Published online by Cambridge University Press: 22 March 2006
Spina bifida myelomeningocele (SBM), a neural tube defect that is the product of a complex pattern of gene-environment interactions, is associated with naturally occurring, systematic variability in the neural phenotype and in environmental factors that lead to systematic variability in the cognitive phenotype. We characterize the basis for variability in the cognitive phenotype of children with SBM with reference to a model of key biological, cognitive, and environmental events unfolding over the course of development from infancy to middle age. The cognitive phenotype is not domain-specific, but represents manifestations of unobservable constructs involving associative and assembled processing, the latter directly reflecting the impact of the neural phenotype on core deficits involving movement, timing, and attention orienting. The expression of the cognitive phenotype is variable, being moderated by features of the neural phenotype involving secondary CNS insults (such as hydrocephalus) that impair assembled processing, as well as by environmental factors (such as poverty, parenting, and education) that impair associative processing. The preservation of strengths in associative processing depends in part on the severity of the CNS deficits in SBM and the impact of the environment. (JINS, 2006, 12, 285–296.)
Spina bifida myelomeningocele (SBM), a neural tube defect that is the product of a complex pattern of gene-environment interactions, is associated with distinctive physical, neural, and cognitive phenotypes (Fletcher et al., 2004). It is a common, severely disabling birth defect, with current prevalence levels in North America of 0.3–0.5 per 1,000 births (post-dietary fortification data, Williams et al., 2005). Unlike other neurogenetic disorders that affect cognition, such as Williams Syndrome or Fragile X Syndrome, SBM has been the topic of relatively little neurocognitive investigation.
While genetic factors are involved in SBM (see Kirkpatrick & Northrup, 2003), the focus of this article is on its cognitive and neural phenotypes. As a group, children with SBM show physical limitations that are usually not accompanied by global mental retardation, and a distinctive cognitive phenotype, expressed both as variations across content domains (e.g., higher Verbal IQ than Performance IQ, Dennis et al., 1981; Fletcher et al., 1992; Soare & Raimondi, 1977), better reading than mathematics (Barnes & Dennis, 1992; Barnes et al., 2002), and variations within content domains (e.g., facility with grammar and vocabulary, but poor pragmatic language; Dennis et al., 1994; Barnes & Dennis, 1998). SBM involves naturally occurring, systematic variability in the genotype, the neural phenotype, and the environment, which is related to systematic variability and individual differences in the cognitive phenotype.
In this article, we present a detailed model of the biological, cognitive, and environmental events relevant to the cognitive phenotype of SBM and to variability in its expression (see Figure 1). The model is composed of biological, cognitive, and environmental components and three types of functional relations connecting the components:
The model has these tenets:
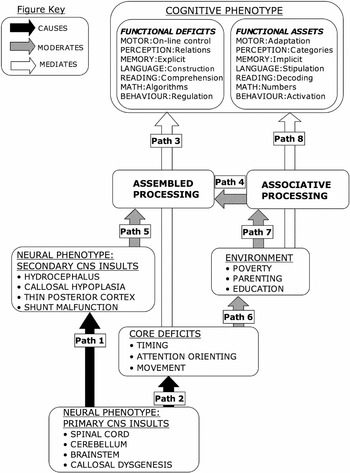
Model of outcome in spina bifida meningomyelocele showing how the cognitive phenotype is related to associative and assembled processing modes, core deficits, environmental influences, and primary and secondary CNS insults of the neural phenotype.
In the following sections, we describe the various components of the model.
The primary CNS insults in SBM (Box, Figure 1) affect both ends of the neural tube. The defining spinal lesion is evident from the first weeks of gestation. It is most commonly a meningomyelocele requiring neurosurgical repair shortly after birth, with subsequent major orthopedic and urologic impairments, including paraplegia of the lower limbs and neurogenic bladder and bowel function (Charney, 1992).
The most common congenital brain anomaly in SBM is the Chiari II malformation (Barkovich, 2000), a deformity of the brainstem and cerebellum that occurs in virtually all births involving meningomyelocele. The Chiari II malformation includes a small posterior fossa, which results in distortion of the posterior fossa contents and their herniation through the tentorial incisure and foramen magnum. The result is a mechanical block to the flow of cerebrospinal fluid (CSF), which in turn leads to hydrocephalus that almost always requires diversionary shunting (Charney, 1992). More than half of children with SBM show partial dysgenesis of the corpus callosum rostrum and/or splenium, which represents congenital disruption of neuroembryogenesis during the time period (7–20 weeks post gestation) of corpus callosum development (Barkovich, 2000; Hannay, 2000). Many individuals with SBM have abnormalities of the brainstem, such as beaking of the tectum, that are related to brain development in a small posterior fossa.
Secondary CNS anomalies occur in response to the primary abnormalities and hydrocephalus associated with the Chiari II malformation. These include hypoplasia (thinning) of the middle or all of the corpus callosum and selective thinning of the posterior cortex. Difficulties regulating CSF because of shunt malfunctions and infections, or other complications associated with SBM (e.g., seizures), produce further secondary brain injury. Although each may be identified before birth, the primary CNS malformations precede and cause the secondary brain insults (e.g., hydrocephalus is a direct result of the Chiari II malformation), so Path 1 in Figure 1 is causal.
The model stipulates that the primary CNS insults lead directly to a set of core deficits (Box, Figure 1) that interfere with cognitive and motor development. We define a core deficit as an impairment that:
On the basis of these criteria, we have identified three core deficits in SBM: movement, timing, and attention orienting.
Movement is a core deficit in children with SBM. It is apparent at birth, and infants with SBM have poorer upper and lower limb movement quality than peers and are slower than peers to learn to move their arm to activate a mobile (Fletcher et al., 2004). Children with SBM have deficits in three effectors: upper and lower limbs (Hetherington & Dennis, 1999; Dennis et al., 2002) and eyes (Biglan, 1995). Movement variability is related to the integrity of brain regions such as the cerebellum that control truncal and axial movement (Miall et al., 2001), and the cerebellum and midbrain that control eye movements (Leigh & Zee, 1999). Variability in movement is related to both spinal cord and brain, such that the higher the spinal lesion, the more severe the motor deficit (Lonton, 1977) and the more severe the brain impairments in the posterior fossa and midbrain (Fletcher et al., 2005). The movement deficits in children with SBM are similar to those in adults with lesions in the brain areas controlling movement.
Sensorimotor experience creates and reinforces internal representations in the cerebellum, which are sensory-motor states that predict the consequences of motor plans or control the motor plans needed for a desired sensory outcome (Iacoboni, 2001). Children with SBM are less able than peers to use motor exploration to discover appropriate ways of responding (Berthier et al., 2005) and thereby fail to capitalize on existing representations or to create new ones. Their movement deficits have significant implications for ecological function because they limit environmental search and exploration normally effected by movement, and thereby truncate input into motor control systems that facilitate learning from the environment.
Timing is a core deficit in children with SBM that is also closely linked with cerebellar abnormalities. Timing deficits are evident over a wide age span, are specific to timing operations, vary with the status of key brain structures, and are similar to those reported in adults with cerebellar lesions.
Throughout childhood and adult life, timing mechanisms are involved in motor control and movement coordination (Mauk et al., 2000; Ivry & Richardson, 2002) and synchrony between sensation and movement is facilitated by short duration timing mechanisms. Compared to typically developing age peers, infants with SBM show less synchronous and more poorly organized upper limb coordination (Fletcher et al., 2004). Children and adolescents with SBM have deficits in short-duration timing (perceiving intervals around a half second) and in motor timing (Dennis et al., 2004). Children with SBM show significantly reduced cerebellar volumes on quantitative MRI studies that are correlated with perceptual and motor timing deficits (Dennis et al., 2004). Deficits in short-duration timing are apparent over the life span in individuals with a variety of cerebellar lesions (Ivry & Keele, 1989; Mostofsky et al., 2000, 2004), so the cerebellar timing system shows little or no age-based change.
The ecological importance of timing deficits is considerable. Temporal information helps generate predictions about the durations of different perceptual events (Ivry & Richardson, 2002) and the sensory consequences of movement (Rao et al., 2001). Early motor development (including aspects of timing) helps to shape cognitive development (Thelen & Smith, 1994), and early deficits in motor synchrony are negatively related to later cognitive search and visual memory abilities in infants with SBM (Landry et al., 2003). Many eye-hand tasks require the constant modulation of motor timing. For example, throwing a ball requires that the opening of the fingers to release the ball must occur within a narrow temporal window with respect to the extension of the arm (Hore et al., 1999). Effective on-line movement coordination is impossible if there is a lack of synchrony between sensation and movement.
Attention orienting is a core deficit in children with SBM. Orienting deficits are apparent over different segments of the life span in individuals with SBM, are specific to the salience orienting function of attention, arise from a specific set of brain abnormalities, and are similar to those reported in adults with midbrain and posterior cortex lesions.
The environment includes both perceptually salient and cognitively interesting information. Visual attention is controlled by two partially segregated neural systems, one involved in responding to salience and requiring automatic, stimulus-driven orienting, the other involved in goal-directed responding that is driven by knowledge, expectations, and goals (Corbetta & Shulman, 2002).
Babies find salience in their visual world, orienting to something new but maximizing their exploration of novelty by not exploring the same location repetitively (Richards, 2003). Compared to typically developing peers, infants with SBM show developmental lags in orienting to salient faces: until 24 months of age, they take longer than age peers to shift from a perceptually salient stimulus (a beeping light) to a second, face stimulus (Landry et al., 2003).
Orienting may involve either overt movements of the head, eyes, or body, or covert shifts of attention whereby the head, eyes, or body remain stationary. Covert orienting, which changes attention priorities, may be either automatic, as when we orient to salient information, or effortful, as with voluntary shifts of attention to something interesting (Posner & Cohen, 1984; Posner & Raichle, 1994). School-aged children with SBM orient more slowly to, and take longer to disengage from, what has captured their attention (Dennis et al., 2005a), but do not show deficits in orienting to cognitively interesting stimuli, which are under goal-directed, top-down control.
Inhibition of return, the additional time required to react to a target in a previously attended location is a mechanism for adaptive visual search, and increases the chance that exploration will occur with new objects and in new locations (Posner & Cohen, 1984; Klein, 2000). Compared to controls, children with SBM show attenuated inhibition of return in the vertical plane (Dennis et al., 2005b). The midbrain, including the superior colliculus, is part of a circuit that controls orienting to salience (Rafal & Henik, 1994). Children with SBM and tectal beaking, the characteristic midbrain malformation of SBM, are compromised in their ability to orient in their environment, both overtly with eye movement, and covertly, in the manner in which they shift attention. These children show difficulties with orienting to salience and a more attenuated inhibition of return in the vertical plane (Dennis et al., 2005a, 2005b). The principled relations between brain dysmorphologies and posterior attention functions such as covert orienting and inhibition of return can be identified not only in adults, but also in children with congenital malformations of the midbrain and posterior cortex. The posterior attention system shows no obvious age-based differences.
Core deficits in attention orienting have ecological significance throughout the life span. Visual foraging in infants has adaptive significance because it provides detailed information about the external world integrated with spontaneous body movement and goal-directed action during a period when the brain is developing rapidly (Robertson et al., 2001). Exploration of the visual environment regulates opportunities for perceptual-cognitive activity (Robertson et al., 2004), but infants and children with SBM explore space inefficiently.
Primary CNS insults cause core deficits (Path 2, Figure 1), the evidence being, first, that each core deficit has been clearly related to one or more of the primary CNS insults and, second, that core deficits vary in the degree of expression according to primary CNS insults (the most obvious example being that, compared to those with lower lesions, children with upper spinal cord lesions have more widespread movement limitations). There is no evidence that the magnitude of core deficits is significantly moderated by secondary CNS insults; for example, timing and attention-orienting deficits are related to measures of primary CNS insult, but not to markers of secondary CNS insult, such as number of shunt revisions to control hydrocephalus.
Core deficits are relatively independent. Motor timing in infancy is not related to attention orienting and shows only small to moderate correlations with movement (Fletcher et al., 2004). Other core deficits, such as covert orienting, are apparent when eye movements are restricted (Dennis et al., 2005a), and occur in children with and without major motor deficits (Dennis et al., 2005b). Studies of short duration timing illustrate the independence of timing and movement. Motor tapping deficits in children with SBM are related to the timing or clock component, concerned with the accuracy of the duration between taps, but not to the performance or motor component, concerned with the initiation and termination of the tapping action (Dennis et al., 2004). Furthermore, perceptual timing deficits occur in children with SBM regardless of their motor status (Dennis et al., 2004). Finally, timing deficits and attention-orienting deficits have different neurological bases (Dennis et al., 2004, 2005a, 2005b).
For typically developing individuals, core processes operate together to enhance ecological coherence, so that the individual is attuned to the environment. For many skills, the synchrony of core processes is important. Motor control, for instance, is a product of movement and timing by which movement is temporally synchronized with external stimuli. However, at both a behavioral and a neural level, core deficits are distinct. In individuals with SBM, deficits in one core process need not entail deficits in another, and, furthermore, intact function in one may be associated with deficits in another.
Associative Processing is data-driven and based on the formation of associations, enhancement, engagement, and categorization. It includes adaptive changes in response to stimulus repetition, as well as the activation and categorization of stimulus information. Examples of associative processing are recognizing faces or decoding familiar words. Assets in associative processing enable motor adaptation, categorical perception, and stipulated language. Assembled Processing, in contrast, is based on dissociation, suppression, disengagement, and contingent relations. It requires the assembling of models of input across various content domains. Examples of assembled processing are performing mental rotations or making inferences between world knowledge and text for oral or reading comprehension. Deficits in assembled processing make it difficult for children with SBM to exert on-line motor control, perceive perceptual relations, and understand constructed language. The two types of cognitive processing are not directly observable, but are inferred from how they affect a constellation of cognitive-behavioral skills.
In Figure 1, the relation between core and functional deficits is mediated by the Assembled Processing construct (Path 3, Figure 1). Associative Processing may exist independent of Assembled Processing, but Assembled Processing operates on the products of Associative Processing, reflecting a directional moderating relationship (Path 4, Figure 1). For example, it is necessary to decode words and activate word meanings in order to understand written texts and assemble meaning, respectively. The cognitive phenotype of SBM is constituted from intact associative processing and deficient assembled processing, so that children with SBM approach the task of constructing a functionally integrated model of the word with associative processing as the default option, and assembled processing as the effortful or unavailable option.
The Figure 1 model includes functional assets (Box, Figure 1) and functional deficits (Box, Figure 1). No content domain is functionally isomorphic with any core deficit, although each domain represents the direct influence of one or more core deficits. In the cognitive phenotype of SBM, assets and deficits may be demonstrated both within and across content domains.
Despite core deficits in movement, children with SBM exhibit a pattern of intact and impaired motor skills that, as Yeates et al. (in press) have suggested, may be decomposed in accordance with the neuroscience of motor skills. Although deficits in upper and lower extremity motor functions (Grimm, 1976; Hetherington & Dennis, 1999) have long been documented, motor assets have only recently been identified.
Motor adaptation is a change in the control of movements that occurs as a result of repeated task exposure or practice not dependent on conscious recall of the earlier exposure. Motor adaptation is a form of implicit learning, or learning without the intention to learn. New findings showing intact motor learning and motor adaptation in both hand and eye suggests that implicit motor learning (associative processing) may be an asset for children with SBM. They perform similarly to age peers on two measures of motor adaptation: biasing in weight judgments after exposure to heavy versus light weight, adaptation in reaching movement after vision is laterally displaced by prisms (Colvin et al., 2003), as well as procedural motor learning (Edelstein et al., 2004). Adaptive motor learning in children with SBM is intact for eye as well as for limb movement. Children with SBM and controls are equally proficient at making saccadic eye movements under controlled environments, and at modifying saccadic programming by adapting to “jumps” in visual targets below the threshold of conscious awareness (Salman et al., 2005a, 2005b).
In contrast to adaptive motor learning, regulated on-line motor performance of limbs and eyes (assembled processing) is impaired in children with SBM. The same children with SBM who showed procedural motor learning nonetheless had lower initial and final motor performance (Edelstein et al., 2004). Smooth pursuit eye movements are slow and conjugate eye movements that stabilize the image of a moving target on the fovea for high definition vision (Sharpe, 1998). Salman et al. (2005c) found that SBM children with nystagmus (who could adapt to jumps in visual targets) have impaired smooth pursuit movements.
The midline cerebellum calibrates reflex movement of the eyes, whereas the cerebellar hemispheres are concerned with voluntary control of eye movements, including visual fixation, ocular alignment, and smooth pursuit movements (Sharpe, 1998). That children with SBM seem to have particular difficulty with motor functions of the cerebellar hemispheres is consistent with significant reduction in their cerebellar hemispheric volumes (Fletcher et al., 2005).
The neural network of the SBM motor profile is not fully understood. In adults, motor adaptation is related to a neural circuit that involves both the cerebellum and the striatum. In children with SBM, adaptive motor learning is unrelated to measures of cerebellar volume (Edelstein et al., 2004). On-line motor control, which is a function of the posterior parietal cortex in the mature brain (e.g., Pisella et al., 2000), is uncorrelated with structural size of the midsagittal areas of the cerebellar vermis in children with SBM (Salman et al., in press; 2005c). In the adult, automatic and voluntary motor control cortical networks show substantial overlap (e.g., there is an overlapping cortical network for saccades and smooth pursuit movements, Berman et al., 1999), but it is not known whether overlap exists in children with SBM.
Although visual perception was traditionally identified as a problem area in children with SBM, recent research has shown that children with SBM (and other neurodevelopmental disorders, e.g. Williams Syndrome; Bellugi et al., 1994) have relative strengths on visual perception tasks involving categorical relations (e.g., face perception, object perception) and relative deficits on visual perception tasks of figure–ground delineation and relational coordinates (e.g., overlapping objects) (Dennis et al., 2002). Children with SBM have difficulty with coordinate or relational perceptual representations (assembled processing). This is a significant impairment because flexible representations of space guide behaviors as diverse as handwriting, establishing figural contours, and throwing a ball to a target. Perceptual difficulties have been correlated with posterior cortex thinning (Dennis et al., 1981; Fletcher et al., 1996).
Impaired movement is not responsible for the impairment on coordinate perception tasks because children with SBM have difficulties not only on drawing tasks that require both visual perception and motor control, but also on tasks with limited motor components (Fletcher et al., 1992). The distinction between categorical perception and flexible perceptual representations has also been demonstrated among classes of illusory stimuli. Children with SBM can perceive illusory object properties (size, length, contour) but not multistable objects, which require perceptual flexibility and the maintenance of multiple fleeting relations, for instance, between figure and ground (Dennis et al., 2001).
Intact associative processing and impaired assembled processing in visual perception may also be demonstrated in auditory perception. In the domain of music, children with SBM show intact ability to extract pitch feature information on a psychophysical task requiring them to judge variations around a target frequency of 300 Hz (Dennis et al., 2004), but are impaired in assembling temporal information into higher-order rhythmic structures (Hopyan et al., 2003), especially if they have reduced cerebellar volumes (Dennis et al., 2005c).
An important theoretical distinction in memory research is that between implicit memory, the learning or facilitation of performance by exposure without intention to remember (associative processing), and explicit memory, the conscious effort to recognize or recall (assembled processing). Children with SBM have relatively intact implicit memory. Using both a perceptual priming task (recognition of fragmented pictures, some of which had been passively encountered earlier) and a conceptual priming task (semantic decisions about words), Yeates and Enrile (2005) found that children with SBM had intact perceptual and semantic priming. In contrast, children with SBM are impaired on explicit memory tests involving different types of content (single word, story, list, design and spatial material) and different indices of memory (cued recall, encoding, recognition, and retrieval) (Yeates et al., 1995; Scott et al., 1998; but see Donders et al., 1991). Memory problems may be the cause of deficits in other domains (e.g., poor working memory is correlated with math problems, Dennis & Barnes, 2002) and/or the results of other domain problems (e.g., memory for designs measured by drawing is likely to be related to spatial and motor problems). Adults with SBM have quite widespread memory problems (Dennis et al., 2000), although the origin of these deficits is not well understood. The neural correlates of memory difficulties in SBM have not been studied.
Although language was once viewed as an asset for children with SBM, recent psycholinguistic and experimental studies of language have suggested a profile of intact and impaired language skills. Children with SBM develop content and function words, and demonstrate facility with grammar and vocabulary, while showing semantic–pragmatic, discourse-level difficulties (Dennis et al., 1994; Barnes & Dennis, 1998). Their strengths are in the formal, fixed structures of grammar, and single words or phrases and meanings that represent stored associations; their weaknesses are in assembling on-line meaning by integrating words, world knowledge, and context.
Meaning may involve the passive activation of frozen meaning or stipulated information, such as is involved in dictionary entries of lexical items. Meaning is also an active process that involves on-line assembly, suppression, and integration of information from the current oral or written text, real-world knowledge, and a representation of the situation the text or conversation describes. Preschoolers with SBM produce fewer story elements than age peers (Fletcher et al., 2004). Children with SBM learn, remember, and activate old knowledge better than they assemble meaning using old and new knowledge (e.g., Dennis et al., 1994; Barnes & Dennis, 1998). Children with hydrocephalus, most with SBM, continue to show particular difficulty with the assembly, suppression, and integration components of language (Barnes et al., 2004). Deficient suppression may preempt other comprehension processing resources and provide incomplete input to mental computations that generate a well-specified semantic representation (Tompkins et al., 2001). In accordance with this, children with SBM have poorly specified semantic representations that contain extraneous, contextually irrelevant information, which might explain why their conversations are referentially underspecified and tangential.
Idiom comprehension provides a clear example of the distinction between intact associative and impaired assembled meaning in children with SBM (Huber-Okrainec et al., 2005). Children with SBM can understand frozen idioms that are processed as a lexicalized unit, but have difficulty understanding novel idioms constructed from context. Specifically, they show a comprehension advantage for decomposable idioms whose meaning can be directly accessed by regular nonfigurative language mechanisms (e.g., a mile a minute) over nondecomposable idioms whose meaning needs to be constructed using context (e.g., kick the bucket) (Huber-Okrainec et al., 2005). Within the domain of figurative language, performance varies with the demands of assembled processing. Huber-Okrainec et al. (2005) report more severe idiom comprehension deficits in children with SBM who had more severe callosal abnormalities.
Reading decoding requires the activation of previously formed associations, in contrast to reading comprehension, in which world knowledge is combined with text to construct meaning and text representations. Reading comprehension, but not reading decoding, is related to general cognitive ability (Shatil & Share, 2003).
Children with SBM can decode words better than they can understand texts. Reading comprehension is poorer in children with SBM than in controls (Barnes & Dennis, 1992), even when reading decoding is precisely matched (Barnes et al., 2001). With increasing age, the reading profile stays relatively constant in individuals with SBM (Wills et al., 1990), although limitations in broader aspects of literacy such as writing ability continue into adult life (Barnes & Dennis, 2004).
Math calculation (associative processing) involves the retrieval of number facts and calculation procedures from memory. Numerical estimation (assembled processing), in contrast, requires that information be accessed and compared with reference to a more abstract rather than exact representation of quantity or extent, and real world estimation requires the integration of quantitative knowledge with knowledge about approximate quantities and sizes of objects. Children with SBM are more accurate on measures of multiplication (presumed to rely on math fact retrieval from semantic memory) than on estimation of size, length, mass, and price, which rely on integration of multiple sources of information (Barnes et al., 2002). Within exact calculation, math fact retrieval is more accurate than is the application of procedures or algorithms such as carrying and borrowing (Ayr et al., 2005; Barnes et al., 2005). Within single digit arithmetic, children with SBM and specific math disability are relatively fast at retrieving small number facts (sums < 10), but are slow in solving larger sum problems (sums between 11 and 18). Because large sum facts are less reliably retrieved from semantic memory than small sum facts, these findings suggest that the distinction between associative and assembled processing is found not only between tasks, but also within tasks in which individual items may draw on different types of processing (Barnes et al., 2006). The neural correlates of mathematics have not been explored in SBM.
Children with SBM are sociable and appear to activate a range of social behaviors, although they have difficulty in behavior regulation. Preschool children with SBM require more hints from a familiar examiner about how to involve a naïve examiner in a social party (Fletcher et al., 2004). In preschoolers, difficulties with behavior regulation are related to an inability to apply rules discovered through instruction and social interaction (Landry, 2005). School-aged children with SBM have a range of difficulties, including problems identifying the rules underlying effective goal-directed behavior in social situations, and maintaining goal-directed activities during play (Landry et al., 1993b); difficulties in unstructured social situations, showing inappropriate social distance, hyperverbosity, inappropriate use of language, tangential speech, and a failure to benefit from feedback or instruction about their behavior (Barnes & Dennis, 1998); and difficulty with social problem solving, including production of contextually appropriate responses to social interactions and comprehension of the social expression of emotion (Landry et al., 1993a; Koval, 2004). Neural correlates of behavioral difficulties have not been identified.
The cognitive phenotype of functional assets and functional deficits is shaped by three factors: deficits in assembled processing mediated by core deficits, the status of associative processing, and two moderators, secondary CNS insults of the neural phenotype, and the environment. Moderators do not exert constant effects, and the variable influence of moderators helps to account for individual differences in the cognitive phenotype.
Secondary CNS insults (Box, Figure 1) moderate assembled processing (Path 5, Figure 1). The effect of secondary insults begins before birth, and may continue throughout life. A thin posterior cortex is associated with more impairment in visual perception and visuo-motor function (Dennis et al., 1981; Fletcher et al., 1996). Young adults with SBM exhibit math deficits, the magnitude of which varies with lifetime shunt revision history (Dennis & Barnes, 2002). The magnitude of some language comprehension deficits in children with SBM is related to corpus callosum hypoplasia (thinning secondary to hydrocephalus), as well as congenital dysgenesis (Huber-Okrainec et al., 2005). Interhemispheric communication as assessed by dichotic listening tests, on the other hand, is related to the presence of splenial dysgenesis and not to corpus callosum thinning (Hannay et al., 2004). Secondary CNS insults appear to have fewer moderating effects on associative processing.
Core deficits exert an influence on the environment of the child with SBM (Path 6, Figure 1), so that environmental challenges are greater for children with more severe core deficits. Children with upper spinal lesions, for example, have more significant limitations on mobility, orthopedic and urologic function (Lonton, 1977), and require more economic and care-giving resources than do children with less impaired movement.
Environmental factors such as poverty, parental education, care giving, and parenting constitute a second set of moderators that begin before birth and continue throughout life. Poverty lowers general ability in children with SBM, especially in language (Fletcher et al., 2005), and disadvantaged children with SBM have lower Verbal IQ (Fletcher et al., 2004). Low socioeconomic status contributes to poor social competence (Greenley et al., 2006; Landry et al., 1994). Cognitive and social development is facilitated by parenting that promotes autonomy and independence (Weiss et al., 1992), and cognitive and language development is accelerated in children who receive flexible and responsive parenting (Landry et al., 1997), including children with SBM (Landry, 2005). Family and care-giving factors moderate the effect of SBM on adjustment (Greenley et al., 2006). Socioeconomic status and the presence or absence of family support is likely to influence the provision of appropriate remediation for specific cognitive and academic skills, such as math. Less is understood about how variations in education moderate the cognitive phenotype. For example, children with SBM rarely meet criteria for dyslexia because they can decode words, but the effect of reading comprehension programs have not been evaluated because children with SBM are rarely enrolled in evaluation studies.
Environmental factors (Box, Figure 1) moderate associative processing (Path 7, Figure 1), which, in turn, mediates the status of functional assets (Path 8, Figure 1). Environmental moderators are important, not because of their influence on assembled processing, but because they reduce SBM assets in associative processing, which serves to elevate the overall level of functional disability.
The model we propose provides a principled account of the characteristics of the SBM cognitive phenotype, and also of individual differences related to neural and environmental moderators of the cognitive phenotype. A model-driven analysis of SBM, which is associated with genetic, neurobiological, and behavioral variability, serves to increase our theoretical understanding of a number of brain-behavior issues and to prompt new research directions and testable hypotheses about these issues.
Genetic heterogeneity in SBM is considerable (see Kirkpatrick & Northrup, 2003). To date, the strongest evidence links genetic variability with variability in spinal lesion level, which has also been linked to variability in the neural phenotype (children with SBM and upper spinal lesions have more abnormalities in the cerebellum, midbrain, and corpus callosum, Fletcher et al., 2005). It is likely that this research can be further extended to more specific relationships of genetic variability and variability in the neural phenotype, as well as to direct relations of genetic variability and variability in the cognitive phenotype, as has been accomplished with other neurodevelopmental disorders (e.g., Crnic & Hagerman, 2004).
Variability in the neural phenotype is linked to variability in the cognitive phenotype for a number of content domains. The links were clearest for core deficits involving movement, timing, and attention orienting, but could also be demonstrated for content domains involving motor, language, and visual perceptual skills. Neural-cognitive relations in other content domains remain to be explored, such as those for mathematics and behavior.
To date, most of the research on neural-cognitive relations has been correlational and based on relations of MRI morphometry and/or qualitative evaluation of the MRI with cognitive performance. Future research questions may exploit newer methods of structural imaging such as diffusion tensor imaging, and functional neuroimaging methods, to refine some of the issues identified in this article, such as the need for functional interhemispheric integration for successfully using context to interpret idioms.
Models such as the one presented here may provide hypotheses about how SBM is related to other neurodevelopmental disorders, which, like SBM, are associated with disruptions of gene, brain, and behavior (e.g., Fragile X Syndrome; Crnic & Hagerman, 2004). A few generalizations are emerging. Neurodevelopmental disorders are often associated with mental retardation (e.g., Williams Syndrome), but may occur with a distinctive cognitive phenotype that is independent of mental retardation and that involves strengths in domains such as face processing or music (Bellugi et al., 1994). Similar links of core deficits related to the development of numeracy to outcomes involving visual perceptual skills and math have been made in children with the chromosome 22q11.2 deletion syndrome (Simon et al., 2005). Dorsal stream visual processing is impaired in a number of disorders in which ventral stream processing is relatively unimpaired (SBM: Dennis et al., 2002; congenital hypothyroidism: Leneman et al., 2001), in agreement with recent proposals of the high vulnerability of dorsal visual processing to developmental perturbations (Braddick et al., 2003). Comparisons across neurodevelopmental disorders and between congenital and acquired disorders (e.g., Ayr et al., 2005) will help evaluate the broader relevance of our proposed model.
SBM is a life span neurodevelopmental disorder, and some of the data discussed in the SBM model have concerned infancy and adulthood, which provides the basis for some testable hypotheses about aging in neurodevelopmental disorders. As adults, individuals with SBM exhibit clinical memory problems (Dennis et al., 2000), and the hypothesis that their cognitive aging is accelerated is supported by proposals such as those arguing for faulty folate metabolism and B-group vitamin deficiency as risk factors for Alzheimer's disease in Down Syndrome (Ward, 2004), and for declines in the corpus callosum splenium as part of the neuropathology of Alzheimer's disease (Rose et al., 2000).
Age-based functional plasticity refers to the hypothesis that functional outcome is better after earlier, compared to later, brain insult. Because few children with SBM experience mental retardation, despite the cascade of insults to the CNS beginning shortly after conception, functional plasticity is evident (although it may not be based on age because, of course, many adult brain lesions are congruent with the maintenance of normal intelligence). The narrower perspective of age-based functional plasticity in specific forms of cognitive processing is perhaps a more interesting question. Because we have conducted neuroscience experiments on children with SBM using many of the same paradigms used in studies of the adult brain, we are able to address such issues for two functions, short-duration timing and attention orienting. The timing functions of the cerebellum appear to be nonplastic because they are evident across the life span after cerebellar lesions. The motor functions of the cerebellum show a range of plasticity levels, from apparent plasticity in terms of saccades and saccadic adaptation to more limited plasticity for smooth pursuit movements. Cortical plasticity in children with SBM has not yet been investigated, although one would hypothesize more plasticity for functions, such as those in the cortex, that are not established at birth and that have a more distributed neural representation. Future research studies might compare neurocognitive and neuroimaging in children and adults for cortical, subcortical, and subtentorial functions in order to establish a fuller view of whether and how functional plasticity in the brain has a principled relation to age.
The model highlights research lacunae. Relatively few studies have addressed how environmental factors such as poverty and parenting affect cognitive outcomes in SBM, especially in the preschool period, an important time point given the early onset of difficulties related to core deficits and the potential for early intervention. The purpose of any model should be not only to enhance scientific understanding of a particular disorder or phenomenon, but also to suggest approaches for enhancing outcomes at optimal time points. Examining environmental factors in relation to the physical and neural phenotypes, as well as interactions of environmental factors and the genotype, may prove particularly fruitful for enhancing the outcomes for individuals with SBM over the life span.
This research was supported by a grant from the National Institute of Child Health and Human Development, P01 HD35946, Spina Bifida: Cognitive and Neurobiological Variability.

Model of outcome in spina bifida meningomyelocele showing how the cognitive phenotype is related to associative and assembled processing modes, core deficits, environmental influences, and primary and secondary CNS insults of the neural phenotype.