Introduction
Female fertility preservation is one of the most common problems addressed by assisted reproductive technologies. Many women suffer from diseases like hormone-dependent malignancies (e.g. breast cancer) or autoimmune diseases (e.g. vasculitis), the treatment for which involves aggressive methods of therapy such as radiotherapy and chemotherapy. As these methods negatively influence the health of gametes and their progenitors, fertility preservation is required in these cases. Moreover, developing new techniques allowing us to preserve fertility may be necessary for women who want to postpone their childbearing.
Although oocyte cryopreservation is commonly used for fertility preservation, this method involves hormonal ovarian stimulation, which cannot be used in some groups of patients, e.g. prepubertal girls and patients who suffer from hormone-dependent types of cancer. This paper presents a review of the current literature and a discussion of the data regarding methods of fertility preservation that are applicable for patients who cannot undergo hormonal stimulation. These techniques refer to two main strategies of female fertility preservation that are being actively developed: ovarian tissue cryopreservation followed by retransplantation, and ovarian tissue in vitro culture.
Main strategies of female fertility preservation
Currently, patients who suffer from different kinds of hormone-dependent malignancies, such as breast cancer or autoimmune diseases like vasculitis, are offered an aggressive form of therapy, which negatively influences the health of both themselves and the gametes. For example, the treatment of breast cancer, which is a hormone-dependent kind of cancer, may involve a drastic method like oophorectomy. This procedure leads to a decrease in the production of sex hormones (Di Leva Gianpiero et al., Reference Di Leva, Piovan, Gasparini, Ngankeu, Taccioli, Briskin, Cheung, Bolon, Anderlucci, Alder, Nuovo, Li, Iorio, Galasso, Ramasamy, Marcucci, Perrotti, Powell, Bratasz, Garofalo, Nephew and Croce2013). Moreover, treatment of other cancer types includes radio- and chemotherapy, which negatively affects the ovarian status. It is well known that most of the alkylating chemotherapeutic agents are gonadotoxic (Bokemeyer et al., Reference Bokemeyer, Schmoll, van Rhee, Kuczyk, Schuppert and Poliwoda1994; Thomson et al., Reference Thomson, Critchley and Wallace2002). Use of these agents in a therapy protocol leads to the degradation of follicles and oocytes (Familiari et al., Reference Familiari, Caggiati, Nottola, Ermini, Di Benedetto and Motta1993; Oktem & Oktay, Reference Oktem and Oktay2007a). Moreover, such treatment may result in the formation of ovarian tissue fibrosis (Meirow, Reference Meirow, Fasouliotis, Nugent, Schenker, Gosden and Rutherford1999; Behringer et al., Reference Behringer, Breuer, Reineke, May, Nogova, Klimm, Schmitz, Wildt, Diehl and Engert2005). Chemotherapeutic agents induce apoptosis in ovarian stroma and in the follicular system (Verga Falzacappa et al., Reference Verga Falzacappa, Timperi, Bucci, Amendola, Piergrossi, D'Amico, Santaguida, Centanni and Misiti2012), which leads to a reduction in follicle numbers or, in other words, results in an increase in the risk of infertility (Oktem & Oktay, Reference Oktem and Oktay2007b).
The use of radiation therapy is dangerous for women over the age of 30, because the effective sterilizing dose of radiotherapy decreases with age (Wallace et al., Reference Wallace, Anderson and Irvine2005). At the same time, the risk of malignancy formation increases with age (Xu et al., Reference Xu, Lawson, Yeoman, Molskness, Ting, Stouffer and Zelinski2013). Given these facts, the use of aggressive antitumour therapy for women over 30 must be supplemented with procedures of fertility preservation.
In contemporary society, many women prefer to delay childbearing, but the number of follicles decrease and reproductive health becomes impaired with age, reducing the probability of normal fertilization and pregnancy (Wallace & Kelsey, Reference Wallace and Kelsey2010). Therefore, methods that allow us to preserve fertility could also help these women to bear healthy children.
In some cases, fertility preservation is assisted with a classical cycle of ovulation induction (Cardozo et al., Reference Cardozo, Thomson, Karmon, Dickinson, Wright and Sabatini2015; Sigismondi et al., Reference Sigismondi, Papaleo, Viganò, Vailati, Candiani, Ottolina, Di Mattei and Mangili2015). Obtained oocytes can be cryopreserved, or fertilized and the developed embryos can subsequently be cryopreserved. Use of such a protocol is possible only in cases in which the woman is absolutely healthy or when her disease was diagnosed as being at an early stage of development and does not depend on gonadotropic and steroid sex hormones. Otherwise, the use of this protocol may lead to growth of the tumour. Moreover, such a strategy of therapy is unsuitable for prepubertal patients (Mahajan, Reference Mahajan2015).
At the present time, a patient requiring fertility preservation is offered cryopreservation of ovarian tissue, followed by retransplantation of thawed fragments into the patient's body (Meirow et al., Reference Meirow, Baum, Yaron, Levron, Hardan, Schiff, Nagler, Yehuda, Raanani, Hourvitz and Dor2007). However, technologies of ovarian tissue in vitro culture are being actively developed (Roy & Treacy, Reference Roy and Treacy1993; Abir et al., Reference Abir, Franks, Mobberley, Moore, Margara and Winston1997, Reference Abir, Roizman, Fisch, Nitke, Okon, Orvieto and Ben Rafael1999, Reference Abir, Fisch, Nitke, Okon, Raz and Ben Rafael2001; Nayudu et al., Reference Nayudu, Wu and Michelmann2003; Xu et al., Reference Xu, Fazleabas, Shikanov, Jackson, Barrett, Hirshfeld-Cytron, Kiesewetter, Shea and Woodruff2011b). The main aim of these technologies is to produce mature oocytes capable of fertilization from pieces of in vitro cultured ovarian tissue (Filatov et al., Reference Filatov, Khramova and Semenova2015). Embryos obtained after the fertilization of oocytes may be cryopreserved and transferred into the patient's uterus after full recovery. Moreover, obtained embryos may be used in surrogacy programmes if the patient would like to have a child before the disease is cured.
Diseases whose treatment requires fertility preservation
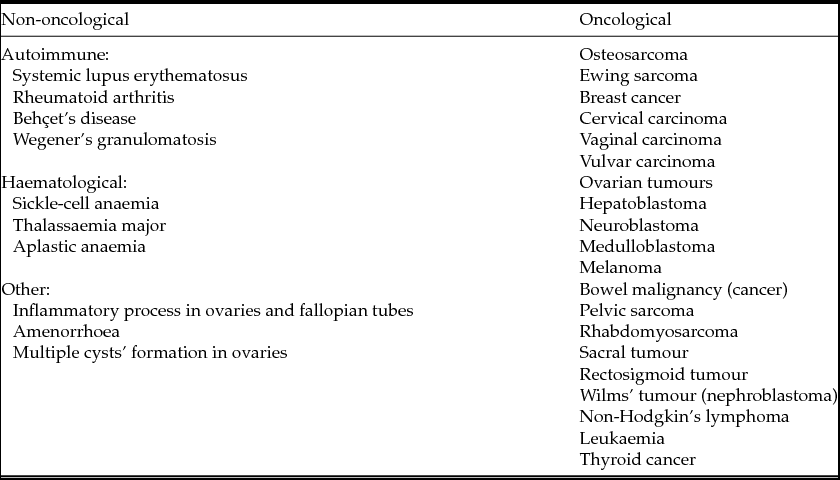
Many diseases whose treatment requires aggressive methods of therapy and procedures of fertility preservation are known. These diseases can be divided into two groups: oncological and non-oncological diseases. Non-oncological diseases include autoimmune and haematological disorders that, like oncological ones, are treated with chemotherapy and radiotherapy (Donnez et al., Reference Donnez, Martinez-Madrid, Jadoul, Van Langendonckt, Demylle and Dolmans2006a,b, 2010a,b). The most common diseases the treatment of which requires procedures of fertility preservation are represented in Table 1.
Table 1 Diseases that require fertility preservation (according to Wallace et al., Reference Wallace, Anderson and Irvine2005; Donnez & Dolmans, Reference Donnez and Dolmans2013)
In many cases, diagnosis of the disease occurs at a late stage, when treatment must begin immediately. In these situations, the usage of standard protocols of assisted reproductive technologies, followed by the cryopreservation of embryos obtained after in vitro fertilization of oocytes received via ovulation stimulation, is not possible (Sigismondi et al., Reference Sigismondi, Papaleo, Viganò, Vailati, Candiani, Ottolina, Di Mattei and Mangili2015, Cardozo et al., Reference Cardozo, Thomson, Karmon, Dickinson, Wright and Sabatini2015). Moreover, the use of classical programmes of assisted reproductive technologies for fertility preservation is not possible for prepubertal girls (Mahajan, Reference Mahajan2015). In these cases, the application of ovarian tissue cryopreservation technologies or the ovarian tissue in vitro culture technique seems to be the best strategy for female fertility preservation.
Methods of ovarian tissue isolation
Isolation of ovarian tissue for further cryopreservation or in vitro culture may be performed in two ways: surgical removal of the whole ovary or biopsy of ovarian tissue pieces. The most commonly used method for obtaining ovarian tissue is laparoscopy (Meirow et al., Reference Meirow, Fasouliotis, Nugent, Schenker, Gosden and Rutherford1999), but minilaparotomy also may be used for this purpose (Bolla et al., Reference Bolla, Deseö, Sturm, Schöning and Leimgruber2012). There is no consensus on the amount of ovarian tissue that must be left in the patient's body after the procedure of ovarian tissue isolation. If treatment requires usage of an aggressive therapy that can negatively influence reproductive function, it is better to isolate larger parts of ovarian tissue (Rosendahl et al., Reference Rosendahl, Andersen, Ernst, Westergaard, Rasmussen, Loft and Andersen2008; Harel et al., Reference Harel, Ferme and Poirot2011). Conversely, parts of ovarian tissue in the body must be preserved in order to provide the better survival of retransplanted fragments.
If the proposed fertility preservation strategy is the cryopreservation of ovarian tissue followed by retransplantation of isolated fragments, in most cases, the cortical part of ovarian tissue is extracted. Cortical ovarian tissue contains the bulk of the follicles, particularly the primordial and primary ones. Furthermore, it is supposed that after the procedure of ovarian tissue cryopreservation and thawing, small follicles survive better than do larger ones (Hovatta et al., Reference Hovatta, Silye, Krausz, Abir, Margara, Trew, Lass and Winston1996, Reference Hovatta, Silye, Abir, Krausz and Winston1997; Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996; Oktay et al., Reference Oktay, Nugent, Newton, Salha, Chatterjee and Gosden1997).
The ovarian tissue biopsy procedure allows the obtaining of fragments about 1 cm × 4–5 mm × 1–1.5 mm (Donnez et al., Reference Donnez, Martinez-Madrid, Jadoul, Van Langendonckt, Demylle and Dolmans2006a, Reference Donnez, Jadoul, Squifflet, Van Langendonckt, Donnez, Van Eyck, Marinescu and Dolmans2010a, Reference Donnez, Dolmans, Pellicer, Diaz-Garcia, Sanchez Serrano, Schmidt, Ernst, Luyckx and Andersen2013). Before cryopreservation, isolated fragments must be separated into small pieces about 0.3–2 mm wide (Hovatta et al., Reference Hovatta, Silye, Krausz, Abir, Margara, Trew, Lass and Winston1996; Silber et al., Reference Silber, Lenahan, Levine, Pineda, Gorman, Friez, Crawford and Gosden2005; Silber, Reference Silber2011). There are two main reasons to cut obtained ovarian tissue fragments into thin slices. First, after retransplantation, thin tissue slices stimulate better neovascularization than thicker ones do. This situation allows a decrease in ischaemia stress in retransplanted tissue (Silber, Reference Silber2011; Andersen et al., Reference Andersen, Silber, Berghold, Jorgensen and Ernst2012; Silber & Barbey, Reference Silber and Barbey2012). Second, the thinness of fragments allows cryoprotectants to penetrate better into the tissue, resulting in less injury to the tissue during the procedures of freezing and thawing. It has been demonstrated that 2 mm thick slices thaw better than 4 mm thick slices (Ferreira et al., Reference Ferreira, Bos-Mikich, Frantz, Rodrigues, Brunetto and Schwartsmann2010).
In some cases, a whole ovary instead of ovarian tissue fragments can be used for retransplantation (Leporrier et al., Reference Leporrier, von Theobald, Roffe and Muller1987; Wang et al., Reference Wang, Chen, Yin, Kim, Lin Tan and Gosden2002; Hilders et al., Reference Hilders, Baranski, Peters, Ramkhelawan and Trimbos2004; Martinez-Madrid et al., Reference Martinez-Madrid, Dolmans, van Langendonckt, Defrere and Donnez2004; Mhatre et al., Reference Mhatre, Mhatre and Magotra2005; Imhof et al., Reference Imhof, Bergmeister, Lipovac, Rudas, Hofstetter and Huber2006; Silber et al., Reference Silber, DeRosa, Pineda, Lenahan, Grenia, Gorman and Gosden2008a,b). Whole ovary retransplantation decreases the risk of ischaemia in ovarian tissue because the immediate revascularization of the transplant is achieved via the vascular pedicle (Leporrier et al., Reference Leporrier, von Theobald, Roffe and Muller1987; Wang et al., Reference Wang, Chen, Yin, Kim, Lin Tan and Gosden2002; Hilders et al., Reference Hilders, Baranski, Peters, Ramkhelawan and Trimbos2004; Martinez-Madrid et al., Reference Martinez-Madrid, Dolmans, van Langendonckt, Defrere and Donnez2004; Mhatre et al., Reference Mhatre, Mhatre and Magotra2005; Imhof et al., Reference Imhof, Bergmeister, Lipovac, Rudas, Hofstetter and Huber2006; Silber et al., Reference Silber, DeRosa, Pineda, Lenahan, Grenia, Gorman and Gosden2008a,b). There is a clinical case of a live birth after the transplantation of a non-cryopreserved whole ovary into the donor's body (Silber et al., Reference Silber, Grudzinskas and Gosden2008b). However, cryopreservation of the whole ovary seems to be impossible because, in the event of microvascular anastomosis failure, all ovarian tissue may be lost with no chance of future fertility restoration (Courbiere et al., Reference Courbiere, Caquant, Mazoyer, Franck, Lornage and Salle2009).
Ovarian tissue cryopreservation techniques
The two most commonly used methods of ovarian tissue cryopreservation are vitrification and slow freezing. The slow freezing technique was developed earlier than vitrification. The slow freezing protocol involves ovarian tissue impregnation with the cryoprotective agent, followed by a step-by-step temperature lowering. Usually, the slow freezing procedure involves the application of programmed freezers, which are special devices that allow the gradual lowering of temperature according to the program's settings. In most cases, tissue is cooled to −140°C and then placed into liquid nitrogen for storage (Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996, Reference Newton, Fisher, Arnold, Pegg, Faddy and Gosden1998; Fuller & Paynter, Reference Fuller and Paynter2004; Hovatta, Reference Hovatta2005).
Both slow freezing and vitrification require the exposure of ovarian tissue to a cryoprotective solution. The principal difference between vitrification and slow freezing is the very rapid cooling of the sample in the former. Rapid cooling allows the avoidance of ice crystal formation in the tissue. The appearance of ice crystals inside cells during cryopreservation has a detrimental effect on their viability: ice has a lower density than water, meaning that frozen water takes up more volume than the liquid form. Ice crystals can impair a cell's membrane and, after thawing, a cell with a destroyed membrane may die.
The vitrification technique has demonstrated good results in the cryopreservation of oocytes and early embryos (Kuwayama et al., Reference Kuwayama, Vajta, Kato and Leibo2005; Cobo et al., Reference Cobo, Domingo, Perez, Crespo, Remohi and Pellicer2009; Abedellhafez et al., Reference Abedellhafez, Desai, Abou-Setta, Falcone and Goldfarb2010; Cobo et al., Reference Cobo, Meseguer, Remohi and Pellicer2010; Edgar & Gook, Reference Edgar and Gook2012), but this method has grave disadvantages. Cryopreservation with a vitrification protocol requires the application of cryoprotective agents in high concentrations, which may negatively affect cryopreserved cells (Fahy et al., Reference Fahy, MacFarlane, Angell and Meryman1984; Isachenko et al., Reference Isachenko, Isachenko, Rahimi and Nawroth2003, Reference Isachenko, Isachenko, Reinsberg, Montag, van der Ven, Dorn, Roesing and van der Ven2007; Gandolfi et al., Reference Gandolfi, Paffoni, Papasso Brambilla, Bonetti, Brevini and Ragni2006; Keros et al., Reference Keros, Xella, Hultenby, Pettersson, Sheikhi, Volpe, Hreinsson and Hovatta2009; Kagawa et al., Reference Kagawa, Silber and Kuwayama2009; Amorim et al., Reference Amorim, David, Van Langendonckt, Dolmans and Donnez2011;). Moreover, the high concentration of cryoprotective agents can cause osmotic shock in cells (Fahy et al., Reference Fahy, Lilley, Linsdell, Douglas and Meryman1990). Only two clinical cases of a successful pregnancy followed by a live birth after the vitrification and retransplantation of ovarian tissue are known (Kawamura et al., Reference Kawamura, Cheng, Suzuki, Deguchi, Sato, Takae, Ho, Kawamura, Tamura, Hashimoto, Sugishita, Morimoto, Hosoi, Yoshioka, Ishizuka and Hsueh2013; Suzuki et al., Reference Suzuki, Yoshioka, Takae, Sugishita, Tamura, Hashimoto, Morimoto and Kawamura2015). Brief descriptions of cryopreservation protocols whose usage led to pregnancies and live births are listed in Table 2. Comparison of the effectiveness of vitrification vs. slow freezing requires further studies, which are to be carried out in the near future.
Table 2 Protocols of cryopreservation procedures that led to live births after the retransplantation of thawed ovarian tissue

DMSO, dimethyl sulphoxide; EGTA, ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid; HSA, human serum albumin; PBS, phosphate-buffered saline; SSS, serum substitute supplement.
Methods of ovarian tissue transplantation
There are three principally different methods of ovarian tissue transplantation: orthotopic retransplantation, heterotopic retransplantation and xenotransplantation. During orthotopic retransplantation, ovarian tissue is placed into the remaining ovary or (if that is not possible) into the pelvic cavity. In other words, ovarian tissue is transplanted into its native surroundings. In the case of heterotopic retransplantation, ovarian tissue is transplanted to a different site, e.g., under the skin of the forearm or under the abdominal wall (Oktay & Karlikaya, Reference Oktay and Karlikaya2000; Callejo et al., Reference Callejo, Salvador, Miralles, Vilaseca, Lailla and Balasch2001; Oktay et al., Reference Oktay, Economos, Kan, Rucinski, Veek and Rosenwaks2001; Radford et al., Reference Radford, Lieberman, Brison, Smith, Critchlow, Russell, Watson, Clayton, Harris, Gosden and Shalet2001; Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and van Langendonckt2004, Reference Donnez, Squifflet, Dolmans, Martinez-Madrid, Jadoul and van Langendonckt2005; Meirow et al., Reference Meirow, Levron, Eldar-Geva, Hardan, Fridman, Zalel, Schiff and Dor2005; Schmidt et al., Reference Schmidt, Andersen, Loft, Byskov, Ernst and Andersen2005; Demeestere et al., Reference Demeestere, Simon, Buxant, Robin, Fernandez, Centner, Delbaere and Englert2006; Kim et al., Reference Kim, Lee, Chung, Lee, Lee and Hill2009; Grynberg et al., Reference Grynberg, Poulain, Sebag-Peyrelevade, le Parco, Fanchin and Frydman2012).
At present, two main orthotopic retransplantation techniques of ovarian tissue exist. In a case where some parts of the ovary remain in the patient's body, thawed pieces of cortical ovarian tissue can be attached to the remaining medulla. Where the medulla has not been saved, ovarian tissue pieces can be placed into the peritoneal window, in the region of small retroperitoneal vessels (Donnez et al., Reference Donnez, Jadoul, Pirard, Hutchings, Demylle, Squifflet, Smitz and Dolmans2012, Reference Donnez, Dolmans, Pellicer, Diaz-Garcia, Sanchez Serrano, Schmidt, Ernst, Luyckx and Andersen2013). The main advantage of orthotopic retransplantation as compared with the heterotopic method is that natural conception may occur when ovarian tissue is transplanted orthotopically. Moreover, in the case of the orthotopic technique, fragments are placed into an environment that is native for ovarian tissue. It is likely that tissue retransplantation into its original physiological surroundings may promote the development of transplanted tissue more effectively than retransplantation into a heterotopic site. The main disadvantage of orthotopic retransplantation is that it is often impossible to determine whether ovulation has occurred in the transplanted tissue or in the remaining ovary fragment, especially in the event of a natural conception.
Heterotopic retransplantation of ovarian tissue has other advantages over the orthotopic method. Firstly, it is easy to observe the actual condition of transplanted tissue. Secondly, heterotopic retransplantation of ovarian tissue does not require the usage of general anaesthesia. Thirdly, it is easier (when required) to remove the transplanted fragment than it is in the case of orthotopic retransplantation.
However, heterotopic retransplantation also has some principal drawbacks. First, conception may occur only with assisted reproduction technologies; moreover, the non-native surroundings of transplanted tissue probably reduce the ability to induce neovascularization in the ovarian fragment than in the case of orthotopic retransplantation. Taken together, these two factors reduce the probability of pregnancy (Oktay et al., Reference Oktay, Economos, Kan, Rucinski, Veek and Rosenwaks2001, Reference Oktem and Oktay2004; Partridge et al., Reference Partridge and Winer2004; Sonmezer & Oktay, Reference Sonmezer and Oktay2004; Kim et al., Reference Kim, Lee, Chung, Lee, Lee and Hill2009; Jemal et al., Reference Jemal, Siegel, Xu and Ward2010; Donnez et al., Reference Donnez, Dolmans, Pellicer, Diaz-Garcia, Sanchez Serrano, Schmidt, Ernst, Luyckx and Andersen2013).
The method of Rosendahl et al., (Reference Rosendahl, Loft, Byskov, Ziebe, Schmidt, Andersen, Ottosen and Andersen2006) shows that ovarian tissue xenotransplantation can also be applied. In this case, ovarian tissue is transplanted under the skin or under the renal capsule of immunodeficient mice (Sonmezer & Oktay, Reference Sonmezer and Oktay2004; Chao et al., Reference Chao, Jiang, Deng, Yu and Zhen2008; Lan et al., Reference Lan, Xiao, Xiao-Hui, Chun-Yan and Hong-Ling2010). Immunodeficient mice do not develop an immune response to tissues transplanted from other species (Bosma et al., Reference Bosma, Custer and Bosma1983).
The main advantage of xenotransplantation is that the transplanted tissue is located outside the patient's body. In the case in which malignant cells have remained in the transplanted tissue, the tumour will grow in the mouse rather than in the patient. For this reason, this technique seems to be very promising for patients who suffer from hormone-dependent types of cancer. Furthermore, it is very simple to realize monitoring of transplanted tissue and to obtain mature oocytes. However, the application of xenotransplantation in routine clinical practice is not possible, due to the insufficiency of data about disease transmission from animals to humans via ovarian tissue (Seli & Tangir, Reference Seli and Tangir2005). Thus, the ovarian tissue xenotransplantation technique may be applicable only in research studies until the question of infection transmission is resolved.
Orthotopic, heterotopic and xenotransplantation methods have several advantages and drawbacks, as discussed above. In summary, orthotopic ovarian tissue retransplantation currently seems to be more optimal than the other techniques. In the case of orthotopic retransplantation, a tissue fragment is placed in a native environment with adequate temperature, blood pressure, mechanical tension and normal hormone and paracrine regulation. Furthermore, in contrast with heterotopic retransplantation and xenotransplantation, natural conception may occur in the case of orthotopic retransplantation. At the present time, xenotransplantation cannot be used in routine clinical practice, and the effectiveness of heterotopic retransplantation still remains unclear: there is only one case of a live birth after IVF procedures with an oocyte obtained from heterotransplanted ovarian tissue (Stern et al., Reference Stern, Gook, Hale, Agresta, Oldham, Rozen and Jobling2013). In all other known cases of live births after ovarian tissue retransplantation, the orthotopic technique was used.
Effectiveness of ovarian tissue cryopreservation
The effectiveness of cryopreservation and thawing procedures can be estimated using different methods. Evaluation of a tissue's condition after cryopreservation and thawing can be performed via histological analysis. However, the only reliable way to prove that a cryopreservation and thawing technique is effective is to obtain mature oocytes capable of fertilization and further development, followed by the live birth of healthy baby.
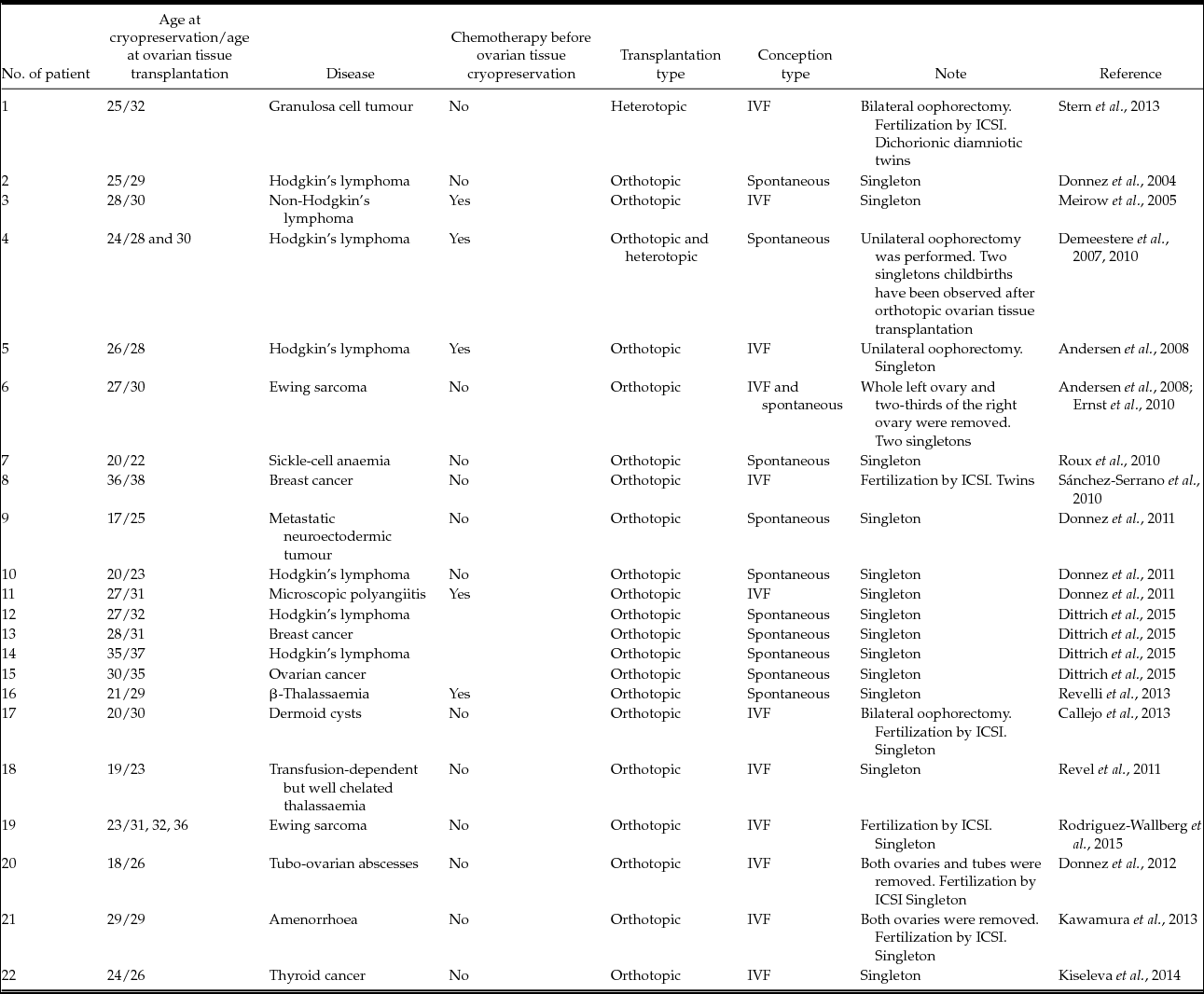
At present, more than 25 cases of successful ovarian tissue cryopreservation that led to childbirth are known (Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and van Langendonckt2004, Reference Donnez, Squifflet, Dolmans, Martinez-Madrid, Jadoul and van Langendonckt2013; Meirow et al., Reference Meirow, Levron, Eldar-Geva, Hardan, Fridman, Zalel, Schiff and Dor2005; Chung et al., Reference Chung, Donnez, Ginsburg and Meirow2013). Most of these cases are listed in Table 3. However, although the data are very promising, it is not known exactly whether the oocytes ovulated from the retransplanted ovarian tissue or the remaining fragment of the ovary. In most cases, pieces of ovary remain in the patient's body after an ovarian tissue biopsy, which is required for better survival rates of retransplanted tissue. It is only in isolated instances that women need a total bilateral oophorectomy, so only in these cases is it possible to declare confidently that an embryo has developed successfully from an oocyte obtained from the thawed ovarian tissue.
Table 3 Cases of successful ovarian tissue cryopreservation that led to live birth
ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
Cryopreservation of ovarian tissue followed by retransplantation is not applicable in several cases. Many scientific groups (Meirow et al., Reference Meirow, Levron, Eldar-Geva, Hardan, Fridman, Zalel, Schiff and Dor2005; Silber et al., Reference Silber, Lenahan, Levine, Pineda, Gorman, Friez, Crawford and Gosden2005; Donnez et al., Reference Donnez, Martinez-Madrid, Jadoul, Van Langendonckt, Demylle and Dolmans2006a, Reference Donnez, Jadoul, Squifflet, Van Langendonckt, Donnez, Van Eyck, Marinescu and Dolmans2010a, Reference Donnez, Dolmans, Pellicer, Diaz-Garcia, Sanchez Serrano, Schmidt, Ernst, Luyckx and Andersen2013; Andersen et al., Reference Andersen, Rosendahl, Byskov, Loft, Ottosen, Dueholm, Schmidt, Andersen and Ernst2008; Sanchez et al., Reference Sanchez, Novella-Maestre, Teruel, Ortiz and Pellicer2008; Schmidt et al., Reference Schmidt, Rosendahl, Ernst, Loft, Andersen, Dueholm, Ottosen and Andersen2011; Silber, Reference Silber2011) agreed that, as a rule, 35 is the upper age limit for the cryopreservation of ovarian tissue. This situation is due to the fact that, after the procedure of cryopreservation of ovarian tissue, follicles are preserved mainly in the early stages of folliculogenesis (primary and primordial follicles), the number of which reduces significantly with age.
In the case of prepubertal girls, obtaining ovarian tissue, followed by its cryopreservation and retransplantation, will negatively influence the patient's health. Such procedures will provoke harmful hormonal stress. In these cases, it seems better to obtain mature oocytes from ovarian tissue cultured in vitro and use them in surrogate programmes.
In some instances, ovarian tissue cryopreservation cannot be used (for example, when the patient is a prepubertal girl) or is not very effective (when the patient is older than 35). In these cases, the ovarian tissue in vitro culture technique can be applied for female fertility preservation.
Ovarian tissue in vitro culture
There are two methods for an ovarian tissue in vitro culture technique: (i) two-dimensional (2D); and (ii) three-dimensional (3D) systems (Hirao, Reference Hirao2012). The principal drawback of 2D systems is the low correspondence between the microenvironment of the cultured fragment and the in vivo conditions. In the case of the 2D method, the ovarian tissue culture's stroma and follicular cells migrate along the plane, follicles lose their normal shape and oocytes lose their normal cellular environment. Regulation of the 2D system's conditions may be performed only by adding various chemical agents (growth factors, hormones, etc.) to the culture medium. Three-dimensional systems allow us to regulate the condition of cultivated ovarian tissue, not only by adding different chemical agents but also by variation of microenvironmental physical properties.
For in vitro culture, the whole ovary (Jin et al., Reference Jin, Lei, Shikanov, Shea and Woodruff2010), ovarian fragments or single follicles can be used (Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006b). In vitro culture of a whole ovary or ovarian fragments has a significant disadvantage. This is due to the difficulties of nutrient delivery and oxygenation in the deep layers of cultivated tissue. Therefore, studies dedicated to isolated ovarian follicles in an in vitro culture seem to be the most effective. In the case of isolated ovarian follicles in vitro culture, all cultivated follicles receive required nutrients that penetrate into all of the follicular compartments via diffusion. Moreover, when single ovarian follicles are cultivated, it is possible to trace the development of each follicle, which is not possible in the cases of a whole ovary or ovarian tissue fragments in vitro culture.
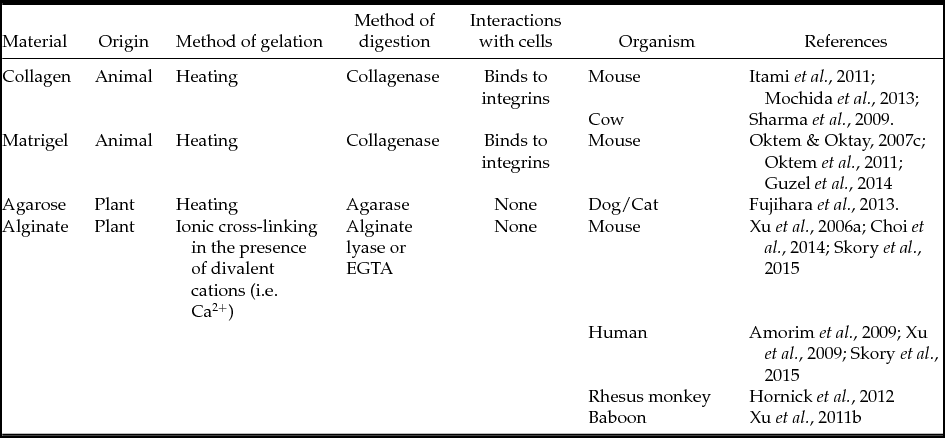
Mammalian ovarian follicles in vitro culture can be performed using various substrates, e.g. agarose, collagen, Matrigel or alginates and their derivatives. The main parameters of different substrates used for ovarian follicles in vitro culture are listed in Table 4. Described substrates belong to the group of hydrogels, which are polymeric compounds that are powdery when dried. If water or another solvent is added to the dry hydrogel, its granules swell, resulting in the formation of a gel-like substance. This gel can be used further for the in vitro culture of various cell types, particularly for the culture of ovarian follicles.
Table 4 Substrates used for in vitro ovarian follicles’ culture
EGTA, ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid.
The described substrates have various limitations in their applications. For instance, agarose gelation requires a high temperature but heating is very harmful to ovarian follicles. The most commonly methods used for hydrogel gelation (exposure to UV radiation, high temperatures and aggressive chemical treatment) are dangerous for the living objects.
Alginate hydrogel gelation does not require toxic agents or application of non-physiological conditions (high temperature, UV radiation, etc.). Only the supplementation of a solution containing cross-linking agents (Ca2+ or Mg2+ ions) is needed for alginate polymerization. The effect of the conditions of hydrogel preparation on living cells is less negative. This is because alginate hydrogel preparation does not require temperatures higher than 37°C (physiological temperature) and polymerization agents (divalent cations, added to most culture mediums) have no toxic effects on cells. Moreover, cultivated cells do not form contacts with an alginate hydrogel. The alginate hydrogel destruction procedure does not impair cultivated follicles, because substances that digest the alginate hydrogel are unable to destroy cell contacts.
One of the principal advantages of alginate substrates is their vegetative origins. Therefore, alginate hydrogels can be used in ‘animal-free’ projects. In some studies, e.g. human ovarian follicle culture, the application of substances of animal origin is forbidden because it may lead to the contamination of the culture medium by viruses, proteins and other molecules that are able to interact with the cultivated tissue. There is no consensus on horizontal gene transfer when tissues or substances from different species are cocultured.
Effectiveness of ovarian tissue in vitro culture
To date, the ovarian tissue in vitro culture technique is not a widely used technique in clinical practice because the developing methods are far from perfect. Furthermore, there are no standardized technique protocols for effectively obtaining oocytes capable of in vitro fertilization. There are two principally distinct strategies of ovarian tissue in vitro culture: in vitro culture of ovarian cortical tissue fragments (strips) and in vitro culture of isolated ovarian follicles.
The cortical compartment of an ovary contains a large number of primordial follicles. Most commonly, ovarian tissue fragments are obtained by dissection of the cortical tissue into strips, with their subsequent culturing in vitro. This approach allows to support the growth of primordial follicles (Telfer et al., Reference Telfer, McLaughlin, Ding and Thong2008).
Another approach to culture of ovarian tissue in vitro is based on the culture of isolated ovarian follicles. Commonly, primary and secondary follicles are used for this technique. For the ovarian tissue in vitro culture technique, researchers use various substrates that allow the stimulation of follicle growth and oocyte maturation, e.g., alginate (Xu et al., Reference Xu, West, Shea and Woodruff2006a, Reference Xu, Barrett, West-Farrell, Kondapalli, Kiesewetter, Shea and Woodruff2009, Reference Xu, Fazleabas, Shikanov, Jackson, Barrett, Hirshfeld-Cytron, Kiesewetter, Shea and Woodruff2011b; Amorim et al., Reference Amorim, Van Langendonckt, David, Dolmans and Donnez2009; Hornick et al., Reference Hornick, Duncan, Shea and Woodruff2012; Choi et al., Reference Choi, Agarwal, Huang, Zhao and He2014; Skory et al., Reference Skory, Xu, Shea and Woodruff2015), collagen (Sharma et al., Reference Sharma, Dubey and Meur2009; Itami et al., Reference Itami, Yasuda, Yoshida, Matsui, Hashiura, Sakai and Tamotsu2011; Mochida et al., Reference Mochida, Akatani-Hasegawa, Saka, Ogino, Hosoda, Wada, Sawai and Shibahara2013), Matrigel (Oktem & Oktay, Reference Oktem and Oktay2007c; Oktem et al., Reference Oktem, Buyuk and Oktay2011; Guzel et al., Reference Guzel, Nur Şahin, Sekeroglu and Deniz2014) and agarose (Fujihara et al., Reference Fujihara, Comizzoli, Wildt and Songsasen2012). There are promising results showing that the ovarian tissue in vitro culture technique allows not only to stimulate follicular growth but to obtain mature oocytes that are competent for development after fertilization (Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006b, Reference Xu, Lawson, Yeoman, Pau, Barrett, Zelinski and Stouffer2011a, Reference Xu, Lawson, Yeoman, Molskness, Ting, Stouffer and Zelinski2013; Shikanov et al., Reference Shikanov, Xu, Woodruff and Shea2009, Reference Shikanov, Xu, Woodruff and Shea2011; Jin et al., Reference Jin, Lei, Shikanov, Shea and Woodruff2010; Choi et al., Reference Choi, Agarwal, Huang, Zhao and He2014).
In comparison with the method of ovarian tissue cryopreservation followed by retransplantation, the method of in vitro culturing of ovarian tissue allows to obtain mature oocytes not only from primary and primordial follicles that can be cryopreserved but also from follicles at advanced stages of development, e.g., from the secondary follicles (Mochida et al., Reference Mochida, Akatani-Hasegawa, Saka, Ogino, Hosoda, Wada, Sawai and Shibahara2013).
It has been shown that the in vitro culturing of murine ovarian follicles can lead to the formation of mature oocytes that are able to undergo successful fertilization and further development. Furthermore, after the transfer of these embryos to a surrogate mother, the live birth of healthy pups has been obtained (Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006b). However, live births of healthy pups after in vitro culturing of ovarian tissue have been shown in only a few studies (Yamamoto et al., Reference Yamamoto, Otoi, Koyama, Horikita, Tachikawa and Miyano1999; Hasegawa et al., Reference Hasegawa, Mochida, Ogasawara and Koyama2006; Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006b; Mochida et al., Reference Mochida, Akatani-Hasegawa, Saka, Ogino, Hosoda, Wada, Sawai and Shibahara2013; Higuchi et al., Reference Higuchi, Maeda, Horiuchi and Yamazaki2015). Such significant results motivate researchers to develop more advanced systems that will allow us to obtain mature oocytes more effectively.
The latest studies of the ovarian tissue in vitro culture technique are dedicated to the reconstruction of the ovarian corticomedullary structure. During folliculogenesis, in vivo growing follicles migrate from the more rigid ovarian cortex to the soft ovarian medulla (Xu et al., Reference Xu, Lawson, Yeoman, Molskness, Ting, Stouffer and Zelinski2013). Therefore, in a natural environment, mechanical stress reduction occurs in follicular cells. Reconstruction of the corticomedullary ovarian structure in vitro can be performed using combined hydrogels; for example, a fibrin-alginate hydrogel can be used (Shikanov et al., Reference Shikanov, Xu, Woodruff and Shea2009, Reference Shikanov, Xu, Woodruff and Shea2011; Jin et al., Reference Jin, Lei, Shikanov, Shea and Woodruff2010). A growing follicle secretes proteases that destroy fibrin, resulting in mechanical stress decreasing around the follicle and thus promoting its development (Shikanov et al., Reference Shikanov, Xu, Woodruff and Shea2011).
Another approach to reconstruct the ovarian corticomedullary structure in vitro is using an alginate-collagen system. The drop in which a single follicle is cultivated contains two compartments. The centre of the drop consists of collagen, and its periphery is formed with alginate. This technique allows to create a system with heterogenic mechanical tensions. Collagen is softer than alginate, so collagen imitates the medulla and alginate imitates the cortex (Choi et al., Reference Choi, Agarwal, Huang, Zhao and He2014). It has been shown that follicular development up to the late antral stage occurs in 28% of cases when an alginate–collagen system is used (Choi et al., Reference Choi, Agarwal, Huang, Zhao and He2014). Preparation of such a complex culture system can be performed using microfluidic technologies (Streets & Huang, Reference Streets and Huang2014). These technologies allow to create drops consisting of various substances in the required proportions by using directed microflows of liquids. The type of microflow is determined by the microfluidic chip configuration.
Technologies of 3D ovary reconstruction in vitro are being actively developed (Vanacker et al., Reference Vanacker, Luyckx, Dolmans, Des Rieux, Jaeger, Van Langendonckt, Donnez and Amorim2012; Luyckx et al., Reference Luyckx, Dolmans, Vanacker, Scalercio, Donnez and Amorim2013). The main conception of this technology is the encapsulation of ovarian follicles with other ovarian cells into the matrix. At the present time, this technique is at the first stage of its development but it seems to be very promising in the future. These systems will allow better reconstitution of in vivo conditions, resulting in the better stimulation of follicles’ growth and maturation.
Rodent ovarian follicles are most commonly used to develop ovarian tissue in vitro culture techniques. However, it is impossible to create an effective technology for medical practice on the basis of rodent ovarian tissue. Firstly, rodents have shorter ovarian cycles than the human female's one. Moreover, the ovarian tissue of primates has a higher density than the rodent one. Therefore, for the development of medical technology, studies on primate ovarian tissue in vitro culture are needed.
There is little scientific research related to primate ovarian follicles in vitro culture. However, the obtained results seem to be very promising. It has been shown that baboon follicles can produce mature oocytes that are capable of fertilization and can develop until the morula stage after fertilization via the intracytoplasmic sperm injection (ICSI) procedure (Xu et al., Reference Xu, Fazleabas, Shikanov, Jackson, Barrett, Hirshfeld-Cytron, Kiesewetter, Shea and Woodruff2011b).
Other research that was performed on rhesus monkeys has demonstrated that more rigid alginate hydrogels allow for better development of follicles than softer ones do (Hornick et al., Reference Hornick, Duncan, Shea and Woodruff2012). Moreover, mature oocytes capable of fertilization and further development up to the morula stage after fertilization by using the ICSI procedure have been obtained during a study on rhesus monkey ovarian follicles, using a fibrin-alginate hydrogel culture system (Xu et al., Reference Xu, Lawson, Yeoman, Molskness, Ting, Stouffer and Zelinski2013).
Research studies dedicated to human ovarian follicles in vitro culture are few in number. However, there are very encouraging results among that research. In the early works on human ovarian follicles in vitro culture, follicles were encapsulated into the agar (Roy and Treacy, Reference Roy and Treacy1993) or collagen gel (Abir et al. Reference Abir, Franks, Mobberley, Moore, Margara and Winston1997, Reference Abir, Roizman, Fisch, Nitke, Okon, Orvieto and Ben Rafael1999, Reference Abir, Fisch, Nitke, Okon, Raz and Ben Rafael2001), and they maintained their morphology. Moreover, some of the follicles developed until the early antral stages. A recent study has shown that alginate hydrogel permits the growth of encapsulated human ovarian follicles, corresponding to the normal folliculogenesis in vivo. Furthermore, an increase of oestradiol, progesterone and inhibin A during the period of cultivation has been observed (Xu et al., Reference Xu, Barrett, West-Farrell, Kondapalli, Kiesewetter, Shea and Woodruff2009). These hormones are required for normal follicular development.
A scientific group from Belgium has shown that human ovarian follicles can grow in alginate hydrogel. Moreover, in 90% of cases, follicular cells and oocytes retained their viability (Amorim et al., Reference Amorim, Van Langendonckt, David, Dolmans and Donnez2009).
In more recent work, researchers from the USA developed an alginate culture system that imitates follicles’ microenvironment in vivo. Moreover, this scientific group (Skory et al., Reference Skory, Xu, Shea and Woodruff2015) has developed alginate hydrogel ovarian follicles in vitro culture system that reconstitutes natural conditions. Hormone levels (17β-oestradiol, progesterone, inhibin A, inhibin B, activin A and anti-Müllerian hormone) of cultured follicles were observed by an enzyme-linked immunosorbent assay. When the level of 17β-oestradiol increased and stabilized on the highest value, ovulation was induced by the addition of human chorionic gonadotropin (hCG) and epidermal growth factor (EGF) into the culture medium. Due to the monitoring of follicular hormone secretion, researchers imitated menstrual cycle conditions in vitro by adding hormones and growth factors to the culture medium.
The human ovary contains a large portion of connective tissue, the amount of which varies greatly from patient to patient. Therefore, it appears impossible to develop a standard protocol of a primordial follicle's isolation from human ovarian tissue. This is due to the fact that the period of enzymatic treatment of human ovarian tissue for the obtaining of primordial follicles varies noticeably in different patients. Moreover, the loss of the integrity of primordial follicles with surrounding somatic cells, which may occur during this procedure, negatively influences their condition (Laronda et al., Reference Laronda, Duncan, Hornick, Xu, Pahnke, Whelan, Shea and Woodruff2014). Therefore, for better female fertility preservation, both techniques can be used: ovarian tissue cryopreservation in the case of primordial follicles, and ovarian follicle in vitro culture for follicles of advanced stages.
A two-step culture system is another approach that can also be performed for primordial follicle preservation. In these systems, small fragments of ovarian cortical tissue that contain primordial follicles are first cultured in order to stimulate follicular development. After the cultivation, follicles of advanced stages of folliculogenesis (primary, secondary, etc.) are isolated from the ovarian tissue. Subsequent cultivation is carried out with the obtained follicles (Telfer et al., Reference Telfer, McLaughlin, Ding and Thong2008; McLaughlin et al., Reference McLaughlin, Kinnell, Anderson and Telfer2014).
Akt-signalling pathway inactivation allows to stimulate growth of primordial follicles in vitro. The Akt-pathway suppressor is PTEN (phosphatase and tensin homologue). It has been shown that PTEN inactivation results in an increase in the number of growing ovarian follicles in the cases of humans (McLaughlin et al., Reference McLaughlin, Kinnell, Anderson and Telfer2014) and mice (Jagarlamudi et al., Reference Jagarlamudi, Liu, Adhikari, Reddy, Idahl, Ottander, Lundin and Liu2009).
Nowadays, mature oocytes that are capable of fertilization can be obtained not only from rodents but also from primates. Furthermore, these oocytes can develop after fertilization. Designing human ovarian tissue in vitro culture systems is a very complicated problem. Firstly, it is more difficult to obtain human ovarian tissue for the experiments than in the case of animal models. Secondly, human follicular development in vivo is longer than that of rodents, for example. Due to these facts, human ovarian tissue in vitro culture systems are at the very earliest stages of their development. However, the growth of human ovarian follicles in vitro has still been observed. It is likely that findings that will allow the obtaining of mature human oocytes from ovarian tissue using in vitro culture may appear in the near future.
Conclusion
The first papers dedicated to the cryopreservation of mammalian ovarian tissue were published in 1994 (Aubard et al., Reference Aubard, Teissier and Baudet1994; Harp et al., Reference Harp, Leibach, Black, Keldahl and Karow1994), more than 20 years ago. However, to date, we know of only about a few dozen live births after ovarian tissue orthotopic retransplantation and one live birth after heterotopic retransplantation. In many cases, after ovarian tissue retransplantation, it is impossible to determine whether ovulation occurred in the transplanted tissue or in a remaining fragment of the ovary, especially in the event of natural conception. Therefore, the efficacy of ovarian tissue cryopreservation followed by retransplantation is low. Moreover, the ovarian tissue retransplantation technique cannot be applied in many cases. Retransplantation of ovarian tissue is an additional surgical procedure that can be dangerous for patients who have undergone chemo- or radiotherapy. Furthermore, it is necessary to detect the absence of malignant cells in transplanted tissue. For this reason, several pieces of tissue should be used for analysis that cannot then be transplanted, which reduces the probability of pregnancy. For a time after retransplantation, ovarian tissue is subjected to weak nutrition until angiogenesis is complete. A deficit of nutrients and oxygen leads to apoptosis and atresia in transplanted tissue, which also reduces the probability of pregnancy.
In comparison with the cryopreservation of ovarian tissue followed by retransplantation of thawed fragment ovarian tissue, the in vitro culture technique has a few principal advantages. Firstly, the retransplantation of ovarian tissue is not needed. Secondly, mature oocytes can be obtained and fertilized in vitro, and then embryos can be cryopreserved or transferred into the patient's uterus or into the womb of a surrogate mother. In the case where ovarian tissue cryopreservation is applied for fertility preservation, surrogate mother programmes require additional procedures such as hormonal stimulation and follicular punctures. These procedures can have a negative effect on patients’ health. An ovarian tissue in vitro culture programme of fertility preservation allows to avoid the risky procedures mentioned above. Moreover, this method permits use of all obtained tissue for the culture. Analysis for the presence of malignant cells in the culture can be performed after tissue culturing. Therefore, the ovarian tissue in vitro culture technique increases the probability of pregnancy when compared with ovarian tissue cryopreservation. The ovarian tissue in vitro culture method allows observation of each follicle individually, and the effective provision of them with necessary nutrients.
Although the ovarian tissue in vitro culture is not currently a well developed technique that is applied widely in clinical practice, there are many results showing the efficacy of this method (Xu et al., Reference Xu, Lawson, Yeoman, Pau, Barrett, Zelinski and Stouffer2011a; Jin et al., Reference Jin, Lei, Shikanov, Shea and Woodruff2010; Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006b; Choi et al., Reference Choi, Agarwal, Huang, Zhao and He2014; Xu et al., Reference Xu, Lawson, Yeoman, Molskness, Ting, Stouffer and Zelinski2013; Shikanov et al., Reference Shikanov, Xu, Woodruff and Shea2011; Shikanov et al., Reference Shikanov, Xu, Woodruff and Shea2009). The ovarian tissue in vitro culture technique allows to obtain mature oocytes capable of fertilization and further development, not only in the case of rodents (Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006b), but also for primates (Xu et al., Reference Xu, Fazleabas, Shikanov, Jackson, Barrett, Hirshfeld-Cytron, Kiesewetter, Shea and Woodruff2011b; Xu et al., Reference Xu, Lawson, Yeoman, Molskness, Ting, Stouffer and Zelinski2013). Moreover, the growth of human follicles was observed when using this method (Xu et al., Reference Xu, Barrett, West-Farrell, Kondapalli, Kiesewetter, Shea and Woodruff2009).
Therefore, the ovarian tissue in vitro culture technique seems more promising than ovarian tissue cryopreservation followed by thawed tissue retransplantation. The principal advantages and drawbacks of the methods mentioned above are listed in Table 5. In contrast with cryopreservation, the ovarian tissue in vitro culture technique allows the avoidance of secondary surgical intervention and patient hormonal stimulation. Moreover, in the case of the ovarian tissue in vitro culture technique, it is easier to provide better nutrition for follicles and to observe their growth and development. All of these advantages help practitioners to yield better results.
Table 5 Female fertility preservation strategies’ comparison: ovarian tissue in vitro culture vs. cryopreservation

Acknowledgements
None.
Financial support
This study was supported by the Russian Science Foundation (project no. 14–50–00029).
Statement of interest
None.







