Stem cells have pluripotency and the property of self-renewal as well as differentiate into multiple cell lineages, including endothelial cells and cardiomyocytes.Reference Weissman1 Mesenchymal stem cell transplantation may improve the ventricular function in patients with acute myocardial infarctionReference Assmus, Honold and Schachinger2 without provoking immune rejection,Reference Tse, Pendleton, Beyer, Egalka and Guinan3–Reference Liechty, MacKenzie and Shaaban6 even in xenograft setting.Reference Saito, Kuang, Bittira, Al-Khaldi and Chiu7 Electrical pacing is of great utility in many cardiovascular diseases.Reference Abraham, Fisher and Smith8, Reference Cleland, Daubert and Erdmann9 However, the effects of mesenchymal stem cell transplantation with concomitant pacing have not been established clearly. We reported earlier that mesenchymal stem cell transplantation induces cardiac nerve sprouting in a swine model of myocardial infarction.Reference Pak, Qayyum and Kim10 In contrast to cardiac sympathetic hyper-innervation may improve ventricular function, it may also induce lethal arrhythmias.Reference Cao, Chen and KenKnight11 Therefore, we hypothesised that human mesenchymal stem cell transplantation changes the cardiac sympathetic nerve, endothelial cells, and gap junction with potential arrhythmia, but concomitant electrical pacing (P) has additional biological effects in canine heart. The purposes of this study were to evaluate the mechanisms of their changes and to determine the risk of pro-arrhythmia after human mesenchymal stem cell transplantation and electrical pacing. We also evaluated the effects of electrical stimulation on human mesenchymal stem cell in vivo and in vitro models by analysing mRNA expressions of nerve growth factor-β, vascular endothelial growth factor, and connexin43.
Methods
All experiments were performed in accordance with the Institutional Review Board and Institutional Animal Care and Use Committee. The investigation conformed to the guidelines of the American Heart Association on human and animal studies.
Isolation and culture of human bone marrow derived mesenchymal stem cell
Human bone marrow was harvested from the posterior iliac crest of healthy volunteers for bone marrow transplantation. Human bone marrow derived mononuclear cells were isolated by density gradient centrifugation using Ficoll separating solution (American Pharmacia Biotech, Piscataway, New Jersey, United States of America). DMEM-LG (Life Technologies, Gibco BRL, Karlsruhe, Germany) supplemented with 10% foetal calf serum (biochrom AG) and cultured at 37°C and 5% CO2. On day 3, non-adherent cells were removed. A small quantity of cells was kept for phenotyping by flow cytometry. The nature of cultured cells was characterised as human mesenchymal stem cells by flow-cytometry (CD29+, CD44+, CD90+, CD105+, CD31−, 34−, and CD45−) and real-time and quantitative polymerase chain reaction (Oct-4 gene expression).Reference Abraham, Fisher and Smith8, Reference Cleland, Daubert and Erdmann9 After seven days, individual colonies were collected, isolated, cultured, and expanded. When mesenchymal stem cells grew to approximately 80% confluency, cells were digested with 0.25% trypsin-1 mM ethylene diamine tetraacetic acid (EDTA) for about 3–5 minutes at 37°C until most of the cells were detached. With two passages, homogeneous mesenchymal stem cells that were devoid of hematopoietic cells were used for cell transplantation.
Device implanation and rhythm monitoring
A total 18 mongrel dogs weighing 28–35 kilograms were implanted an epicardial pacemaker (Epicardial lead-IS1 B1 BBL 083659B, St Jude Medical Inc., Minnetonka, Minnesota, United States of America, Generator AFFINITY DR 5330L DDDR, St Jude Med Inc.) by left lateral thoracotomy. Pacing electrodes were fixed on the left atrial appendage and left ventricular mid-anterolateral wall, respectively (Fig 1a and 1b). The pacemaker generator was implanted in the lateral chest after closing the pericardium. We programmed DDD pacemaker with the minimal A-V delay (50 milliseconds) to set the atrial sensing and ventricular pacing with the pacing output 3.5 volts (Fig 1d). Pacemaker was followed up at 24 hours, 1 week, and 2 weeks after surgery and the appropriate pacing was confirmed.
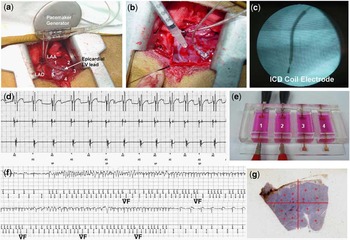
Figure 1 Experimental procedures. (a) Epicardial pacemaker leads were sutured on the left ventricular anterolateral wall. Human mesenchymal stem cell injection sites were marked with suture material around the ventricular pacing lead (numbers 1–5). (b) Human mesenchymal stem cells were transplanted by direct epicardial injection. (c) The implantable cardioverter defibrillator lead was positioned through the left internal jugular venous approach, and the position of the implantable cardioverter defibrillator coil electrode was confirmed by fluoroscopic imaging. (d) Pacemaker electrogram shows successful ventricular pacing preceded by atrial sensing with short atrio-ventricular delay (70 milliseconds: DDD mode). (e) A specially designed culture device for in vitro electrical pacing of human mesenchymal stem cell. Electrical stimulation was delivered to the left side culture wells (1 and 2), and right side cultures (3 and 4) were used as control human mesenchymal stem cell. (f) Stored implantable cardioverter defibrillator electrogram recorded from dogs that were transplanted with human mesenchymal stem cell. Implantable cardioverter defibrillator electrogram reveals an episode of non-sustained ventricular fibrillaton. This dog survived for 4 weeks. (g) We selected the microscopic fields at four different sites in each quadrants for the quantification of immunohistochemical staining.
At the same time of pacemaker implantation, an implantable cardioverter defibrillator (ICD: Model 7275 VVED-DDDR, Medtronic Inc., Minneapolis, Minnesota, United States of America) was implanted in all the experimental animals by the left internal jugular transvenous approach for the rhythm monitoring. The implantable cardioverter defibrillator coil electrode was positioned at right ventricular apex endocardially (Fig 1c). The implantable cardioverter defibrillator generator was implanted in the lateral chest. The ventricular fibrillation was detected when ventricular rate exceeds 210 beats per minute (Fig 1f). To prevent multiple implantable cardioverter defibrillator shock induced cellular or biological changes, we set implantable cardioverter defibrillator with monitoring mode without therapy. Implantable cardioverter defibrillator were followed up at 24 hours, 1 week, and 2 weeks after surgery. The documented arrhythmic events in implantable cardioverter defibrillator were manually analysed.
Human mesenchymal stem cell transplantation in vivo canine ventricle
We injected cells or vehicle at the same time as pacemaker/implantable cardioverter defibrillator implantation, immediate after fixation of pacing electrode. For mesenchymal stem cell transplantation, the dog hearts were selected five sites at least 15 millimetres away from epicardial pacing lead, left ventricular mid-anterolateral wall. These five sites were marked with suture material (Fig 1a); 1 × 107 human mesenchymal stem cells in 1 millilitre of medium, 0.2 millilitre in each point, were injected to the epicardially at five different sites (Fig 1b). We did not use immune suppression on the basis of the previous study result.Reference Saito, Kuang, Bittira, Al-Khaldi and Chiu7, Reference Plotnikov, Shlapakova and Szabolcs12
The animals were assigned to three different groups: sham-operated group, implantations of pacemaker and implantable cardioverter defibrillator, suture marking at injection sites, injection of same volume of cell depleted solution, and pacemaker off (sham; n = 5); human mesenchymal stem cell without pacing group (hMSC; n = 6); and human mesenchymal stem cell with pacing group (hMSC + P; n = 7). After 4 weeks of survival, the animals were killed 32.1 plus or minus 5.7 days after the first surgery and cardiac tissue were analysed for immunohistochemistry, real-time and quantitative polymerase chain reaction. However, the cardiac tissues taken from three dogs those died suddenly within 4 weeks were not included for the analyses.
Three-dimensional activation mapping
In two dogs of human mesenchymal stem cell group, three-dimensional activation maps were generated by left ventricular endocardial bipolar mapping with NavX system (St Jude Medical Inc.) at 4 weeks of survival after human mesenchymal stem cell transplantation. After making left ventricular endocardial geometry with roving catheter, the activation electroanatomical map was generated by point-by-point contact mapping more than 100 points on the left ventricular endocardium during high right atrial pacing with pacing cycle length 500 milliseconds. The area of human mesenchymal stem cell transplantation was marked on the three-dimensional electroanatomical map at the locus of the fluoroscopically visible pacing electrode, where human mesenchymal stem cells were transplanted.
Electrical pacing of human mesenchymal stem cell in vitro culture
To determine whether pacing alone can change mRNA expressions related to cardiac nerve sprouting, angiogenesis, and gap junction, five sets of human mesenchymal stem cell (6 × 106 cells per millilitre, low glucose DMEM (1 gram per litre glucose, l-glutamine, 110 milligrams per litre Sodium Pyruvate)+ 1% antibiotics) were cultured in a specially designed culture device for electrical pacing (2.5 volts, 2 milliseconds pulse width, 80 beats per minute; Fig 1e). After 5 days of human mesenchymal stem cell culture with or without electrical pacing, real-time and quantitative polymerase chain reaction for human nerve growth factor-β, vascular endothelial growth factor, and human connexin43 (Taqman Gene Expression Assays, Applied Biosystem Inc., Foster City, California, United States of America) were examined.
Immunohistochemical study
The hearts were fixed with 4% formalin. They were then sampled, paraffin embedded, and processed routinely for immunohistological examinations. We performed tyrosine hydroxylase staining to detect sympathetic nerves, von Willebrand factor immunostaining for endothelial cells, connexin43 immunostaining for gap junctions, and human nucleolin staining to detect human mesenchymal stem cell, respectively. Formalin-fixed, paraffin embedded transmural 5 micrometres thick sections were stained using a modified immunocytochemical AB complex method for tyrosine hydroxylase, von Willebrand factor, connexin43, and human nucleolin as described previously.Reference Hamabe, Okuyama and Miyauchi13 Primary antibody concentrations were 1:200 for tyrosine hydroxylase (Abcam Inc., Cambridge, United Kingdom), 1:500 for von Willebrand factor (Dako, Copenhagen, Denmark), 1:100 for connexin43 (Chemicon International Inc., United States of America), and 1:400 for human nucleolin (Abcam Inc.), respectively. The immunoreactive products were visualised by incubating the tissue sections with the DAKO Liquid DAB Substrate Chromogen system (Dako) and counterstained with diluted haematoxylin.
mRNA isolation and quantitative real-time polymerase chain reaction
The expressions of mRNAs of nerve growth factor β, von Willebrand factor and connexin43 were quantified using real-time and quantitative polymerase chain reaction. Reverse transcription was carried out to produce the cDNA using TaqMan reverse-transcription kit (Applied Biosystems, Massachusetts, United States of America). Relative quantification of mRNA levels was determined by the prism 7700 Sequence Detection System (Applied Biosystems) directly monitored the fluorescent signal. Real-time and quantitative polymerase chain reaction was performed with AmpliTaq Gold polymerase (Perkin-Elmer ABI, Foster City, California, United States of America) using 20 nanogram of cDNA per reaction (Taqman Gene Expression Assays, Applied Biosystem Inc.).Reference Zhou, Chen and Miyauchi14 The cycle threshold (Ct) values for 18 seconds rRNA and the mRNA of interest were compared and calculated using sequence detector software (Perkin-Elmer ABI). Relative transcript levels were calculated as ![]() , where ΔΔCt = ΔE−ΔC and ΔE = Ct experimental − Ct 18s rRNA; ΔC = Ct control − Ct18s rRNA. For comparison, the Ct-value of the sham-operated group was used as the normal control. ΔE is the Ct value for any sample normalized to the endogenous housekeeping gene
, where ΔΔCt = ΔE−ΔC and ΔE = Ct experimental − Ct 18s rRNA; ΔC = Ct control − Ct18s rRNA. For comparison, the Ct-value of the sham-operated group was used as the normal control. ΔE is the Ct value for any sample normalized to the endogenous housekeeping gene ![]() ΔE = Ct (experimental target) − Ct (normaliser/calibrator/reference); ΔC is the Ct value for the calibrator also normalized to the endogenous housekeeping gene
ΔE = Ct (experimental target) − Ct (normaliser/calibrator/reference); ΔC is the Ct value for the calibrator also normalized to the endogenous housekeeping gene ![]() ΔC=Ct (control target)−Ct(normaliser/calibrator/reference).
ΔC=Ct (control target)−Ct(normaliser/calibrator/reference).
Data analyses
Tyrosine hydroxylase, von Willebrand factor, and connexin43-stained slides were evaluated for the densities of cardiac sympathetic nerves, vascular endothelial cells, and connexin43-positive gap junctions, respectively. The selection and analyses of digital microscopic images were performed by a single student, J-H Park, who was blinded to the experimental information of histological slides with consistent method. To quantify the tyrosine hydroxylase-positive cardiac sympathetic nerves, we selected the microscopic fields with the highest nerve density, and took the image pictures at a ×100 objective in the right upper and lower, left upper and lower quadrants of each slide. In each of the four fields, the cardiac nerves were identified as tyrosine hydroxylase immunostaining-positive fibrillar structures, between myocardial cells, that were longer than 10 micrometres and stained brown; RGB values: Red 22–125, Green 4–77, and Blue 4–55. The von Willebrand factor immunostaining-positive endothelial cells were quantified with the similar method at a ×200 power magnification. We chose the medium sized arteriole, diameter 20–60 micrometres at the centre of the image, and took 16 pictures in each slide (Fig 1g). The connexin43-positive gap junctions were identified by connexin43 immunostaining-positive linear structures located on the cell membranes of each myocardial cell, and we obtained digital pictures of the tissue slides at a ×400 power magnification and quantified the percent area of connexin43-positive gap junctions in four quadrants of each slide. The immunostained percent areas of cardiac sympathetic nerves, endothelial cells, and connexin43-positive gap junctions were quantified using Image Pro software (Media Cybernetics Inc., Silver Spring, Maryland, United States of America). These densities were derived from the nerve, vessel or gap junction areas divided by the total area examined, percent area, respectively. To determine the immune rejection, a pathologist who is a specialist for rejection after organ transplantation, G.-I. Kim, reviewed all slides and found no evidence of cellular rejection. All values were expressed as mean plus or minus standard deviation. Between-group comparison was carried out with analysis of variance post hoc analysis Tukey method for continuous variables. To compare the risk of ventricular fibrillation or sudden cardiac death, Fisher’s exact test was performed. Statistical significance was defined as a p < 0.05.
Results
Human mesenchymal stem cell transplantation increases nerve growth factor-β expression and cardiac sympathetic nerves
Real-time and quantitative polymerase chain reaction for mRNA of nerve growth factor-β was performed with tissue removed from the area of human mesenchymal stem cell injection. In comparison with the sham-operated group, nerve growth factor-β mRNA expressions in the human mesenchymal stem cell group was 56.0 plus or minus 66.83 (p < 0.02) fold higher (Fig 2a). We evaluated tyrosine hydroxylase-positive sympathetic cardiac nerve densities at areas of human mesenchymal stem cell injection, with 1052 digital images at areas of the highest cardiac nerve densities on 263 slides. Figure 2c–2e shows the distributions of tyrosine hydroxylase (TH)-positive cardiac sympathetic nerves in each group. Human mesenchymal stem cell hearts and mesenchymal stem cell with pacing group hearts showed very high sympathetic nerve densities. There were actively arborising large nerves and tyrosine hydroxylase-positive ganglia. By contrast, dogs in the sham-operation group rarely had tyrosine hydroxylase-positive sympathetic nerve twigs. The calculated percent areas cardiac sympathetic nerves in the tissue of human mesenchymal stem cell group, 0.51 plus or minus 0.40%, was significantly higher than those of sham group, 0.15 plus or minus 0.13%, p < 0.05; Fig 2b. The cardiac sympathetic nerve densities taken from human mesenchymal stem cell injection site, left ventricular mid anterolateral wall, and right ventricular mid free wall were not different significantly, 0.47 plus or minus 0.39% versus 0.51 plus or minus 0.40%; p-value is non-significant.

Figure 2 (a) mRNA expression levels for nerve growth factor-β demonstrated a significantly higher expression of nerve growth factor-β in the human mesenchymal stem cell group compared with sham-operated group. (b) Calculated percent area of tyrosine hydroxylase-positive (sympathetic) nerves was significantly higher in the human mesenchymal stem cell transplanted tissues compared with the sham group. (c–e) Tyrosine hydroxylase immunostaining of ventricular tissue close to the area of human mesenchymal stem cell injection at ×100 magnification power. The human mesenchymal stem cell and human mesenchymal stem cell with epicardial pacing groups show significant sympathetic hyper-innervation after 4 weeks of survival. By contrast, the sham group did not show any significant increase of sympathetic nerves.
Human mesenchymal stem cell transplantation increases vascular endothelial growth factor expression and vascular endothelial cells
The expression of vascular endothelial growth factor mRNA was 7.09 plus or minus 6.94-fold higher in human mesenchymal stem cell group as compared with sham-operated group (p < 0.001, Fig 3a). To quantify von Willebrand factor-positive endothelial cells, we calculated percent area of von Willebrand factor at the area of medium sized arterioles in 247 digital images taken from 15 slides (Fig 3c–3e). The percent areas of endothelial cells were significantly higher in human mesenchymal stem cell (0.69 plus or minus 0.49%, p < 0.001) and human mesenchymal stem cell with pacing group (0.32 plus or minus 0.29%, p < 0.001) as compared with sham group (0.16 plus or minus 0.18%, Fig 3b). The density of endothelial cells in human mesenchymal stem cell was also higher then that in human mesenchymal stem cell with pacing group (p < 0.001).

Figure 3 (a) mRNA expression levels for vascular endothelial growth factor demonstrated a significantly higher expression of vascular endothelial growth factor in the human mesenchymal stem cell group compared with sham-operated group. (b) Calculated percent areas of von Willebrand factor-positive endothelial cells were significantly larger in the human mesenchymal stem cell transplanted tissues compared with sham or human mesenchymal stem cell with pacing groups, and significantly larger in the human mesenchymal stem cell with pacing group than sham group. (c–e) von Willebrand factor-positive endothelial cells immunostaining of ventricular tissue close to the area of human mesenchymal stem cell injection at ×200 magnification power. The human mesenchymal stem cell and human mesenchymal stem cell with pacing groups show significant angiogenesis. By contrast, the sham group did not show any significant increase of von Willebrand factor-positive endothelial cells.
Human mesenchymal stem cell transplantation reduces connexin43 expression and connexin43-positive gap junctions
The expression of connexin43 mRNA was significantly lower in human mesenchymal stem cell group (0.59 plus or minus 0.29-fold, p < 0.0001) than in sham-operated group (Fig 4a). In contrast, connexin43 expression was higher in mesenchymal stem cell with pacing group (2.04 plus or minus 1.39-fold, p < 0.02) as compared with sham-operated group. In the quantification of connexin43-positive gap junction at 416 digital images on 26 slides stained with connexin43 immunostaining, the percent area of connexin43-positive gap junction was significantly lower in human mesenchymal stem cell group (1.64 plus or minus 0.79%) compared with sham-operated group (2.12 plus or minus 1.07%, p < 0.001; Fig 4b–4e). However, the density of connexin43-positive gap junction was higher in mesenchymal stem cell with pacing group (2.62 plus or minus 1.59%) than in human mesenchymal stem cell group (1.64 plus or minus 0.78%, p < 0.001) or sham (2.12 plus or minus 1.07%, p < 0.001, Fig 4b).

Figure 4 (a) mRNA expression levels for connexin43-demonstrated a significantly lower expression of connexin43 in human mesenchymal stem cell group, and higher expression in human mesenchymal stem cell with pacing group compared with sham-operated group. (b) Calculated percent areas of connexin43-positive gap junction were also smaller in human mesenchymal stem cell group, but larger in human mesenchymal stem cell with pacing group compared with sham group. (c–e) Connexin43 immunostaining of ventricular tissue close to the area of human mesenchymal stem cell injection at ×400 magnification power. The density of connexin43-positive gap junction is lower in human mesenchymal stem cell transplanted tissue, but higher in human mesenchymal stem cell with pacing tissue compared with sham after 4 weeks of survival.
In the three-dimensional activation mapping study of left ventricle in two dogs of human mesenchymal stem cell group, the remarkable conduction delays were noted at the area of human mesenchymal stem cell injection (Fig 5a).

Figure 5 (a) Activation map of left ventricular endocardium during high right atrial pacing, cycle length 500 milliseconds, in right anterior oblique view and right anterior oblique cranial view in the animals with human mesenchymal stem cell group. As showed in the color scale bar, the impulse conducts from white color to purple color, and the earliest activation site is a septum. However, there is a significant conduction delay at left ventricular anterior wall, black dotted circle, where human mesenchymal stem cell was transplanted and connexin43 expression was reduced. (b–d) The effects of electrical pacing on mRNA expressions of nerve growth factor-β, vascular endothelial growth factor, and connexin43 in vivo and in vitro models. The electrical pacing did not affect the expressions of mRNA of nerve growth factor-β and vascular endothelial growth factor in both in vivo and in vitro experiments (b) and (c). However, in vitro electrical pacing reduced the expression of connexin43 mRNA significantly (d).
Electrical pacing increases connexin43 expression in vivo, but reduces connexin43 expression in vitro
Figure 5b–5d show the effects of electrical pacing in vivo and in vitro studies. In the quantification of mRNA of nerve growth factor-β and vascular endothelial growth factor, the effects of electrical pacing were not significantly different in vivo and in vitro studies. In terms of connexin43 mRNA expression, in vitro electrical pacing of human mesenchymal stem cell culture reduced connexin43 expression (0.76 plus or minus 0.11-fold) as compared with human mesenchymal stem cell culture without pacing (p < 0.02, Fig 5d). In contrast, in vivo electrical pacing tended to be higher expression of connexin43 (2.04 plus or minus 1.39-fold) than in vivo human mesenchymal stem cell group without pacing (0.59 plus or minus 0.29-fold, p-value is non-significant; Fig 5d). This discrepancy suggests the paracrine action of electrical pacing on connexin43 mRNA expression and stretch induced up-regulation of connexin43 expression.
Ventricular fibrillation and sudden cardiac death after human mesenchymal stem cell transplantation
There was no surgical mortality in this study. Table 1 summarises the incidences of ventricular fibrillation and sudden cardiac death. Among 13 animals transplanted with human mesenchymal stem cell, three dogs died suddenly, 8 plus or minus 8.7 days after surgery; implantible cardioverter defibrillator recorded ventricular fibrillation at the time of all sudden cardiac death episodes. In the necropsy, no other cardiovascular reason for sudden cardiac death other than ventricular fibrillation was found. Another two dogs manifested non-sustained ventricular tachycardia/ventricular fibrillation, but did not die suddenly (Fig 1f). Implantible cardioverter defibrillator electrograms revealed spontaneous ventricular tachycardia/ventricular fibrillation in 5/13 dogs (38.5%) and sudden cardiac death related to ventricular fibrillation in 3/13 dogs (23.1%) of those dogs that underwent human mesenchymal stem cell transplantation. In comparison, no ventricular fibrillation or sudden cardiac death episode was documented in the sham-operated group (0/5; 0%; p-value is non-significant).
Table 1 Frequencies of ventricular tachyarrhythmias and sudden cardiac death for each study group.
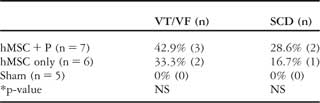
NS, non-significant; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia
*Sham versus hMSC + P or hMSC only
Discussion
In this study, we found that cellular transplantation of human mesenchymal stem cell in a canine heart resulted in nerve growth factor-β induced cardiac sympathetic hyper-innervation, vascular endothelial growth factor induced angiogenesis, but reduced expressions of connexin43 mRNA and connexin43-positive gap junctions. In contrast, concomitant electrical pacing increased connexin43-positive gap junction by paracrine action. Human mesenchymal stem cell transplantation with or without electrical pacing encountered ventricular fibrillation and sudden cardiac death in this model.
Limitations of human mesenchymal stem cell in cardiac cell therapy
As the human mesenchymal stem cell has a capability to differentiate into many cell types, including cardiomyocytesReference Pittenger, Mackay and Beck15 without provoking immune rejection,Reference Tse, Pendleton, Beyer, Egalka and Guinan3–Reference Saito, Kuang, Bittira, Al-Khaldi and Chiu7 it has been considered as a good candidate for the cardiac cell therapy. However, both the risk of proarrhythmia and the issues for the cell to cell coupling with the recipient heart should be resolved. The risk of proarrhyhmia was first reported after transplantation of skeletal myoblast.Reference Menasche, Hagege and Vilquin16 The observed arrhythmias may be due to the lack of gap junctions expressed in transplanted cells, which results in large excitable gaps in the recipient heart and aberrant electrical activity. Consistent to the previous studies,Reference Chang, Tung and Sekar17, Reference Beeres, Atsma and van der Laarse18 gap junction expression was reduced and a local conduction was delayed at the sites of human mesenchymal stem cell transplantation in this study. Even though gap junctions exist, poor differentiation to the functioning cardiomyocyte or insufficient ion channel expression may induce conduction delay, localised re-entry, and proarrhythmia. The localised high density of transplanted human mesenchymal stem cell by local intra-myocardial injection may exaggerate the problems of cell to cell conduction and proarrhythmia,Reference Fukushima, Varela-Carver and Coppen19 because the previous studies utilising intra-coronary delivery of endothelial progenitor cells did not report any lethal arrhythmic event.Reference Assmus, Honold and Schachinger2, Reference Schachinger, Erbs and Elsasser20, Reference Lunde, Solheim and Aakhus21 However, intra-coronary infusion of human mesenchymal stem cell has a risk of coronary embolism due to the large size of human mesenchymal stem cell.
Cardiac sympathetic hyperinnervation and sudden cardiac death
Sympathetic hyperinnervation may contribute to the improvement of ventricular function in the failing heart. However, sympathetic nerve sprouting, and its heterogeneity, is a known substrate for lethal ventricular arrhythmias.Reference Cao, Chen and KenKnight11, Reference Zhou, Chen and Miyauchi14 Sympathetic nerve activation exerts significant effects on the electrophysiologic properties such as automaticity, triggered activity, refractoriness, and conduction velocity of myocardial cells.Reference Martins and Zipes22, Reference Opthof, Misier and Coronel23 Although we previously reported enhanced nerve sprouting in a mesenchymal stem cell transplanted swine model,Reference Pak, Qayyum and Kim10 sympathetic hyper-innervation and its mechanism were not proven at that time. In this study, we first proved the elevation of nerve growth factor-β expression and sympathetic hyper-innervation in a canine model of transplanted human mesenchymal stem cell.
Change of connexin43-positive gap junction after human mesenchymal stem cell transplantation
The cell to cell coupling of transplanted cells with recipient cardiac cell is essential to maintain the conduction and mechanical function, and short of gap junctions may result in local conduction delay and proarrhythmia. It has been reported that human mesenchymal stem cell expresses gap junction,Reference Valiunas, Doronin and Valiuniene24, Reference Pijnappels, Schalij and van Tuyn25 and can be enhanced by growth factors.Reference Hahn, Cho and Kang26 However, Beeres et alReference Beeres, Atsma and van der Laarse18 demonstrated that mesenchymal stem cells express gap junctions with sufficient coupling between recipient and donor cells, but the impulse was propagated passively over rather short distances. As the cells are not excitable, they will be unable to carry the action potential over larger distances, and unlikely to support contractility due to the absence of a well-developed functional contractile apparatus. Chang et alReference Chang, Tung and Sekar17 also reported potential arrhythmia in an optical mapping study of co-cultured mesenchymal stem cell and ventricular myocytes, as a result of reduced conduction velocity due to increased tissue heterogeneity; this was observed despite the presence of functional gap junctions involving mesenchymal stem cells. Consistent to the previous studies, both the expression of gap junction and a local conduction were delayed at the sites of human mesenchymal stem cell transplantation in this study.
The effects of electrical pacing
In this study, in vitro electrical pacing of human mesenchymal stem cell culture reduced mRNA expression of connexin43 as compared with human mesenchymal stem cell culture without pacing. In contrast, in vivo electrical pacing tended to be higher expression of connexin43 than in vivo human mesenchymal stem cell group without pacing. This discrepancy suggests that localised electrical pacing of left ventricular epicardium may result in focal strain and stretch induced up-regulation of connexin43 expressionReference Zhuang, Yamada, Saffitz and Kleber27 or the paracrine action of human mesenchymal stem cell in vivo model. The reason for the reduction of connexin43 expression in vitro electrical stimulation is not clear. Although there have been reports that in vitro electrical stimulation co-culture of myofibroblasts with rabbit ventricular myocyte or myoblast express gap junction,Reference Chilton, Giles and Smith28, Reference Kawahara, Yamaoka and Iwata29 pacing in human mesenchymal stem cell culture has not yet been reported. Under the same condition, the incidence of ventricular tachycardia/ventricular fibrillation tended to be higher in mesenchymal stem cell with pacing group than sham, p-value is non-significant, in spite of better gap junction expressions. There are several potential explanations for this outcome, such as, cardiac sympathetic nerve sprouting, transmural dispersion of repolarisation by epicardial pacing, or unknown effect of xenograft cell therapy in mesenchymal stem cell with pacing group as compared with sham. However, we do not know the exact mechanism. It was reported that electrical pacing induces capillary growth by mechanical factor and release of angiogenic factors,Reference Hudlicka, Wright and Ziada30 and percent area of von Willebrand factor-positive endothelial cells was significantly higher in mesenchymal stem cell with pacing group than sham, but lower than in human mesenchymal stem cell in this study. Therefore, it is hard to say the physiological meaning of electrical pacing on angiogenesis in this specific animal model without direct comparison of sham and pacing alone.
Study limitations
There were multiple factors for ventricular tachycardia/ventricular fibrillation in human mesenchymal stem cell transplanted hearts independently of sympathetic hyper-innervation. However, sympathetic hyper-innervation and reduced gap junction after human mesenchymal stem cell transplantation are novel findings, and might be contributing (not only) factors those cause ventricular fibrillation. Although a pathologist determined no evidence of immune rejection, we cannot absolutely exclude undetected proarrhythmic effects from the cellular xenograft. We applied left ventricular mid anterolateral wall epicardial pacing in normal canine heart, instead of cardiac resynchronisation therapy in the heart with left ventricular dysfunction. For the activation mapping, endocardium was mapped due the post-surgical fibrosis on the corresponding epicardium in the limited number of animal. Marking with suture material can be the cause of obstacle formation, which provokes ventricular arrhythmia. The increase of connexin43 protein expression does not mean the increase of gap junctions directly. Although we performed quantitative real-time–polymerase chain reaction in this study, we did not do additional quantification of nerve growth factor-β, connexin43, and vascular endothelial growth factor by western blot. We also did not characterise the potential differentiation of human mesenchymal stem cell to osteoblast, adipocyte, or cardiomyocyte. We did not perform long-term monitoring of the experimental animals.
Conclusion
We first reported a potential proarrhythmia of human mesenchymal stem cell transplantation in canine model, associated with nerve growth factor-β induced cardiac sympathetic hyper-innervation and reduced connexin43-positive gap junction. However, the combined electrical pacing in vivo model rather increased connexin43-positive gap junctions by paracrine action, suggesting potential benefits of combined electrical pacing with cardiac cell therapy.
Acknowledgements
This work was supported by a grant of the Korea Health 21 R&D Project (A085136), Ministry of Health & Welfare, Republic of Korea, and Korean Society of Circulation Industry-University Cooperation. We thank Mr Do Young You for a technical assistance and Mr John Martin for his linguistic assistance.








