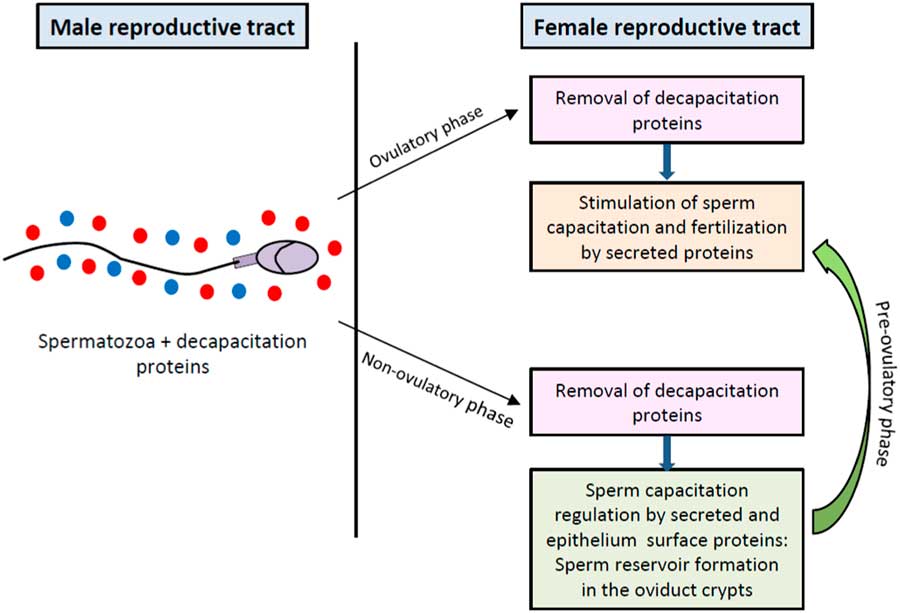
Figure 2 Regulation of sperm capacitation by proteins from male and female reproductive tracts. Proteins from male reproductive tract that associate to sperm plasma surface act as decapacitation factors. After removal of decapacitation factors in the female reproductive tract, secreted and oviductal epithelium surface proteins differentially regulate the progress of capacitation in accordance to the menstrual/estrous cycle stage to synchronize sperm maturation with ovulation.
Uterine proteins
Although GdA is abundant in the amniotic fluid, it is also present in the endometrial secretions of non-pregnant females. It has immunosuppressive properties that protect the spermatozoa from immune attack in the female reproductive tract (Bolton et al., Reference Bolton, Pockley, Clough, Mowles, Stoker, Westwood and Chapman1987), but is also able to inhibit sperm binding to the ZP (Oehninger et al., Reference Oehninger, Coddington, Hodgen and Seppala1995). However, as it is a progesterone-dependent protein, it is mainly secreted during the luteal phase of the menstrual cycle and therefore spermatozoa are not exposed to significant concentrations of GdA during the fertile window (Durand et al., Reference Durand, Koistinen, Chirinos, Rodriguez, Zambrano, Seppala and Larrea2010). Another progesterone-dependent protein expressed and secreted in the human uterus and cervix is the cysteine protease inhibitor cystatin C (CST3), which binds to sperm enhancing motility but inhibits cholesterol efflux and sperm protein tyrosine phosphorylation, indicating CST3’s ability to inhibit sperm capacitation (Lee et al., Reference Lee, Tseng, Hwu, Fan, Lin, Yu, Yeh and Li2018). Interestingly, this protein is also prominently expressed in the male reproductive tract and has been associated with human prostasomes (Carlsson et al., Reference Carlsson, Ronquist, Eliasson, Egberg and Larsson2011), but its potential role as a decapacitation factor has not been investigated. Other proteins of uterine origin that have been shown to inhibit sperm–ZP interaction are LTF (Teng et al., Reference Teng, Gladwell, Beard, Walmer, Teng and Brenner2002; Zumoffen et al., Reference Zumoffen, Gil, Caille, Morente, Munuce and Ghersevich2013) and glucose-regulated protein 78 (Grp78) (Marin-Briggiler et al., Reference Marin-Briggiler, Gonzalez-Echeverria, Munuce, Ghersevich, Caille, Hellman, Corrigall and Vazquez-Levin2010), and it has been proposed that such inhibition may contribute to regulate the number of sperm with the ability to interact and fertilize the oocyte (Zumoffen et al., Reference Zumoffen, Gil, Caille, Morente, Munuce and Ghersevich2013). Conversely, heat shock protein 60 (Hsp60), a chaperone protein secreted by uterus and oviduct epithelial cells from human and bovine (Boilard et al., Reference Boilard, Reyes-Moreno, Lachance, Massicotte, Bailey, Sirard and Leclerc2004; Lachance et al., Reference Lachance, Bailey and Leclerc2007), binds to spermatozoa and increases the intracellular calcium concentration with no apparent effects on sperm motility or acrosome reaction (Lachance et al., Reference Lachance, Bailey and Leclerc2007).
Endometrial cells also secrete interleukin-6 (IL-6), a mediator of the inflammatory response that increases during the periovulatory phase (Tabibzadeh and Sun, Reference Tabibzadeh and Sun1992). IL-6 induces sperm capacitation by increasing protein tyrosine phosphorylation and enhances spontaneous as well as calcium ionophore-induced acrosome reaction (Naz and Kaplan, Reference Naz and Kaplan1994; Laflamme et al., Reference Laflamme, Akoum and Leclerc2005). Moreover, uterine secretions contain the sialic acid binding protein (SABP), a protein that binds to spermatozoa head plasma membrane (Banerjee and Chowdhury, Reference Banerjee and Chowdhury1994) and stimulates in vitro sperm capacitation, increases the percentage of motile cells and the subsequent acrosome reaction (Banerjee and Chowdhury, Reference Banerjee and Chowdhury1995). Furthermore, SABP induces the exposure of mannose ligand receptors on the sperm surface and increases the production of the superoxide anion (Banerjee and Chowdhury, Reference Banerjee and Chowdhury1997). Furthermore, recent investigations indicate a widespread presence of fibroblast growth factor 2 (FGF2) in the mouse uterus and oviduct, and that this protein is able to increase sperm motility, intracellular calcium levels and acrosomal loss in vitro (Saucedo et al., Reference Saucedo, Sobarzo, Brukman, Guidobaldi, Lustig, Giojalas, Buffone, Vazquez-Levin and Marin-Briggiler2018).
In addition to uterine secreted proteins, uterosomes may also contribute to sperm function regulation, as they carry proteins that may promote sperm capacitation (Martin-DeLeon, Reference Martin-DeLeon2016). Moreover, it has been shown that uterosome-like vesicles secreted by endometrial epithelial cells are able to fuse with human spermatozoa, prompting their fertilizing capacity by increasing protein tyrosine phosphorylation and the acrosome reaction (Franchi et al., Reference Franchi, Cubilla, Guidobaldi, Bravo and Giojalas2016). In mouse, uterosomes contain sperm adhesion molecule 1 (SPAM1 or PH-20) (Griffiths et al., Reference Griffiths, Galileo, Reese and Martin-Deleon2008a), a protein that binds to spermatozoa on the acrosome and the midpiece of the flagella (Griffiths et al., Reference Griffiths, Miller, Galileo and Martin-DeLeon2008b). SPAM1 increases sperm intracellular calcium in macaque (Cherr et al., Reference Cherr, Yudin, Li, Vines and Overstreet1999) and enables sperm penetration of the cumulus oophorus surrounding the oocyte in mouse (Lin et al., Reference Lin, Mahan, Lathrop, Myles and Primakoff1994). Moreover, SPAM1 is required for hyaluronic acid enhancement of progesterone-induced acrosome reaction in human sperm (Sabeur et al., Reference Sabeur, Cherr, Yudin and Overstreet1998).
Oviduct proteins
When spermatozoa reach the oviduct, they either migrate to the ampulla region to meet the oocyte or are retained in the isthmus region by oviduct epithelial cells to give rise to the sperm reservoir (Jeulin et al., Reference Jeulin, Soumah and Jouannet1985). Sperm interactions with the oviductal epithelial cells increase the intracellular superoxide dismutase and glutathione peroxidase activities as protective mechanisms against reactive oxygen species (Huang et al., Reference Huang, Zhao, Lee, Lee, Lam, Ko, Yeung, Ho and Chiu2013). It has been shown that spermatozoa bound to oviductal epithelial cells show normal morphology and lower DNA fragmentation (Ellington et al., Reference Ellington, Evenson, Wright, Jones, Schneider, Hiss and Brisbois1999), but the role of the oviductal epithelium on sperm capacitation has been a controversial issue. It has been shown that incubation of sperm with oviductal cells enhances sperm viability and hyperactivated motility (Kervancioglu et al., Reference Kervancioglu, Djahanbakhch and Aitken1994), but other investigations have indicated that, although there is enhancement in sperm motility, there is a delay in capacitation (Murray and Smith, Reference Murray and Smith1997) by reducing protein tyrosine phosphorylation and avoiding follicular fluid-induced acrosome reaction (Morales et al., Reference Morales, Palma, Salgado and Villalon1996; Zumoffen et al., Reference Zumoffen, Caille, Munuce, Cabada and Ghersevich2010). These observations are in agreement with oviduct cells’ role as a sperm reservoir, where oviduct cells must stimulate some early capacitation-associated features but delay capacitation late events that are expected to happen in the oocyte vicinity.
As the time of ovulation approaches, the sperm in the reservoir must reassume capacitation. In mouse, hyperactivation assists the sperm to pull off the epithelium and escape out of mucosal pockets (Demott and Suarez, Reference Demott and Suarez1992). At this stage of the journey, oviductosomes transfer proteins to the head and midpiece of spermatozoa (Al-Dossary et al., Reference Al-Dossary, Bathala, Caplan and Martin-DeLeon2015), delivering calcium ATPase 4 (PMCA4), a membrane protein whose deletion leads to severe loss of hyperactivated motility and male infertility (Al-Dossary et al., Reference Al-Dossary, Strehler and Martin-Deleon2013).
Oviduct also secretes oviductin, a high-molecular-weight protein that binds to the head and middle piece of the sperm (King and Killian, Reference King and Killian1994; Lyng and Shur, Reference Lyng and Shur2009). Bovine oviductin promotes in vitro sperm viability and total motility (Abe et al., Reference Abe, Sendai, Satoh and Hoshi1995). Moreover, this protein stimulates sperm–ZP interaction in bovine (Martus et al., Reference Martus, Verhage, Mavrogianis and Thibodeaux1998) and mice (Ensslin et al., Reference Ensslin, Lyng, Raymond, Copland and Shur2007), but has an inhibiting effect in pig (Kouba et al., Reference Kouba, Abeydeera, Alvarez, Day and Buhi2000) and hamster (Kimura et al., Reference Kimura, Matsuda, Ogura, Asano and Naiki1994), indicating that its function may not be conserved among mammalian species. In human, the expression of oviductin at the time of ovulation has been demonstrated (Briton-Jones et al., Reference Briton-Jones, Lok, Yuen, Chiu, Cheung and Haines2001; Lok et al., Reference Lok, Briton-Jones, Yuen and Haines2002). Recently, a secretory form of human oviductin expressed in HEK293 cells has been shown to enhance tyrosine phosphorylation and the incidence of acrosome-reacted sperm induced by calcium ionophore (Zhao et al., Reference Zhao, Yang, Jia, Reid, Leclerc and Kan2016). Finally, sperm binding glycoprotein (SBG) that is synthesized at the apical surface of pig isthmic and ampullar epithelial cells (Perez et al., Reference Perez, Roma, Cabada and Marini2006), binds to carbohydrates on the sperm plasma membrane (Marini and Cabada, Reference Marini and Cabada2003) to increase tyrosine phosphorylation of a polypeptide with an apparent molecular mass of 97 kDa, although suppressing motility (Teijeiro et al., Reference Teijeiro, Cabada and Marini2008).
Follicular fluid proteins
In the ampulla of the oviduct, sperm should meet the cumulus–oocyte complex containing the oocyte surrounded by the hyaluronic acid matrix, cumulus cells and follicular fluid. The hyaluronic acid matrix works as a mechanical filter for the elimination of sperm peripheral proteins that are no longer necessary for ZP penetration and fusion with the oocyte. In addition, follicular fluid stimulates the acrosome reaction rate (Calvo et al., Reference Calvo, Vantman, Banks, Tezon, Koukoulis, Dennison and Sherins1989), as a consequence of its high concentrations of progesterone (De Jonge, Reference De Jonge2005) that stimulates a biphasic calcium influx (Kirkman-Brown et al., Reference Kirkman-Brown, Bray, Stewart, Barratt and Publicover2000) and acrosome reaction in capacitated sperm (Blackmore, Reference Blackmore1993).
There are also secreted proteins in the follicular fluid content that interact with the sperm. Atrial natriuretic peptide (ANP) has been identified in human follicular fluid (Sundsfjord et al., Reference Sundsfjord, Forsdahl and Thibault1989) and pig oviductal fluid in which it increases the acrosome reaction, the oocyte penetration rate and decreases polyspermy (Zhang et al., Reference Zhang, Hong, Zhou, Jin, Wang, Fu, Wang and Xia2006). Another follicular fluid protein that interacts with the sperm is Gd, existing as two isoforms: GdF that prevents premature acrosomal reaction and interferes with sperm–ZP interaction (Chiu et al., Reference Chiu, Koistinen, Koistinen, Seppala, Lee and Yeung2003), and GdC that promotes sperm–ZP interaction (Chiu et al., Reference Chiu, Chung, Koistinen, Koistinen, Seppala, Ho, Ng, Lee and Yeung2007). It has been proposed that cumulus cells may use GdF as a substrate in the production of GdC, which is then released during cumulus expansion and displaces sperm previously bound Gd isoforms (GdS and/or GdA) to promote sperm–ZP interaction (Yeung et al., Reference Yeung, Lee, Koistinen, Koistinen, Seppala and Chiu2009).
Glycoprotein fibronectin (Fn) is secreted during cumulus expansion (Sutovsky et al., Reference Sutovsky, Flechon and Pavlok1995) and in the fallopian tube (Makrigiannakis et al., Reference Makrigiannakis, Karamouti, Petsas, Makris, Nikas and Antsaklis2009). Its receptor, the integrin α5β1, has been detected on both male and female human (Fusi et al., Reference Fusi, Vignali, Gailit and Bronson1993, Reference Fusi, Tamburini, Mangili, Montesano, Ferrari and Bronson1996) and bovine gametes (Thys et al., Reference Thys, Nauwynck, Maes, Hoogewijs, Vercauteren, Rijsselaere, Favoreel and Van Soom2009). By contrast, it has been demonstrated that Fn strongly inhibits sperm penetration during bovine IVF by diminishing sperm binding to ZP and oolemma as well as sperm–oocyte fusion (Tanghe et al., Reference Tanghe, Van Soom, Duchateau, Nauwynck and de Kruif2004; Thys et al., Reference Thys, Nauwynck, Maes, Hoogewijs, Vercauteren, Rijsselaere, Favoreel and Van Soom2009), although its mechanism of action under physiological conditions remains unknown. Recent investigations have indicated that Fn is present in the oviductal fluid as well as in the extracellular matrix of oviductal epithelial cells and that it is a key regulator of sperm binding to the epithelium when forming the sperm reservoir (Osycka-Salut et al., Reference Osycka-Salut, Castellano, Fornes, Beltrame, Alonso, Jawerbaum, Franchi, Diaz and Perez Martinez2017).
Interestingly, despite CRISP1 being considered as a male reproductive tract protein, recent studies have shown that is also expressed by mouse cumulus cells and that fertilization of CRISP1 knockout females is impaired because of sperm failure to penetrate the cumulus. Apparently, CRISP1 produced in the cumulus cells may regulate sperm orientation by modulating sperm hyperactivation through a mechanism that involves inhibition of CatSper and TRPM8 calcium channels, indicating a novel role for this protein in mammalian fertilization (Ernesto et al., Reference Ernesto, Weigel Munoz, Battistone, Vasen, Martinez-Lopez, Orta, Figueiras-Fierro, De la Vega-Beltran, Moreno, Guidobaldi, Giojalas, Darszon, Cohen and Cuasnicu2015).
Conclusion
In order to fertilize, sperm must undergo changes that are sequentially regulated by the male and the female reproductive tracts that allow them to meet the oocyte and fuse with it. During that journey, the glycoprotein calyx surrounding the sperm plasma membrane is constantly remodelling to modulate the progress of capacitation. Years of investigation carried out in different models have indicated that mammalian sperm interaction with proteins from the male reproductive tract mostly inhibits sperm capacitation (see Table 1), while those from the female reproductive tract stimulate this process (see Table 2), but not all proteins characterized so far behave as expected. In addition, it is evident that there is still much work ahead to be carried out to fill the gaps that would allow an understanding of the mechanisms that transform the ejaculated spermatozoa into a fully competent cell able to fertilize.
Table 1 Proteins from male reproductive tract and their participation in sperm capacitation and gamete interaction
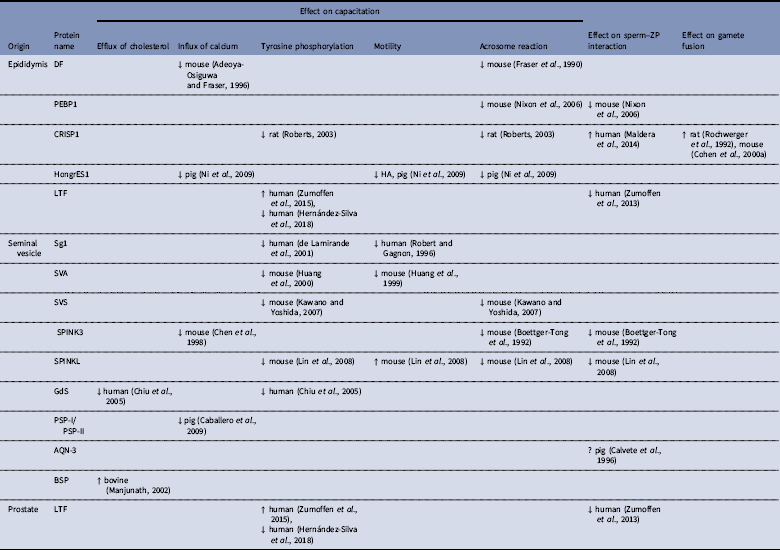
↓: inhibition; ↑: stimulation; ?: unknown; HA=hyperactivation.
Table 2 Proteins from mammalian female reproductive tract and their participation in sperm capacitation and gamete interaction.
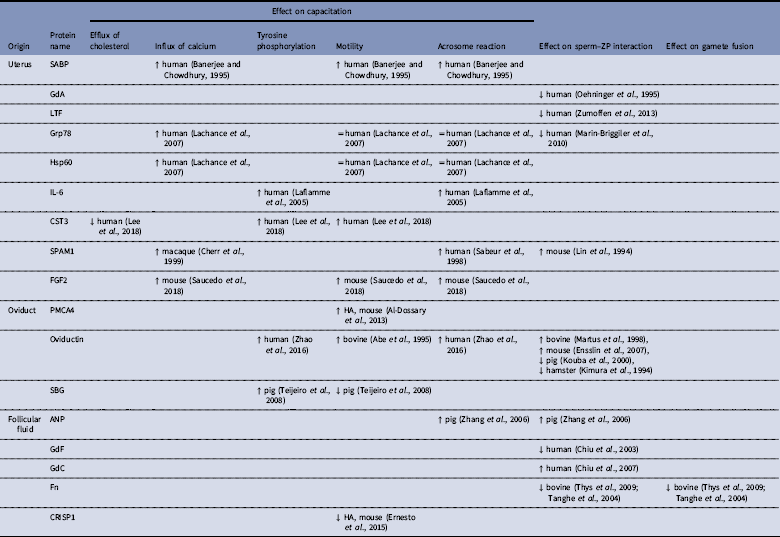
↓: inhibition; ↑: stimulation; =: no effect; HA=hyperactivation.
The capacitation progress is regulated by inputs received from the extracellular milieu. An adequate balance between decapacitating and capacitating factors is required and therefore plasma membrane-associated proteins acquired during sperm transit along the male reproductive tract need to be removed to allow interaction with molecules of female origin that must modulate the progress of capacitation. However, some proteins have been found to be expressed in both male and female reproductive tracts, suggesting that there are multifunctional pieces in the capacitation machinery. In addition, protein post-translational modifications such as changes in the glycosylation pattern could be responsible for differential effects of a protein on capacitation, as has been described for Gd. Moreover, the variations in the abundance of certain proteins in each segment of the road and their particular affinity for sperm surface receptors may also contribute to regulate the evolution of sperm capacitation.
The evidence presented here indicates that there are several proteins that clearly regulate the sperm’s ability to fertilize. Some of these such as Eppin and CRISP1 are of great interest due to their potential as male contraceptive targets because of its specificity and location on the human sperm surface. Nonetheless their participation during in vivo fertilization has not been sufficiently investigated. Furthermore, the in vitro studies reviewed evaluated the effects of single proteins on sperm, but the simultaneous or sequential interaction with several proteins competing for sperm membrane receptors, the involvement of non-protein molecules contained in the reproductive fluids and the presence of protein complexes (as in the case of Eppin/LTF/clusterin) are elements that should be considered in the future to fully understand spermatozoa changes that culminate in a successful gamete encounter and fusion. The findings currently revised shall contribute to improve understanding of the mechanisms that regulate sperm function, delineate novel approaches to study sperm physiology and identify potential biomarkers for the diagnosis of infertility.
Acknowledgements
GHS is recipient of a scholarship from CONACyT (México) for postgraduate studies at the Posgrado en Ciencias Biológicas of the Universidad Nacional Autónoma de México (UNAM, México).
Conflict of interests
None.
Financial support
This work was partially supported by the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (México).
Ethical standards
Not applicable