After percutaneous implantation of an atrial septal defect occlusion device, the healing process is an important step to allow the formation of a neotissue covering the device, preventing the occurrence of thrombus or endocarditis. Reference Foth, Quentin and Michel-Behnke1 The Nit-Occlud ASD-R occluder (PFM medical, Cologne, Germany) consists of two disks manufactured from a single nitinol wire and two polyester membranes, without central screw. It was recently approved for clinical use in Europe.
We present the histological workup of one of eight atrial septal defect occlusion devices that were percutaneously implanted in sheep after trans-septal puncture and dilation of the fossa ovalis. After explantation at 1 or 3 months, the specimens were embedded in the hard resin methylmethacrylate. Histological slides were produced by sawing and grinding; standard staining was performed with Richardson blue.
On gross examination (Fig 1), most of the right-sided disks of the devices were covered with neotissue (Panel A) whereas all left-sided disks were completely covered with tissue (Panel B). The Richardson blue staining allowed to identify cellular and extracellular matrix components (blue), metal struts (black), and polyester membrane (grey) (Panel C). Fibroblasts were found around the metal frame (Panel D). Neo-endothelium was present with endothelial cells organised in a thin superficial cell layer (Panel D, arrow). The sub-endothelial tissue consisted of fibroblasts, macrophages and giant cells surrounded by extracellular matrix material (Panel D) with longitudinal arrangement of the cells parallel to the surface (Panel E). Fibrin was also present and so was thrombus material.
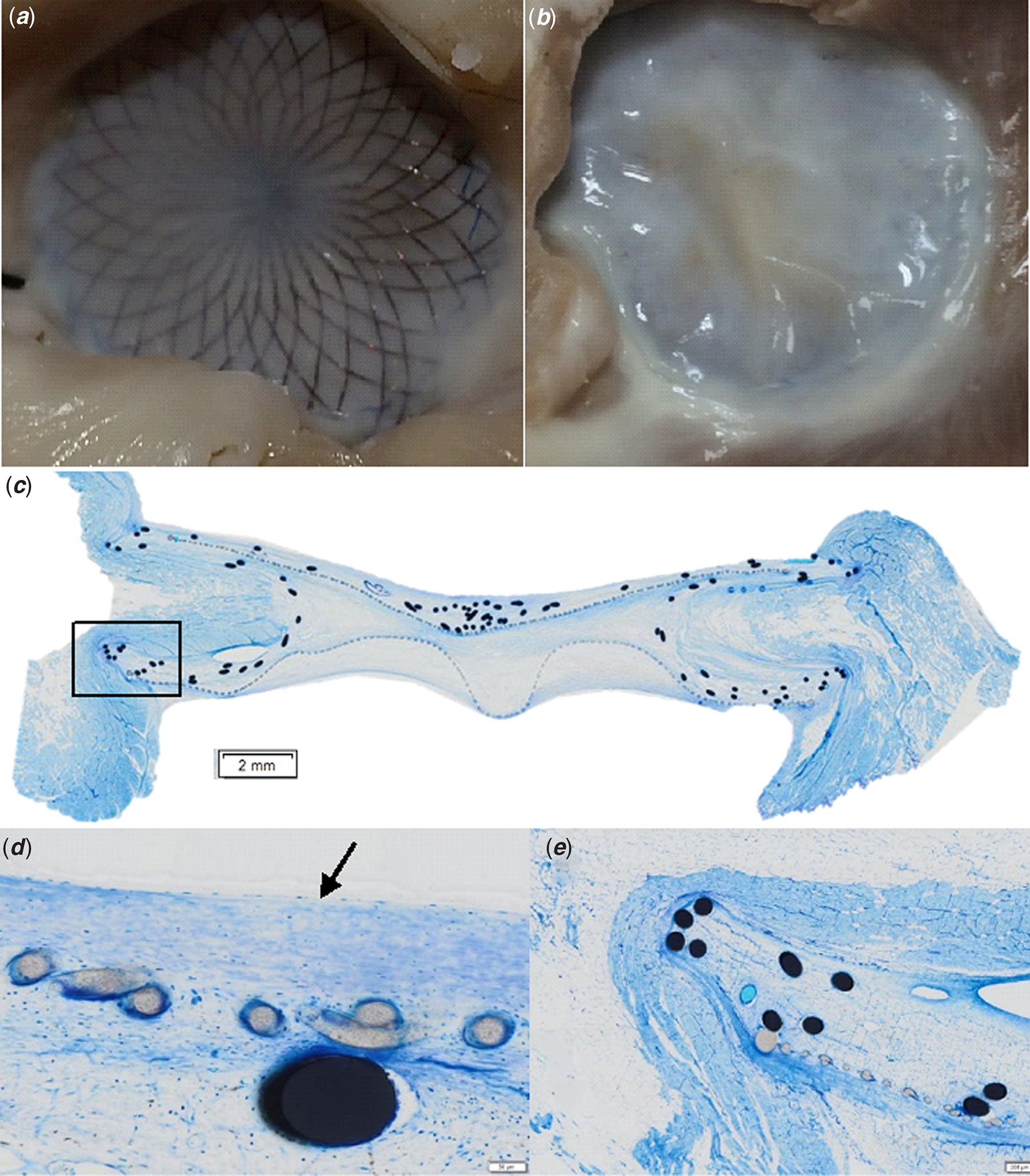
Figure 1. Macroscopic examination and histological analysis. Panel A : right-sided disk. Panel B : left-sided disk. Panel C : histological slice after Richardson blue staining. Panel D et E : neo-endothelium (arrow) and sub-entothelial tissue made of fibroblasts and extracellular matrix.
The healing process described above seems like other devices of this type, confirming the good biocompatibility of Nit-Occlud ASD-R occluder.
Acknowledgements
We thank Pr Jean-Benoît Thambo for his support.
Financial support
This study received financial supports from the French Government as part of the “Investments of the Future” program managed by the National Research Agency (ANR), Grant reference ANR-10-IAHU-04 and the grant “Aide à la recherche par équipe 2018, Cardiopathies de l’enfant” from the French Federation of Cardiology.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (2010/63/UE; 2010) and has been approved by the institutional committee (Ethical Committee of Bordeaux CEEA 50, reference number: APAFIS#15508-201806140929827v2).




