Bats constitute one of the most diverse recent mammal groups; indeed, they represent over 20 % of all extant species (Simmons Reference Simmons2005). In contrast, their fossil record is notably incomplete (Teeling et al. Reference Teeling, Springer, Madsen, Bates, O'Brien and Murphy2005; Eiting & Gunnell Reference Eiting and Gunnell2009; Brown et al. Reference Brown, Cashmore, Simmons and Butler2019). In the Mio–Pliocene of Spain (Table 1), their record mainly consists of indeterminate taxa presented as faunal lists (Sevilla Reference Sevilla2002).
Table 1 Mio–Pliocene sites from Spain with remains of bats, excluding this work (Pons-Moyà et al. Reference Pons-Moyà, Moyà-Solà, Agustí and Alcover1981; Sigé & Legendre Reference Sigé and Legendre1983; Aguilar et al. Reference Aguilar, Brandy and Thaler1984; Sesé Reference Sesé1986; Martínez-Salanova Reference Martínez-Salanova1987; Alcalá et al. Reference Alcalá, Sesé, Herráez and Adrover1991; Robles et al. Reference Robles, Belinchón, García-Flor and Morales1991; Sevilla Reference Sevilla2002; Dam & Rubio Reference Dam, Rubio, López-Martínez, Peláez-Campomanes and Hernández-Fernández2003; Murelaga et al. Reference Murelaga, Astibia, Sesé, Soria and Pereda-Suberbiola2004a, Reference Murelaga, Larrasoaña and Garcésb, Reference Murelaga, Pérez-Rivarés, Vázquez-Urbez and Zuluaga2008; Álvarez-Sierra et al. Reference Álvarez-Sierra, García-Paredes, Hoek Ostende, Meulen, Peláez-Campomanes and Sevilla2006; Agustí et al. Reference Agustí, Santos-Cubedo, Furió, De Marfá, Blain, Oms and Sevilla2011; Mansino et al. Reference Mansino, Crespo, Lázaro, Ruiz-Sánchez, Abella and Montoya2016; Hoek Ostende et al. Reference Hoek Ostende, Álvarez-Sierra, García-Paredes, Montoya, Ruiz-Sánchez and Peláez-Campomanes2017; Crespo et al. Reference Crespo, Sevilla, Mansino, Montoya and Ruiz-Sánchez2018a).

Despite their current abundance, fossil bats are one of the least common groups in fluvio-lacustrine sediments and typically only represent 0.01 % or less of the small mammal assemblages (Sigé & Legendre Reference Sigé and Legendre1983; Sevilla Reference Sevilla2002; Crespo et al. Reference Crespo, Sevilla, Mansino, Montoya and Ruiz-Sánchez2018a). The scarcity of bats in the Neogene fluvio-lacustrine record enhances the importance of each discovery to improve the knowledge of this group and its biogeographic history. The relative abundance of chiropterans in the Ribesalbes-Alcora Basin (eastern Spain) provides an exceptional opportunity to understand bat assemblages in their ecological environment during the early Miocene in Europe.
1. Geological setting
The Ribesalbes-Alcora Basin is an intramontane basin (Fig. 1) located in the eastern Iberian Peninsula (Agustí et al. Reference Agustí, Anadón, Ginsburg, Mein and Moissenet1988). The sections that yielded bat fossils include Mas dels Coixos, Mas de Torner, Barranc de Campisano, Foieta la Sarra, Mas d'Antolino B, and Corral de Brisca, all located in the Campisano ravine. They consist of nearly 120 m of grey mudstone, sandstone, and limestone belonging to the ‘Unit Three’ sensu Anadón (Reference Anadón1983) (Crespo et al. Reference Crespo, Fagoaga, Montoya and Ruiz-Sánchez2019a).
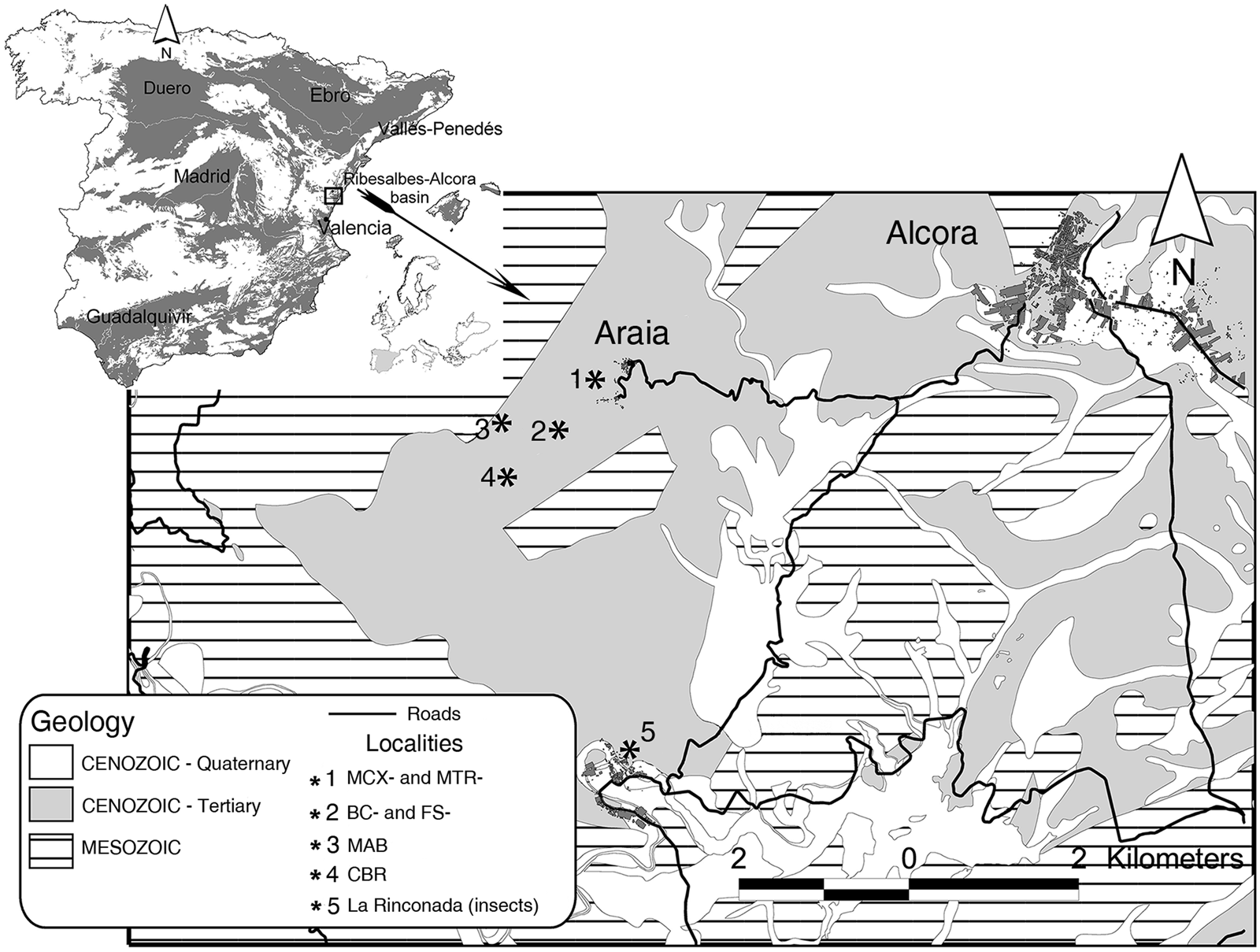
Figure 1 Cenozoic basins from Spain, with location of the Ribesalbes-Alcora Basin and the schematic distribution of sediments. Abbreviations: MCX = Mas dels Coixos; MTR = Mas de Torner; BC = Barranc de Campisano; FS = Foieta la Sarra; MAB = Mas d'Antolino B; CBR = Corral de Brisca. La Rinconada is the classical fossil-lagerstätte site with remains of insects, plants, and amphibians. Modified from Crespo et al. (Reference Crespo, Fagoaga, Montoya and Ruiz-Sánchez2019a). Scale bar = 2 km.
Fossil mammals in the Ribesalbes-Alcora Basin were first described by Agustí et al. (Reference Agustí, Anadón, Ginsburg, Mein and Moissenet1988). During the recent campaigns carried out between 2008 and 2011, up to 45 new mammal sites were found in this basin near the classic localities described by Agustí et al. (Reference Agustí, Anadón, Ginsburg, Mein and Moissenet1988). It is important to note that we have not yet been able to find the original sites described by these authors (Crespo et al. Reference Crespo, Fagoaga, Montoya and Ruiz-Sánchez2019a). The recent campaigns have provided the southernmost occurrence of the herpetotheriid Amphiperatherium frequens (von Meyer, 1846) (Furió et al. Reference Furió, Ruiz-Sánchez, Crespo, Freudenthal and Montoya2012; Crespo et al. Reference Crespo, Goin, Montoya and Ruiz-Sánchez2020), a new species of the dimylid Plesiodimylus ilercavonicus Crespo, Furió, Ruiz-Sánchez & Montoya, 2018 (Crespo et al. Reference Crespo, Furió, Ruiz-Sánchez and Montoya2018b), talpids (Crespo et al. Reference Crespo, Marquina-Blasco, Ruiz-Sánchez and Montoya2019b), as well as soricids and heterosoricids (Crespo et al. Reference Crespo, Suárez-Hernando, Murelaga, Ruiz-Sánchez and Montoya2019c), erinaceids (Crespo et al. Reference Crespo, Goin, Montoya and Ruiz-Sánchez2020), among other findings.
According to Crespo et al. (Reference Crespo, Fagoaga, Montoya and Ruiz-Sánchez2019a), the study of the mammalian assemblages from these sections resulted in two biozones correlated with the local biozone C from Calatayud-Montalbán Basin (Meulen et al. Reference Meulen, García-Paredes, Álvarez-Sierra, Hoek Ostende, Hordijk, Oliver, López-Guerrero, Hernández-Ballarín and Peláez-Campomanes2012). The first local biozone is characterised by the association of Ligerimys florancei Stehlin & Schaub, 1951, together with Megacricetodon primitivus (Freudenthal, 1963) and Democricetodon decipiens (Freudenthal & Daams, 1988), in Mas dels Coixos, Mas de Torner, Barranc de Campisano, and the earlier part of Mas d'Antolino B. The other local biozone is characterised by the replacement of L. florancei by Ligerimys ellipticus Daams, 1976, in the later part of Mas d'Antolino B, Foieta la Sarra, and Corral de Brisca. Both biozones are indicative of an early Miocene age (lower Aragonian, MN4, ca.16.6–16 Ma, according to Meulen et al. Reference Meulen, García-Paredes, Álvarez-Sierra, Hoek Ostende, Hordijk, Oliver, López-Guerrero, Hernández-Ballarín and Peláez-Campomanes2012; Crespo et al. Reference Crespo, Fagoaga, Montoya and Ruiz-Sánchez2019a).
2. Materials, methods, and abbreviations
The fossils under study are currently held at the Museum of the University of Valencia of Natural History (MUVHN) with the field labels MCX-, MTR-, BC-, FS-, MAB-, and CBR-. We have followed the nomenclature (Fig. 2) and measurement methods of Sevilla (Reference Sevilla1988), Fracasso et al. (Reference Fracasso, Oliveira-Salles and Perini2011), and Gunnell et al. (Reference Gunnell, Eiting and Geraads2011). Biozonation follows the MN system described in Mein (Reference Mein, Rössner and Heissig1999, Reference Mein2000). We follow Maitre's (Reference Maitre2014) definition of myotodonty, submyotodonty, and nyctalodonty.

Figure 2 Terminology of the Chiroptera teeth after Sevilla (Reference Sevilla1988), Fracasso et al. (Reference Fracasso, Oliveira-Salles and Perini2011), and Gunnell et al. (Reference Gunnell, Eiting and Geraads2011).
Abbreviations: BC, Barranc de Campisano; CBR, Corral de Brisca; FS, Foieta la Sarra; L, length; MAB, Mas d'Antolino B; MCX, Mas dels Coixos; MGUV, Museu de Geologia de la Universitat de València; MN, European Neogene land mammal units; MTR, Mas de Torner; W, width. Lower teeth are indicated as i, c, p, and m, and upper teeth as I, C, P, and M.
3. Systematic palaeontology
Order Chiroptera Blumenbach, 1779
Suborder Yangochiroptera Koopman, 1984
Superfamily Vespertilionoidea Gray, 1821
Family Molossidae Gervais, in de Castelnau, 1855
Genus Cuvierimops Legendre & Sigé, 1982
Cuvierimops penalveri sp. nov.
(Fig. 3a, b)
Type species. Cuvierimops parisiensis Cuvier in Pictet, 1844
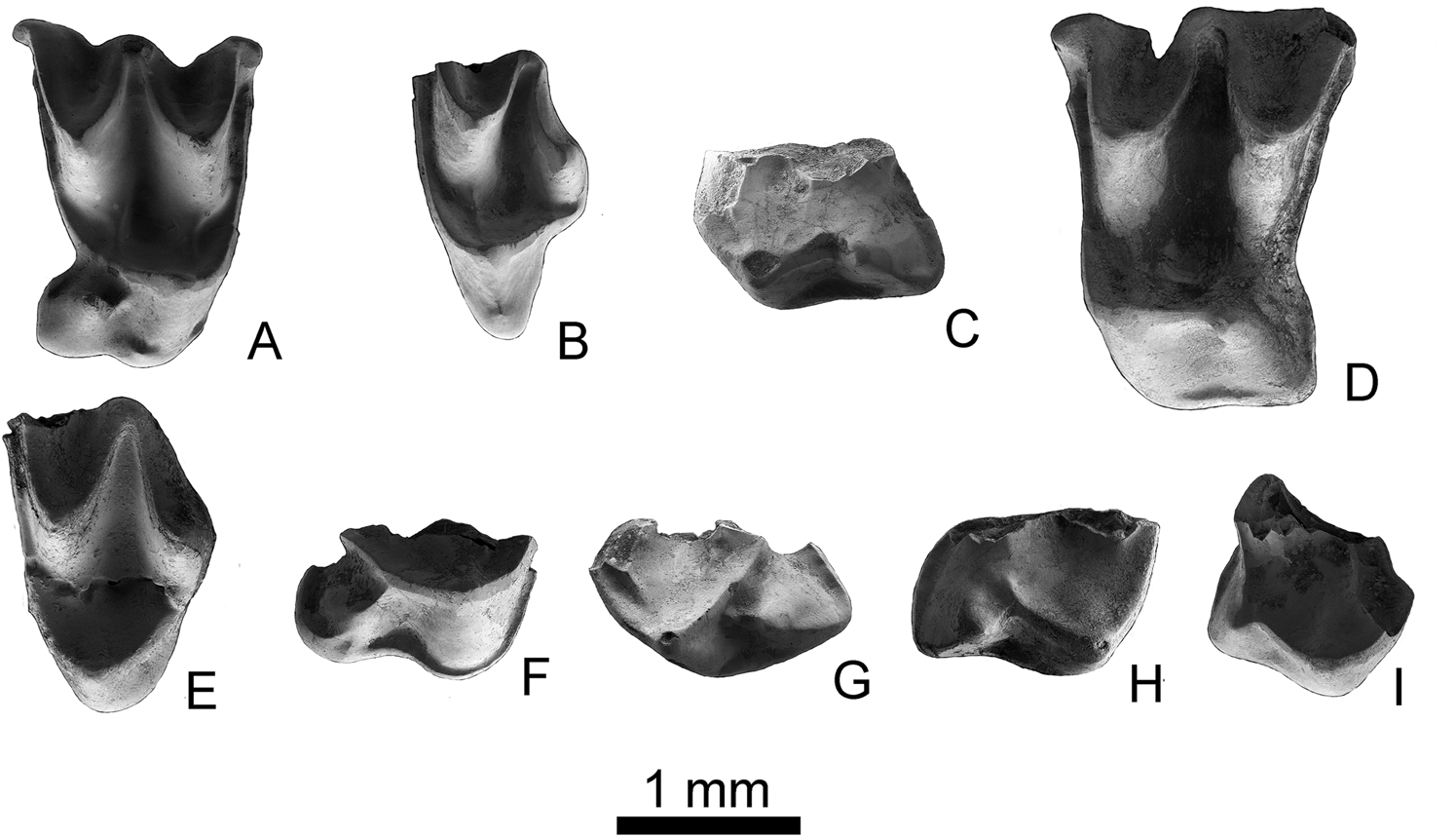
Figure 3 Molossid teeth from the Ribesalbes-Alcora Basin. Cuvierimops penalveri sp. nov.: (A) right M2 (MAB11-99; holotype); (B) left M3 (MAB11-100). Hydromops helveticus: (C) left M1 (MAB0A-85); (D) left M2 (MAB5-386); (E) left M3 (MAB5-397). Rhizomops cf. brasiliensis: (F) right M2 (MAB5-443). Chaerephon sp.: (G) left M1 (MAB11-104); (H) right M2 (MAB5-437). Tadarida sp.: (I) right M2 (MTR2-37). Scale bar = 1 mm.
2017 Tadarida (Rhizomops) cf. brasiliensis (in part) Crespo, Reference Crespo2017.
Etymology. Named after Enrique Peñalver, our colleague, friend, and one of the scientists who studied insects from the classical sites of Ribesalbes-Alcora Basin.
Holotype. Right M2, MAB11-99 (MGUV-38486; 1.37 × 1.87).
Type locality. Mas d'Antolino B 11, Ribesalbes-Alcora Basin, Spain, MN4, early Miocene.
Material and measurements. 1 M2 (MAB11-103; MGUV-38490); 2 M3 (MAB11-98 (MGUV-38485); MAB11-100 (MGUV-38487)). Doubtful remains: 1 c (MAB11-140 (MGUV-38527); 0.86 × 0.81); 1 p3 (MAB11-138 (MGUV-38525); 0.74 × 0.52).
Diagnosis. The youngest and smallest species of Cuvierimops, upper molars with less developed lingual cingula.
Differential diagnosis. Differs from all other species of Cuvierimops in terms of its smaller-sized upper molars and less developed lingual cingula from Rhizomops in its M1/2 conical hypocone.
Stratigraphic and geographic range. MN4, early Miocene; Ribesalbes-Alcora Basin, eastern Spain.
3.1. Description
M2. Tooth with a subquadrate outline in occlusal view. The parastyle is curved. The paracingulum starts close to the parastyle. It connects lingually to the protocone and continues with the postprotocrista and the metacingulum. A well-developed talon is present; the hypocone is a tall cusp and the postprotocrista is a small crest between the protocone and metaconule. The hypocone has a small precingulum, while the lingual cingulum is poorly developed in one specimen and divided into two cingula in the other. The metaloph and the paraloph are developed. The metastyle shows a right-angle shape with the postmetacrista.
M3. The teeth are broken and the parastyle is not preserved; their posterior part is reduced regarding the upper molars of the other. The metastyle is absent and the premetacrista and metacone are well developed and similar in length to the postparacrista. The paracone and the metacone are of similar height. The paraloph is developed. An independent paracingulum is present and ends at the upper part of the protocone. A short labial cingulum on the posterior part of the protocone is present.
3.2. Description of the tentatively referred material
c. A broken tooth without the main cusp. It presents a triangular outline in occlusal view. The cingulid is continuous, surrounding most of the base of the tooth; it is briefly interrupted on its posterior margin where the cingulid has thickened and forms a small triangular cuspule. The root is wider than it is elongated.
p3. An elongated tooth with only one root preserved; a single main cusp and a well-developed cingulid. In the posterior part of the tooth, a small basin divided by a crest is present between the cusp and the cingulid.
3.3. Discussion
Cuvierimops is a typical genus from the early Oligocene of Europe. Until now, it was considered to have disappeared at the end of the Oligocene (MP26, Saint-Privat-des-Vieux, France; Maitre Reference Maitre2014; Vianey-Liaud et al. Reference Vianey-Liaud, Comte, Marandat, Peigne, Rage and Sudre2014). It is characterised by a strong hypocone on the M1 and M2, isolated from the posprotocrista.
Cuvierimops differs from Tadarida in the presence of a metaloph and a strong paraloph, although both genera share a cuspate hypocone (Legendre Reference Legendre1985). On the other hand, the extant Rhizomops differs from its ancestor Cuvierimops with better development of the talon and the presence of a crestiform hypocone on the upper molars, which has only been detected in some specimens of Cuvierimops legendrei Maitre, Reference Maitre2014. This species is likely the ancestor of Rhizomops (Legendre Reference Legendre1985; Maitre Reference Maitre2014).
Maitre (Reference Maitre2014) describes one lineage of this genus characterised by the simplification of the anterior part of the jaw through anteroposterior compression of the premolars, the appearance and the increasing proportion of submyotodonty, and, subsequently, myotodonty on the lower molars, as well as an increase in size (Table 2). This lineage includes the species C. parisiensis and C. legendrei. The small size of Cuvierimops penalveri sp. nov. enables us to discard the possibility that it might pertain to this lineage. Nevertheless, Maitre (Reference Maitre2014) remarks on the existence of a small undetermined species described by Sigé (Reference Sigé1995) from Le Garouillas (29.1–27.6, MP25, France), which has been considered to be one of the last representatives of the genus. Due to its small size, we suggest that C. penalveri sp. nov. belongs to a new second lineage together with Cuvierimops indet. A from Le Garouillas, and that this lineage was the only one to survive the end of the Oligocene.
Table 2 Measurements of the M2 of the different species, subspecies, and populations of the genus Cuvierimops (Maitre Reference Maitre2014; this publication). Abbreviations: L = length; W = width.

1 In this population, in specimens with the same length/width as Ribesalbes-Alcora Basin material, the width/length is considerably larger.
Genus Hydromops Legendre, 1984
Hydromops helveticus (Revilliod, 1920)
(Fig. 3c–f)
2017 Miniopterus sp. nov. Crespo, Reference Crespo2017.
2017 Mormopterus (Hydromops) cf. helveticus (in part) Crespo, Reference Crespo2017.
Localities. MAB0A, MAB5.
Material and measurements. 3 m1 or m2 (MAB0A-13 (MGUV-36900); MAB5-428 (MGUV-25218): -x1.40; MAB5-746 (MGUV-38169): -x1.24); 1 M1 (MAB0A-85; MGUV-36972); 1 M2 (MAB5-386 (MGUV-25176); 1.71 × 2.20); 1 M3 (MAB5-397 (MGUV-25187); 1.19x-). Doubtful remains: 1 mandible (MAB0A-17; MGUV-36904), 1 c (MAB5-434 (MGUV-25224); 1.01x-); 1 P2/P3 (MAB5-903 (MGUV-38326); 0.68 × 0.78).
3.4. Description
m1 or m2. Only the medium-sized trigonid is preserved. The paralophid is curved. The trigonid basin is open lingually. The metaconid and the paraconid are of similar size and are aligned. The protoconid is the highest cusp. The labial cingulid is well developed and straight in lateral view. The crista obliqua meets the trigonid near the centre of the crown.
M1. A broken tooth with complex protocone and hypocone. The precingulum is absent. A very weak metaloph is present; the paraloph is better developed. The postprotocrista links the protocone with the hypocone, the second cusp being lower. The lingual cingulum is well developed. The posterior crest of the hypocone joins the metacingulum.
M2. Tooth with a subrectangular outline in occlusal view. The parastyle is straight. The paracone and the metacone are similar in size and connected by a well-developed mesostyle. Only the valley of the paracone (paraflexus) shows a notch in the labial side of the tooth. The paracingulum almost joins the parastyle; lingually, it connects to the protocone. The paraloph is poorly developed. A well-developed talon is present. The hypocone is a poorly developed cusp; the postprotocrista is reduced to a small crest between the protocone and hypocone. The protocone has a narrow lingual and posterior cingulum linked to the metastyle. The metaloph is not present. The metastyle is broken.
M3. The tooth is broken and shows a reduced posteriorly, with no metastyle present. The parastyle is broken. The premetacrista and the metacone are well developed; the former is as long as the postparacrista. The paracone and the metacone are of similar height. The paraloph is well developed. An independent paracingulum is present, ending at the top of the protocone.
3.5. Description of the tentatively referred material
Mandible. Fragment of mandible with a large mental foramen, situated between the canine and the p2, near the alveolar margin.
c. Fragment of tooth with a principal cusp and a broken well-developed cingulid.
P2/P3. Subtriangular medium tooth; its principal cusp shows three sides, with a well-developed cingulum and a small cusp in the posterolingual part of the cingulum.
3.6. Discussion
The genus Hydromops, originally a subgenus of Mormopterus, is represented in the European record by two species, Hydromops stehlini (Revilliod, 1920) and M. helveticus (Storch Reference Storch, Rössner and Heissig1999). The first one was recorded from the nearby locality of Buñol (Robles et al. Reference Robles, Belinchón, García-Flor and Morales1991). Here we align with the common opinion (Legendre Reference Legendre1985; Sigé et al. Reference Sigé, Aguilar, Matandat and Astruc1991; Storch Reference Storch, Rössner and Heissig1999; among others) that considers these species as likely belonging to the same lineage, the first of which is found in MN1-2, while the second is distributed from MN3 to MN7/8 (Storch Reference Storch, Rössner and Heissig1999).
This genus is characterised by an open protofossa, the absence of the metaloph, a weak paraloph, and the hypocone included in the postprotocrista (Legendre Reference Legendre1984a). The crestiform hypocone differs from those shown by Mormopterus (Neomops) faustoi (‘Tadarida’ faustoi after Paula Couto, 1956) (Legendre Reference Legendre1985) and Cuvierimops (Maitre Reference Maitre2014). Hydromops helveticus differs from the recent Hydromops species in the absence of metaloph, the posterior opening of the protofossa, and the connection between the hypocone and the protocone (Legendre Reference Legendre1985). In general, the material from the Ribesalbes-Alcora Basin is similar in morphology and size to the material described from Anwil (Switzerland, MN7/8; Engesser Reference Engesser1972).
Genus Rhizomops Legendre, 1984
Rhizomops brasiliensis (Geoffroy, 1824)
Rhizomops cf. brasiliensis
(Fig. 3g)
2017 Chaerephon sp. Crespo, Reference Crespo2017.
Locality. MAB5.
Material. 1 M2 (MAB5-443; MGUV-25233).
3.7. Description
M2. Only a complex hypocone–protocone is preserved. The protofossa is closed. The paraloph and the metaloph are present. The hypocone is included in the postprotocingulum, which makes contact with the postprotocrista. The talon is well developed. The lingual cingulum is also well developed and has contact with the precingulum.
3.8. Discussion
Rhizomops species are characterised by M1/2 with a closed protofossa and the presence of a metaloph and a paraloph. Overall, these species are very similar to those of the stratigraphically older genus Cuvierimops. They differ from the latter in having a crestiform hypocone, which is included in the postprotocingulum; the talon is better developed, whereas the lingual cingulum is less developed in comparison (Legendre Reference Legendre1985; Maitre Reference Maitre2014). Accordingly, the material from MAB5 cannot be assigned to Cuvierimops, nor to the genus Tadarida, because of the presence of a paraloph and a metaloph in the Ribesalbes-Alcora tooth – two features that are absent in Tadarida upper molars (Legendre Reference Legendre1984a). Other similar genera can be discarded: Sauromys has no lingual cingulum and Otomops by its different morphology of the talon, being more similar to the first of the genera (Legendre Reference Legendre1984a; Hand Reference Hand1990). For discussion about whether European fossils belong to a Rhizomops or a different but convergently evolved taxon, see Owen et al. (Reference Owen, Chesser and Carter1990).
The record of Rhizomops cf. brasiliensis is scarce in the Paleogene and Neogene fossil record of Europe, with the only two records originating from Venelles (latest Oligocene, MP30, France) and Collet Redon (middle Miocene, MN7/8, France) (Storch Reference Storch, Rössner and Heissig1999; Maitre Reference Maitre2014). On the other hand, morphological studies involving more character systems of the cranium, mandible, and molecular genetic studies suggested that R. brasiliensis, although found today only in America, is a member of an Old World Tadarida clade closely related to Tadarida aegyptiaca, Sauromys, and Otomops, and separated from the New World Tadarida's clade (Freeman Reference Freeman1981a; Ammerman et al. Reference Ammerman, Lee and Tipps2012). Therefore, we have provisionally assigned this tooth to R. cf. brasiliensis, awaiting new material collected in the Ribesalbes-Alcora Basin that might enable a more precise description and characterisation of this taxon.
Genus Chaerephon Dobson, 1874
Chaerephon sp.
(Fig. 3h–i)
2017 Tadarida (Rhizomops) cf. brasiliensis (in part) Crespo, Reference Crespo2017.
2017 Mormopterus (Hydromops) cf. helveticus (in part) Crespo, Reference Crespo2017.
Localities. MAB5 and MAB11.
Material and measurements. 1 M1 (MAB11-104; MGUV-38491). 1 M2 (MAB5-437; MGUV-25227). Doubtful remains: 1 c (MAB5-427 (MGUV-25217); 1.15 × 1.18), 2 p3 (MAB5-158 (MGUV-24948): 0.57 × 0.71; MAB5-907 (MGUV-38330): 0.5 × 0.82), 3 p4 (MAB5-431 (MGUV-25221): 0.86 × 0.91; MAB5-764 (MGUV-38187): 0.89 × 0.7; MAB5-903 (MGUV-38326)), 1 I (MAB5-772 (MGUV-38195): 0.68 × 0.48), 1 C (MAB5-426 (MGUV-25216): 1.18 × 1.56; MAB5-912 (MGUV-38335): 1.18 × 1.58).
3.9. Description
M1. Only a complex hypocone–protocone is preserved. The protofossa is almost closed. The paraloph is well developed, while the metaloph is less apparent. The hypocone is included in the postprotocingulum that makes contact with the metacingulum. The talon is well developed. The lingual cingulum is also well developed and the precingulum is short in length.
M2. Incomplete tooth with well-developed protocone and hypocone. The posterior opening of the protofossa is wider than in the M1. The hypocone is included in the postprotocingulum, which is connected to the postprotocrista. The paraloph and the metaloph are small in size. The talon is well developed. The lingual cingulum is present but more poorly developed than in the M1; a short precingulum is present.
3.10. Description of the tentatively referred material
c. Subrectangular outline in occlusal view. The cingulid is continuous around the tooth, although less developed on the lingual side and protruding on the anterolabial part of the tooth. The cusp is straight and displays a distinct crest on its anterior side. A strong triangular root is preserved.
p3. A large subtriangular tooth, whose main cusp has three sides with a well-developed cingulum that bears a cuspule on its posterolingual margin.
p4. A subtriangular tooth with a well-developed cingulid, elevated anteriorly with the lingual side being wider than the labial side. As for the p3, the cingulid protrudes on the posterolingual side, forming a small cusp.
I. Tooth elongated with two cusps; the anterior cusp is better developed than the posterior cusp; the cingulum is well developed, except on the anterolingual side where it is absent.
C. A large tooth, subtriangular in occlusal view. A well-developed continuous cingulum is present around the tooth; the mesio-lingual part of the cingulum thickens and protrudes. A single cusp is present, in which a postero-lingual crest that runs from the tip to the base of the crown is clearly observable.
3.11. Discussion
The genus Chaerephon is the only molossid in which the protofossa may be either closed or open in the M1–2, depending on whether or not the postprotocrista reaches the metacingulum (Legendre Reference Legendre1984a). In contrast, in Mops and Hydromops, the postprotocrista is always in contact with the hypocone (Legendre Reference Legendre1984a, Reference Legendreb). In our material, the two morphotypes are observed: the M1 has a closed protofossa and the paraloph is better developed, while the M2 has an open protofossa and the paraloph is less apparent. In both molars, the metaloph is absent.
Chaerephon is currently distributed in Asia, Africa, and Australia (Hand Reference Hand1990), and, according to Eiting & Gunnell (Reference Eiting and Gunnell2009), no fossil specimen exceeding 12,000 years has yet been found. As a result, the teeth excavated at Mas d'Antolino B 5 and 11 are the first pre-Quaternary remains of Chaerephon described in the fossil record. For this reason, the genus can be considered thus far to be a stratigraphic ‘Lazarus taxon’ (Fara Reference Fara2001) due to the absence of records from the early Miocene to the early Holocene.
Genus Tadarida Rafinesque, 1814
Tadarida sp.
(Fig. 3j)
2017 Mormopterus (Hydromops) cf. helveticus (in part) Crespo, Reference Crespo2017.
Locality. MTR2.
Material. 1 M2 (MTR2-34 (MGUV-38781) and MTR2-37 (MGUV-38784)).
3.12. Description
M2. Two fragments of a tooth; one of them has a right-angled parastyle and a tall paracone. The other fragment shows a well-developed protocone and a small hypocone joined by a short postprotocingulum. The postprotocrista connects to the metacingulum and closes the protofossa. The paraloph is small. The hypocone has a small posterolingual cingulum that is unconnected to the metacingulum.
3.13. Discussion
The species of this genus are characterised by a closed protofossa and a well-developed hypocone isolated from the protocone, as well as by a short postprotocingulum with a well-developed talon (Legendre Reference Legendre1984a).
Tadarida is represented by two lineages: among the New World species, the paraloph and the metaloph converge at the lingual side, while the species of the Old World are characterised by a weak paraloph and the absence of a metaloph (Legendre Reference Legendre1984a). Additionally, Legendre (Reference Legendre1984a) and Arroyo-Cabrales et al. (Reference Arroyo-Cabrales, Gregorin, Schlitter and Walker2002) indicated that in certain African species, such as Tadarida lobata (Thomas, 1891) and particularly in Tadarida fulminans (Thomas, 1903) and Tadarida ventralis (Heuglin, 1861), the hypocone is smaller than in the other species of the genus.
Some features of the hypocone complex observed in our material, such as a small, low hypocone with a short postprotocingulum and a poorly developed talon, are also characteristic of the African species of Tadarida. For this reason, we suggest that this material belongs to an indeterminate species of the African lineage of the genus Tadarida. However, the near-contemporary African species Tadarida rusingae Arroyo-Cabrales, Gregorin, Schlitter & Walker, Reference Arroyo-Cabrales, Gregorin, Schlitter and Walker2002 shows a more developed hypocone and postprotocrista (Arroyo-Cabrales et al. Reference Arroyo-Cabrales, Gregorin, Schlitter and Walker2002).
Family Vespertilionidae Gray, 1821
Genus Submyotodon Ziegler, Reference Ziegler2003
Submyotodon sp.
(Fig. 4a–d)
Localities. BC1, MAB3, MAB5, and MAB11.
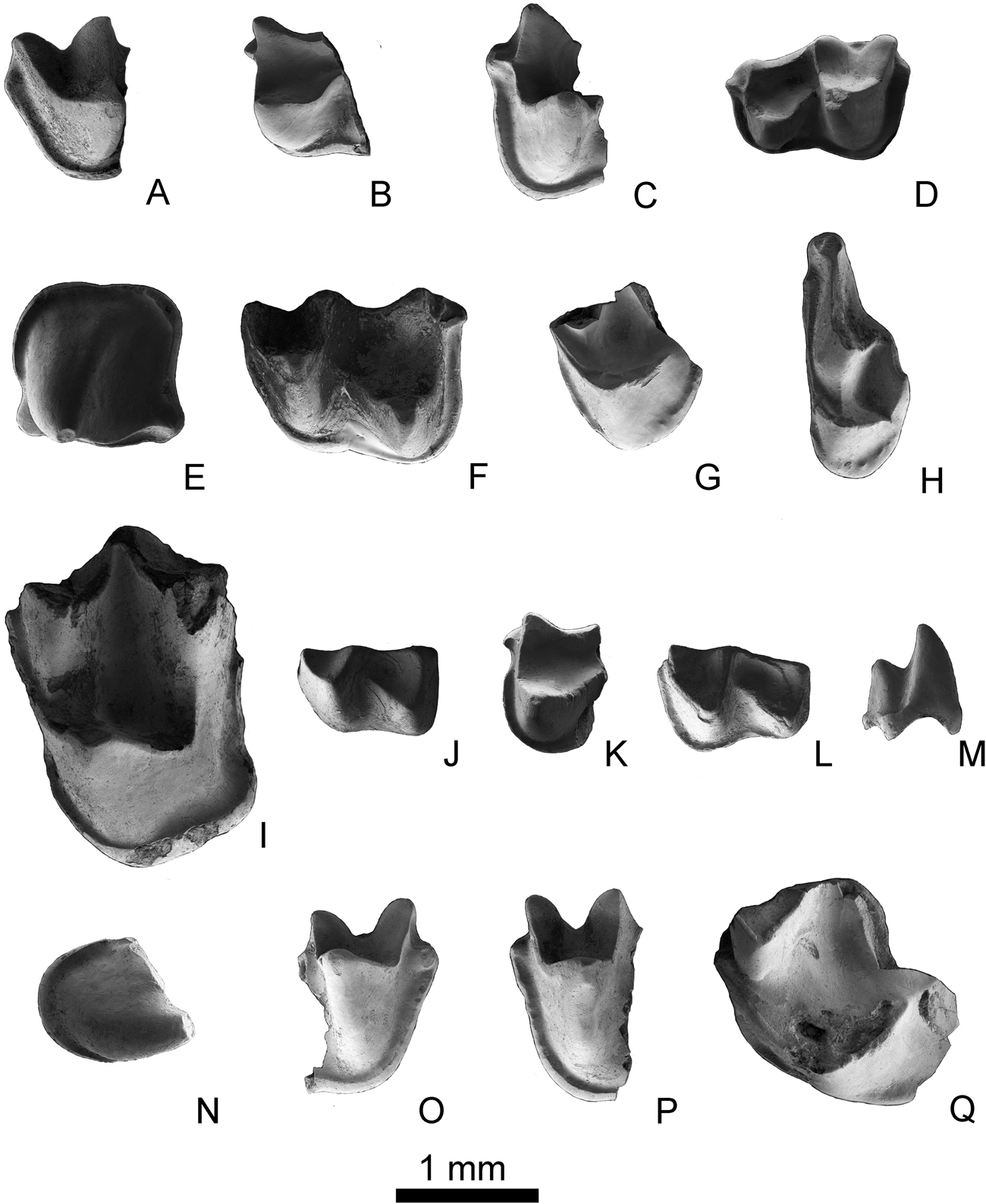
Figure 4 Non-molossid teeth from the Ribesalbes-Alcora Basin. Submyotodon sp.: (A) left m1/2 (MAB3-737); (B) right m1/2 (MAB3-750); (C) right m1/2 (MAB11-117); (D) right m3 (MAB3-725). Myotis cf. intermedius: (E) left p4 (MAB3-817); (F) left m1/2 (MAB3-752); (G) left M1/2 (MAB3-719); (H) left M3 (MAB3-712). Miostrellus aff. Petersbuchensis: (I) left M1 (CBR1-38). Plecotus sp.: (J) left m1/2 (MAB5-891); (K) right m1/2 (MAB5-381); (L) right m1/2 (MAB5-438); (M) I (MAB5-774); (N) C (MAB5-149). Rhinolophus sp.: (O) right m1/2 (MTR2-196); (P) left m1/2 (MAB3-741); (Q) left M1 (MAB3-708). Scale bar = 1 mm.
Material and measurements. 5 m1 or m2 (MAB3-737(MGUV-37745): -x 1.26, MAB3-750 (MGUV-37758), MAB3-754 (MGUV-37762): -x1.22, MAB5-747 (MGUV-38170): -x1.32, MAB11-117(MGUV-38504)); 1 m3 (MAB3-725 (MGUV-37733): 1.33 × 1.02). Doubtful remains: 1 c (BC1-169 (MGUV-26249); 0.85 × 1.03); 2 p2 (MAB5-430 (MGUV-25220): 0.84 × 0.91; MAB5-773 (MGUV-38196): 0.82 × 0.84); 1 P2/P3 (MAB5-908 (MGUV-38331): 0.59 × 0.60).
3.14. Description
m1 or m2. Submyotodont, medium-sized teeth. The paralophid is not sharp (it has a U-shape in labial view). The trigonid valley is wide and open lingually. The paraconid and the metaconid are similar in size and both are arranged in line with the dental axis. The protoconid is straight and constitutes the tallest cusp. The labial cingulum is well developed. In both the m1 and m2, the cristid obliqua ends in the middle of the tooth. The entocristid is concave and well developed. The labial valley of the talonid, entoconid, and hypoconid are well developed, the latter cusp being the highest on the talonid. The hypoconulid is poorly developed and the labial cingulid is wide in occlusal view and straight in lateral view.
m3. Submyotodont and medium-sized tooth. In occlusal view, the paraconid and the metaconid are parallel with the dental axis. The trigonid basin is large and open, and the paraconid stands wide apart from the metaconid. The paralophid is not sharp (it is a U-shape in labial view). The protoconid is the highest cusp of the trigonid. The metaconid and the paraconid are of similar height. Due to the reduction of the entoconid, the talonid is as narrow as the trigonid. The entocristid is well developed and straight. The hypoconulid, hypoconid, and entoconid are also well developed. The anterior end of the cristid obliqua terminates at the middle of the tooth. The labial valley of the talonid is well developed. The labial cingulum is wide and straight in lateral view and thickened anteriorly.
3.15. Description of the tentatively referred material
c. A broken tooth lacking the top of the cusp, sub-triangular in occlusal view. The cingulid is well developed and surrounds the tooth. The main cusp has two sections of thickening in the posterior part of the tooth that confer a subtriangular appearance. The root is longer than it is wide, and is laterally flattened.
p2. Tooth of subquadrangular outline with one main cusp that shows three faces. The cingulum is present and wide posteriorly, but absent anteriorly. Lingually, the cingulid is well developed. It is elevated anterolingually and displays a protrusion posterolingually.
P2/P3. A round small tooth with a rounded cusp and a well-developed cingulum with a small posterior expansion.
3.16. Discussion
Among the bat remains recovered in the Ribesalbes-Alcora Basin, the submyotodont lower molars are of particular interest. Vespertilionidae typically have nyctalodont lower molars, except for some species of the genera Pipistrellus and Lasiurus and all species of the genera Myotis, Miostrellus, Paleptesicus, Plecotus, and Submyotodon; the condition of the postcristid is unknown in Samonycteris (Ziegler Reference Ziegler2003). Other Miocene bats with submyotodont teeth are the molossids Cheiromeles and Mormopterus (Legendre Reference Legendre1984a). In contrast to the Spanish fossils, Cheiromeles is characterised by a small paraconid (Legendre Reference Legendre1984a), while the Miocene Mormopterus species from Europe had lower molars showing myotodonty (Hand Reference Hand1990). On the other hand, to date, no submyotodont molossid bats have been reported in the Miocene of Europe. In terms of size, the material studied in the Ribesalbes-Alcora Basin is larger than Submyotodon petersbuchensis Ziegler, Reference Ziegler2003, the only species of this genus recognised during the Miocene (Ziegler Reference Ziegler2003; Rosina & Rummel Reference Rosina and Rummel2012).
Therefore, Submyotodon is thus far the only Miocene genus of Vespertilionidae with submyotodont lower molars. Although the m3 is always submyotodont, the m1 and m2 may be either submyotodont, nyctalodont, or myotodont (Ziegler Reference Ziegler2003; Rosina & Rummel Reference Rosina and Rummel2012). For this reason, it has been previously misplaced within the genera Myotis or Vespertilio. Recently, it has been identified among the extant faunas from Asia, represented by the extant species Submyotodon latirostris (Kishida, 1932) and Submyotodon caliginosus (Tomes, 1859), in which m1 and m2 have a nyctalodont pattern and a submyotodont m3 (Benda & Gaisler Reference Benda and Gaisler2015; Ruedi et al. Reference Ruedi, Csorba, Lin and Chou2015). According to molecular clocks, the appearance of this genus is dated between 23 and 18 Ma (Lack et al. Reference Lack, Roehrs, Stanley, Ruedi and Bussche2010; Ruedi et al. Reference Ruedi, Stadelmann, Gager, Douzery, Francis, Lin, Guillé- Servent and Cibois2013). This date is in agreement with the age proposed for the material of the Ribesalbes-Alcora Basin. For these reasons, this material has been assigned to an indeterminate species of Submyotodon.
Subfamily Vespertilioninae Gray, 1821
Tribe Myotini Tate, 1942
Genus Myotis Kaup, 1829
Myotis intermedius Ziegler, 2000
Myotis cf. intermedius
(Fig. 4e–h)
2017 Myotis cf. intermedius Crespo, Reference Crespo2017.
2017 Miniopterus sp. nov. (in part) Crespo, Reference Crespo2017.
Locality. MAB3
Material and measurements. 1 p4 (MAB3-817 (MGUV-37825): 1.21 × 1.24); 5 m1 or m2 (MAB3-733 (MGUV-37741), MAB3-747 (MGUV-37755), MAB3-752 (MGUV-37760): 1.59 × 1.22, MAB3-755 (MGUV-37763), MAB3-798 (MGUV-37806)); 4 M1 or M2 (MAB3-719 (MGUV-37727), MAB3-723 (MGUV-37731), MAB3-776 (MGUV-37784), MAB3-789 (MGUV-37797)); 1 M3 (MAB3-712 (MGUV-37720): 0.73 × 1.74). Doubtful remains: 1 c (MAB3-816 (MGUV-37824): 0.80 × 1.05); 2 p2/p3 (MAB3-782 (MGUV-37790): 0.79 × 0.89, MAB3-790 (MGUV-37798)).
3.17. Description
p4. Tooth with a quadrate outline and one main cusp located at the centre-lingual side of the crown. The cusp shows four faces; a crest runs down towards the postero-lingual side of the cusp. The postero- and anterolingual margin of the cingulid present a swelling at the base. The cingulid is well developed around the tooth. Two roots are preserved.
m1 or m2. Myotodont teeth with a trigonid narrower than the talonid. The paralophid is rounded. Although both the paraconid and the metaconid are low, the former is lower while the latter protrudes lingually. The trigonid valley is open lingually. The entocristid is straight. The entoconid and the hypoconid are relatively large but the latter is better developed. The hypoconulid is developed, and a cingulid start at its base. The labial cingulid is wide in the talonid, narrow in the trigonid, and straight and wide in lateral view.
M1 and M2. The teeth are fragmented. The parastyle is short and angular, the precingulum starts in the parastyle. The paraloph and the metaloph are present, but are low and short. The precingulum and the postcingulum are well developed. The preprotocrista is connected to the paracingulum. The postprotocrista is short and does not join the metacingulum. The hypocone is absent.
M3. The tooth is short and wide with a reduced posterior part. The parastyle is extended and ends with a curve. The paracingulum is narrow; it starts near the end of the parastyle and is connected to the protocone, which exhibits a small precingulum. The paracone is the largest cusp and is connected to a small metacone by the mesostyle. A small paraloph is present. The protocone is well developed, and shows a distinct labial cingulum. The postmetacrista is absent.
3.18. Description of the tentatively referred material
c. Subtriangular tooth in occlusal view with a rounded anterior part and is somewhat flattened posteriorly. The cingulid is absent on the labial side but well-developed towards the posterior. A distinct labial crest runs all along the main cusp.
p2/p3. Tooth with a C-shape outline and a single main cusp with three faces located in the centre of the crown. The cingulid is present and wide except on the anteriormost part of the tooth, where it is absent. On the posterolingual side, the cingulid has a cuspule. The main cusp has a crest directed towards the anterior part of the tooth.
3.19. Discussion
Myotis is one of the most common bat genera in the fossil record and has a wide stratigraphic range (Gunnell et al. Reference Gunnell, Smith and Smith2017). It is characterised by myotodont lower molars that have a medium to wide cingulid. The upper molars typically have no basined talon nor hypocone. The paraloph and the metaloph are occasionally present and the M3 is always distally reduced with only three lingual crests, without postmetacrista (Sevilla Reference Sevilla1988). All of these features appear in the studied material.
Both Myotis and the similar genus Leuconoe are highly diverse. The comparison with Myotis species enables us to assign the material described here to Myotis (aff.) murinoides since this species is characterised by a cuspulated cristid obliqua, a small crest between the hypoconulid and the entoconid, well-developed paraloph and metaloph, and the presence of a hypocone in the M1 (Ziegler Reference Ziegler1994; Sevilla Reference Sevilla2002). Myotis elegans Hall, 1962 differs by its well developed and advanced protocone (Sevilla Reference Sevilla2002). In Myotis boyeri Mein, 1964, the postprotocrista is connected to the distal cingulum (Sevilla Reference Sevilla2002). The fossils of Myotis aff. minor from Casetón are smaller than our material and show a less reduced M3 (Sevilla Reference Sevilla2002). Myotis minor Ziegler, Reference Ziegler2000 is smaller and the paraloph and metaloph are better developed. Myotis major Ziegler, Reference Ziegler2000 is slightly larger and the protocone is more rounded (Ziegler Reference Ziegler2000). Myotis korotkevichae Rosina & Semenov, Reference Rosina and Semenov2012 is characterised by a more developed metaloph and paraloph, and a less reduced M3 (Rosina & Semenov Reference Rosina and Semenov2012). Myotis bavaricus Ziegler, Reference Ziegler2003 shows an angular paraloph on the upper molars; the M3 is only moderately reduced and the upper molars lack both the paraloph and metaloph (Ziegler Reference Ziegler2003). Leuconoe antiquus (Gaillard 1899) shows a protolophid and a metalophid with a very low notch, and a premetacrista that is shorter than the postparacrista (Sevilla Reference Sevilla2002). Leuconoe sanctialbani (Viret, 1951) reveals conules in the lingual cingulum of the upper teeth (Baudelot Reference Baudelot1972; Ziegler Reference Ziegler2000).
The species closest to our material is Myotis intermedius from the upper Oligocene of Herrlingen 8 and 9, although these populations display a slightly smaller size. Although the diagnosis of this species indicates the absence of the paraloph and the metaloph, the presence of both small crests can be seen in the photographic images given by Ziegler (Reference Ziegler2000; pl. 6, figs 65, 66). Due to these reasons and the scarcity of the studied material, we have left it to allow open nomenclature and assign the material to Myotis cf. intermedius.
Tribe Vespertilionini Gray, 1821
Genus Miostrellus Rachl, 1983
Miostrellus petersbuchensis Rosina & Rummel, Reference Rosina and Rummel2012
Miostrellus aff. Petersbuchensis
(Fig. 4i)
2017 Miostrellus cf. petersbuchensis Crespo, Reference Crespo2017.
Locality. CBR1.
Material. 1 M1 (CBR1-38; MGUV-36794).
3.20. Description
M1. Broken tooth without parastyle and metastyle. The postparacrista and premetacrista are connected by a well-developed mesostyle. The paracingulum is directly connected to the preprotocrista without a paraconule. The paraloph and metaloph are absent. The precingulum starts at the anterolabial side of the protocone, joins the postcingulum, and reaches the metacingulum; the posterior side of the lingual cingulum is slightly wider than the anterior. The postprotocrista is connected to the metacone, thus forming a V-shape in lateral view. The protofossa is closed and deep.
3.21. Discussion
Miostrellus is characterised with respect to upper molars without paraloph and metaloph, and an almost complete postprotocrista (Horáček Reference Horáček2001). These features appear in the specimen described here, although the postprotocrista is complete and is connected to the metacone.
Regarding the specific ascription, we can discard: (1) Miostrellus risgoviensis Rachl, 1983, which shows a more extended posterior part of the protocone; (2) Miostrellus egerensis Horáček, Reference Horáček2001, which has a postprotocrista that does not reach the metacone; and (3) Miostrellus noctuloides (Lartet, 1851), which presents conules and lophs (Rosina & Rummel Reference Rosina and Rummel2012). The most similar species is Miostrellus petersbuchensis, although the junction of the postprotocrista and metacone is weaker than in the tooth described here.
Tribe Plecotini Gray, 1866
Genus Plecotus Geoffroy Saint-Hilaire, 1813
Plecotus sp.
(Fig. 4j–n)
Localities. BC1, MAB3, and MAB5.
Material and measurements. 11 m1 or m2 (BC1-19 (MGUV-25334): -x1.23, MAB3-738 (MGUV-37746): -x1.37, MAB3-749 (MGUV-37757), MAB3-753 (MGUV-37761), MAB3-754 (MGUV-37762): -x1.22, MAB5-381 (MGUV-25171), MAB5-417 (MGUV-25207), MAB5-420 (MGUV-25210), MAB5-438 (MGUV-25228), MAB5-745 (MGUV-38168), MAB5-748 (MGUV-38171), MAB5-891 (MGUV-38314)); 1 m3 (MAB5-438 (MGUV-25228): 1.17 × 0.83), 1I (MAB5-774 (MGUV-38197): 0.73 × 0.41), 2 C (MAB5-149 (MGUV-24939): 0.87 × 0.94; MAB5-432 (MGUV-25222): 0.91 × 0.87). Doubtful remains: 1 p2/p3 (MAB5-771; MGUV-38194).
3.22. Description
m1 or m2. Broken small-sized teeth with a curved paralophid. The paraconid is similar in size to the metaconid and both are aligned with the entoconid. The protoconid is the highest cusp. The labial cingulid is narrow and well developed. The obliqua cristid starts mid-width of the tooth. The lingual cingulid is well developed with regular thickness in lateral view. The talonid is myotodont and the entocristid is concave lingually. The talonid basin, entoconid, and hypoconid are large and distinct, whereas the hypoconulid is poorly developed.
m3. The tooth is myotodont and small in size. The line formed by the paraconid and the metaconid is parallel to the dental axis and similarly sized. The paralophid is curved. The protoconid is the tallest cusp of the trigonid. The talonid is narrower than the trigonid, although the entoconid is reduced. The entocristid is well developed and straight. The obliqua cristid joins the trigonid at the middle of the metalophid. The hypoconid and the lingual valley of the talonid are well developed. The labial cingulid is wide and straight in lateral view.
I. Teeth with an elliptical outline, with the main cuspid divided into two subcuspids, one of which is distinctly less developed. The cingulum is complete on the labial side and absent on the lingual side; posteriorly, there is a small cuspule.
C. A long tooth with two well-developed cusps and a distinct cingulum on the labial side; there is a small basin on the lingual side.
3.23. Description of the tentatively referred material
p2/p3. A fragment of tooth with a well-developed cingulid, wider posteriorly, and protruding posterolingually.
3.24. Discussion
Species of the Plecotus are characterised by myotodont lower molars with a wide cingulid and reduced m3 (Sevilla Reference Sevilla1988), as well as bicuspid upper incisors and small canines (Menu & Popelard Reference Menu and Popelard1987). The material from the Ribesalbes-Alcora Basin fits with this description; however, the diagnostic material is insufficient for a specific attribution. The Miocene fossil record of Plecotus is limited, the oldest species being Plecotus schoepfelii Rosina & Rummel, Reference Rosina and Rummel2012 from the early Miocene of Petersbuch 28 (Rosina & Rummel Reference Rosina and Rummel2012). The late Miocene record has yielded Plecotus atavus Topál, Reference Topál1989 in Polgárdi 4 (Topál Reference Topál1989) and Kohfidish (Storch Reference Storch, Rössner and Heissig1999), and a similar taxon, Plecotus aff. atavus, from the middle Miocene of Petersbuch 6 and upper Miocene of Gritsev (Ziegler Reference Ziegler2003; Rosina et al. Reference Rosina, Kruskop and Semenov2019). The indeterminate species of Plecotus from Ribesalbes-Alcora Basin is likely an ancestral form pre-dating the first major split of the lineage that took place in the middle Miocene according to genetic studies (Spitzenberger et al. Reference Spitzenberger, Strelkov, Winkler and Haring2006) and made way for to the different extant species currently distributed through Europe.
Suborder Yinpterochiroptera Koopman, 1984
Family Rhinolophidae Gray, 1825
Genus Rhinolophus Lacépède, 1799
Rhinolophus sp.
(Fig. 4o–q)
Localities. MTR2 and MAB3
Material and measurements. 7 m1 or m2 (MTR2-192 (MGUV-38939), MTR2-196 (MGUV-38943), MTR2-201 (MGUV-38948), MAB3-741 (MGUV-37749): -x1.50, MAB3-743 (MGUV-37751): -x1.64; MAB3-744 (MGUV-37752): -x1.60; MAB3-760 (MGUV-37768)), 1 M1 (MAB3-708; MGUV-37716), 1 M1/2 (MTR2-202; MGUV-38949) Doubtful remains: 1 i3 (MAB3-800 (MGUV-37808): 0.77 × 0.59), 1 c (MAB3-25 (MGUV-25281): 0.59 × 0.74), 1 P4 (MAB3-793; MGUV-37801).
3.25. Description
m1 or m2. The teeth are broken. The paralophid is curved and the trigonid basin is open. The paraconid is lower than the metaconid and slightly protruding. The protoconid is the most developed cusp. The lingual cingulid is wide and well developed; on its anterior, there is a small cusp that is most developed in specimen MTR2.
M1. A fragment of a large tooth. It has a wide paracingulum without paraconule, which is connected to the protocone. The precingulum is small. The postprotocrista is well developed. The talon is broken and the protofossa is wide and deep.
3.26. Description of the tentatively referred material
i3. A single tooth with a subtrapezoidal occlusal outline. It has four cusps, three of which are in line, the central one being larger than the other two. The valleys between cusps are wide and deep. There is an additional shallow valley between the cingulid and the cusps.
c. A single tooth with a subtriangular occlusal outline. The cingulid is continuous, but poorly developed on both the lingual and labial sides. The main cusp is straight and has a crest on the anterior face that runs from the tip of the crown to the base. The root is triangular in section, flattened posteriorly.
P4. A fragmented tooth; the talon and anterior side are missing. The paracone is well developed and cone-shaped; the postparacrista is directed towards the cusp and ends in a curved metastyle. The preserved anterior part of the lingual cingulum is wide and is directed towards the talon.
3.27. Discussion
Rhinolophus material is common in Neogene fossil assemblages and easily identified due to its characteristic morphology. Although the fossil material in Ribesalbes-Alcora Basin is scarce, Rhinolophus material has been reported at a nearby locality of Buñol (Robles et al. Reference Robles, Belinchón, García-Flor and Morales1991). The morphology of the upper molars with an expanded talon and without hypocone is present in two common genera of the Neogene in Europe, Rhinolophus and Hipposideros (Sevilla Reference Sevilla1988). Although similar at first sight, there are differences that enable us to distinguish both genera in fossil material. For instance, whereas in Hipposideros collongensis (Depéret, 1892) the postprotocrista runs towards the metacone in the upper molars, in Rhinolophus, including the material described here, it runs posteriorly (Sevilla Reference Sevilla1988; Ziegler Reference Ziegler2003; Rosina & Rummel Reference Rosina and Rummel2012) and lacks a precingulum (Sevilla Reference Sevilla1990; Álvarez-Sierra et al. Reference Álvarez-Sierra, García-Paredes, Hoek Ostende, Meulen, Peláez-Campomanes and Sevilla2006). Although in modern Rhinolophus species the cingulid in the lower molars tend to be straight and narrow (Sevilla Reference Sevilla1988), some Miocene species display a thicker cingulid, such as in Rhinolophus grivensis (Depéret 1892) from Petersbuch 6 or Rhinolophus lemanensis Revilliod, 1920 from Petersbuch 62 (Ziegler Reference Ziegler2003; Rosina & Rummel Reference Rosina and Rummel2012), a feature that is also observed in the material described here.
As such, the material under study clearly belongs to a large species of the genus. These remains are larger than R. lemanensis, which is the largest species in Petersbuch (Ziegler Reference Ziegler2003; Rosina & Rummel Reference Rosina and Rummel2012), or R. grivensis and Rhinolophus delphinensis Gaillard, 1899 from Escobosa de Calatañazor (Spain; Sesé Reference Sesé1986). Our material is similar in size to the extant species Rhinolophus ferrumequinum (Schreber, 1774) and Rhinolophus macrorhinus Topál, 1967, and even to the Plio–Pleistocene species Rhinolophus postdelphinensis Topál, 1979 (Sevilla Reference Sevilla1988).
Therefore, we can assume that this material comes from perhaps one of the largest Rhinolophus species of the Miocene; however, the scarcity of the material precludes any specific classification.
4. Discussion
4.1. Palaeoecological and palaeobiogeographic considerations
Considering the fact that bat remains are uncommon in fluvio-lacustrine fossil sites, it can be said that they are extraordinarily frequent in the Ribesalbes-Alcora Basin. They constitute 6 % of the small mammal assemblages in MAB11 and around 2–4 % of the other sites (all sites with more than 100 specimens of small mammals recovered), whereas they usually account for only 0.01 % of the small mammal fossils in fluvio-lacustrine sites (Sigé & Legendre Reference Sigé and Legendre1983; Sevilla Reference Sevilla2002). The discovery of fluvio-lacustrine sites containing fossil bats is interesting because the assemblages in this type of site typically contain taxa that are either absent or uncommon in karstic localities, thus providing important data to obtain a clearer picture of the past diversity of Chiroptera (Sigé & Legendre Reference Sigé and Legendre1983; Maitre Reference Maitre2014).
One of the particularities of the Araia assemblage is the high abundance and diversity of typically rare molossids. The combination of a high number of species and a low number of individuals is common in present day Neotropical, Ethiopian, and Indo-Australian molossid faunas (Freeman Reference Freeman1981b). This family includes high, fast, and direct fliers, which likely have few competitors (Freeman Reference Freeman1981b; Fenton Reference Fenton1983). The ability for similar and related species to live in syntopy is probably favoured by their different habitat preferences, and feeding and roosting strategies; however, generally this family tends to avoid thickly forested habitats or subcanopy areas since they are fast flyers specialised in insect hawking (Freeman Reference Freeman1981b; Hill & Smith Reference Hill and Smith1984; Norberg & Rayner Reference Norberg and Rayner1987; Hand Reference Hand1990). The highly diverse molossid assemblages consisting of five species suggests tropical climatic conditions (Freeman Reference Freeman1981b) for the Ribesalbes-Alcora paleolake and the surrounding area. The diversity is higher in sites starting from the end of the local biozone C (end of early Miocene), with four species, than in those from the lower part of the local biozone C, with two species. The relatively cool climatic event Mi-2, which was likely recorded in site MAB5 (Ríos Reference Ríos2013; Crespo Reference Crespo2017), does not seem to affect the diversity of molossids.
The genus Myotis currently shows a widespread distribution, which suggests that its fossil species might have lived in many different habitats (Norberg & Rayner Reference Norberg and Rayner1987; Ziegler Reference Ziegler2000). The genus Plecotus can roost both in caves and trees, always in small groups; it is a slow surface hunter in thick woods (Norberg & Rayner Reference Norberg and Rayner1987; Sevilla Reference Sevilla1988). The genus Submyotodon occurs nowadays in southeastern Asia and is commonly misidentified as Myotis. Both genera might share similar modes of life, hunting small flying insects, drinking directly from the surface of the waters where insects tend to trawl (Richardson Reference Richardson1993; Bonaccorso Reference Bonaccorso1998; Benda & Gaisler Reference Benda and Gaisler2015). The extant rhinolophids are more diverse in tropical to subtropical regions, but are also found in temperate areas of Europe; they can roost in caves or forests (Sevilla Reference Sevilla1988, Reference Sevilla1990) and are specialised in hunting flying insects and occasionally stationary prey on foliage or the ground (Norberg & Rayner Reference Norberg and Rayner1987). Myotis, Plecotus, and Rhinolophus are common taxa in the European fossil record and are consistent with the ecological interpretation inferred for the sites being studied, since all are either generalised in their ecological requirements or are dependent on the presence of tree cover or bodies of water. According to Crespo (Reference Crespo2017), these were the conditions in the area at the time during the sites were formed.
Studies of habitat use demonstrate that bats spend significant time near water bodies (Walsh & Harris Reference Walsh and Harris1996; Vaughan et al. Reference Vaughan, Jones and Harris1997). It is likely that bats benefit from shallow warm waters with macrophytes since these environments provide abundant insects (Zimmer et al. Reference Zimmer, Hanson and Butler2000). The fossil record of bats associated with lacustrine sites where a high diversity of species is observed may be related to the favourable conditions provided by these water bodies, in which different ecological niches sustained a high level of diversity and abundant resources. As such, for bats, these environments ensured a variety of prey consisting of both insects and fish, water to drink, and places to roost. The remains of the animals that died either due to predation or natural causes and had ended up in the lake were preserved with other vertebrate remains as a result of the fast burial that ensured the preservation of their delicate bones and teeth. Therefore, the presence of a paleolake in the Ribesalbes-Alcora Basin may have played an important role in the presence of bats in its fossil assemblages.
Compared to other sites of similar age from central and southern Europe, the bats from the Ribesalbes-Alcora Basin show interesting and unusual differences, for instance, with those from Petersbuch 28 and 62, Wintershof-West, Cremat, Bouzigues, Vieux-Collonges, and Merkur-North, among others (Sigé & Legendre Reference Sigé and Legendre1983; Sigé et al. Reference Sigé, Aguilar, Matandat and Astruc1991; Ziegler Reference Ziegler1993; Horáček Reference Horáček2001; Rosina & Rummel Reference Rosina and Rummel2012; Fortelius Reference Fortelius2016). The main differences are found in the relatively high abundance of molossids, while non-molossid families appear to be less common in the material described here than in central and other southern European localities. The fossil record of molossids in Africa, which starts in the early Miocene, is also rather scarce (Arroyo-Cabrales et al. Reference Arroyo-Cabrales, Gregorin, Schlitter and Walker2002). This particular feature of the Ribesalbes-Alcora Basin assemblages cannot be a consequence of the type of sedimentary environment, since non-molossids in the Miocene are predominant both in karstic and fluvio-lacustrine sites. Furthermore, certain molossids are cave dwellers (Freeman Reference Freeman1981b) and might be expected to be better represented in karstic sites. However, molossids are not rare in the early Oligocene sites of western Europe (Maitre Reference Maitre2014), but seem to have undergone a regression in central and western Europe from the late Oligocene onwards (Sevilla Reference Sevilla1990; Ziegler Reference Ziegler2000), thus becoming increasingly less common. Other fossil assemblages with bats from the middle Miocene of the Iberian Peninsula differ from the material described here in their proximity to modern faunas in taxonomic representation with a predominance of rhinolophids and vespertilionids (Sesé Reference Sesé1986; Sevilla Reference Sevilla2002). The particularity of the assemblages in the Ribesalbes-Alcora Basin could be explained by the presence of tropical forests in the area, which would favour a higher proportion of molossids in the assemblages compared to the dominance of taxa linked to more arid environments represented in other localities. Highly diverse molossid communities are seen today in tropical Neotropical regions (Freeman Reference Freeman1981b). This interpretation is supported by the fact that in the Ribesalbes-Alcora Basin, molossids are most abundant and diverse in sites known to correspond to densely forested areas according to the associated fauna, such as eomyids, beavers, and some kinds of dormouse and insectivores, although they are scarce elsewhere (Crespo Reference Crespo2017; Crespo et al. Reference Crespo, Fagoaga, Montoya and Ruiz-Sánchez2019a). The development of open forests and grassland habitats during the late early Miocene and the middle Miocene at the expense of pre-existing dense forest areas (Barrón et al. Reference Barrón, Rivas-Carballo, Postigo-Mijarra, Alcalde-Olivares, Vieira, Castro, Pais and Valle-Hernández2010) provoked a dramatic change in these early Miocene bat assemblages, in which molossids were well represented, resulting in faunas more similar to recent ones where they are practically non-existent.
5. Conclusions
The order Chiroptera is one of the least abundant mammal groups in the Ribesalbes-Alcora Basin in the early Miocene record. Nevertheless, ten different taxa – one of which is a new species – have been identified in the localities studied. This constitutes, thus far, the first and largest collection of fossil bats from the early Miocene of the Iberian Peninsula.
The ten taxa identified in the sites from the Ribesalbes-Alcora Basin are Cuvierimops penalveri sp. nov., Hydromops helveticus, Rhizomops cf. brasiliensis, Chaerephon sp., Tadarida sp., Submyotodon sp., Myotis cf. intermedius, Miostrellus aff. petersbuchensis, Plecotus sp., and Rhinolophus sp. The genera Cuvierimops, Rhizomops, and the Lazarus genus Chaerephon are reported globally for the first time in the early Miocene.
The richness of molossids recorded in this material reveals the high diversity attained by this group in the Miocene of Europe, which had been largely unrecognised as a result of such fossils being typically underrepresented in the Neogene fluvio-lacustrine fossil record. The abundance of these bats in the Ribesalbes-Alcora Basin is consistent with the presence of a tropical forest surrounding a paleolake, as suggested also by the presence of other mammal taxa such as eomyids, certain types of dormouse, and insectivores.
6. Acknowledgements
The survey and excavation campaigns in the area of Araia d'Alcora were funded by the Conselleria de Cultura i Esports of the Generalitat Valenciana from 2008 to 2011, by projects 2008/0433-CS, 2010/0528-CS, 2011/0230-CS, GV06/304, and GVPRE/2008/320. This research was also supported by the Spanish Ministerio de Ciencia, Innovación y Universidades PGC2018-094122-B100 (AEI/ FEDER, UE). Thanks are also due to the helpful comments of T. Brock, J. Guillem, N. J. Czaplewski, and two anonymous reviewers on the original manuscript. V. D. C. is beneficiary of a postdoctoral fellowship from the Argentinian Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).









