The past decade has seen great efforts to elucidate mechanisms that underlie psychopathology. One key premise guiding these efforts is that identifying such mechanisms would guide treatment and prevention, and inform early identification of high-risk cases. To this end, endophenotypes that presumably lie along a causal pathway between genes and their distal manifestations (i.e., phenotypes) have received particular attention (Dubin et al., Reference Dubin, Weissman, Xu, Bansal, Hao and Liu2012; Gottesman & Gould, Reference Gottesman and Gould2003; Simmons & Drevets, Reference Simmons and Drevets2012; Stewart, Bismark, Towers, Coan, & Allen, Reference Stewart, Bismark, Towers, Coan and Allen2010). According to Gottesman and Gould (Reference Gottesman and Gould2003), the following criteria identify an endophenotype: association with an illness (Criterion A), state independence (Criterion B), heritability (Criterion C), higher rates among family members at high risk for the disorder than in the general population (Criterion D), and cosegregation with illness within families (Criterion E).
Although the search for endophenotypes was initially conceived as a “placeholder” to accommodate complex, polygenic effects and environmental influences for developing schizophrenia (Gottesman & Gould, Reference Gottesman and Gould2003), this approach has expanded to depression (Lenzenweger, Reference Lenzenweger2013). A depression endophenotype should predict depressive symptoms and be linked to depression histories and current depression; evidence heritability between family members; be more prevalent among high-risk family members; and cosegregate with depression within families. The search for depression endophenotypes has identified promising candidates, including biased experience of stressful events (e.g., Conway et al., Reference Conway, Hammen, Espejo, Wray, Najman and Brennan2012; Wichers et al., Reference Wichers, Myin-Germeys, Jacobs, Peeters, Kenis and Derom2007), frontal electroencephalographic asymmetry (Stewart et al., Reference Stewart, Bismark, Towers, Coan and Allen2010), and atypical structural and functional characteristics in selected neural regions (Dubin et al., Reference Dubin, Weissman, Xu, Bansal, Hao and Liu2012; Simmons & Drevets, Reference Simmons and Drevets2012). However, few candidate processes have satisfied all the definitional criteria for an endophenotype. In the present article, we propose atypical patterns of parasympathetic nervous system (PNS) activity during rest and in response to negative affect triggers as endophenotypes for depression.
Measuring PNS Activity
One index of PNS activity is heart rate variation that occurs during the breathing cycle. This variation is driven by the influence of the vagus nerve on the sinoatrial node of the heart, and respiratory patterns that potentiate or inhibit its activity, which is respiratory sinus arrhythmia (RSA). In the absence of environmental demands, efferent outflow of the vagus modulates the cardiac cycle by serving as a “brake” on heart rate (resting RSA; Porges, Doussard-Roosevelt, Portales, & Greenspan, Reference Porges, Doussard-Roosevelt, Portales and Greenspan1996). An efficient vagal brake, indexed by high “resting RSA” levels, is associated with lower heart rate and is believed to maintain an organism's visceral homeostasis in the form of energy conservation (Porges, Reference Porges2007). It is also believed to promote good self-regulation (Beauchaine, Reference Beauchaine2001). Poor vagal inhibition of the heart is associated with increased resting heart rate, and it may be a risk factor for excessive autonomic arousal and dysregulated affective states (Beauchaine, Reference Beauchaine2001). In response to environmental demands, such as situations involving threat or loss, the vagal brake is withdrawn. Conversely, the brake is augmented in response to positive hedonic stimuli (see Kreibig, Reference Kreibig2010; Overbeek, van Boxtel, & Westerink, Reference Overbeek, van Boxtel and Westerink2012). Broadly termed RSA reactivity, such normative changes in vagal activity are believed to support optimal deployment of attention resources and to modulate the stress response (see Beauchaine, Reference Beauchaine2001; Porges, Reference Porges2007). Atypical reactivity (e.g., excessive vagal withdrawal) is associated with an increased risk for emotional lability (Beauchaine, Reference Beauchaine2001). Thus, while resting RSA levels signal potential energy reserves that can be marshaled for self-regulation, RSA reactivity reflects the efficiency with which such inner reserves can be accessed.
RSA patterns
Recent theoretical and empirical work suggests that studying resting RSA and RSA reactivity individually provides an incomplete picture of the relationship between the PNS and adaptive functioning (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011; El-Sheikh & Erath, Reference El-Sheikh and Erath2011; Hinnant & El-Sheikh, Reference Hinnant and El-Sheikh2009, Reference Hinnant and El-Sheikh2013). This notion was raised in Lacey's (Reference Lacey, Rubinstein and Parloff1959) seminal work, which showed that a single index of a physiological system may obscure the extent of interindividual differences. Since then, others also have suggested that some combination of resting RSA levels and RSA reactivity may be needed to elucidate the role of the PNS in risk for psychiatric disorders (e.g., El-Sheikh & Erath, Reference El-Sheikh and Erath2011).
Using this foundation, we have previously proposed that the combination of RSA withdrawal to negative mood triggers and high resting RSA represents an optimal or normative RSA pattern that supports successful self-regulation and buffers against depression; conversely, combinations of high resting RSA and RSA augmentation, or low resting RSA and RSA withdrawal in the face of negative mood triggers, represent suboptimal or atypical patterns for self-regulation that heighten risk for depression (Yaroslavsky, Bylsma, Rottenberg, & Kovacs, Reference Yaroslavsky, Bylsma, Rottenberg and Kovacs2013). RSA withdrawal to an interpersonal stressor (normative response) in the context of high resting RSA levels has been shown to prospectively predict lower internalizing symptoms in children (Hinnant & El-Sheikh, Reference Hinnant and El-Sheikh2009), and membership in the normative symptom trajectory group relative to those with sustained childhood internalizing problems across middle to late childhood (Hinnant & El-Sheikh, Reference Hinnant and El-Sheikh2013). In both of these studies, combined RSA indices provided incremental prediction beyond individual indices of resting RSA or RSA reactivity. Likewise, Cribbett, Williams, Gunn, and Rau (Reference Cribbet, Williams, Gunn and Rau2011) noted the incremental utility of combining RSA indices to predict adjustment during emotional stress. We have also found that patterns of RSA activity incrementally predict mental health outcomes, relative to single RSA indices (Yaroslavsky, Rottenberg, & Kovacs, Reference Yaroslavsky, Rottenberg and Kovacs2013; Yaroslavsky et al., Reference Yaroslavsky, Rottenberg, Bylsma, Jennings, George and Bajo2014).
Do RSA patterns meet criteria for a depression endophenotype?
While the practice of examining patterns of RSA indices is relatively novel, there is preliminary evidence that atypical RSA patterns (i.e., high resting RSA + augmentation or low resting RSA + withdrawal) have the characteristics of depression endophenotypes. In the following sections, we review the findings on this topic using Gottesman and Gould's (Reference Gottesman and Gould2003) criteria for an endophenotype as the framework.
Association with illness (Criterion A)
We are unaware of studies by others that examined the association of RSA patterns and clinical depression. However, in our work, we found that RSA patterns predict depression symptom severity among adults and adolescents. In a large sample of adults with histories of juvenile onset depression and healthy controls, we have shown that in the context of high resting RSA, subjects who exhibited RSA augmentation to a sad film (atypical response) had high depression levels, whereas those who exhibited RSA withdrawal (normative response) reported low levels of depressive symptoms (Yaroslavsky, Bylsma, et al., Reference Yaroslavsky, Bylsma, Rottenberg and Kovacs2013). These findings were replicated at trend levels in a sample of Hungarian youths with and without histories of pediatric onset depression (Yaroslavsky et al., Reference Yaroslavsky, Rottenberg, Bylsma, Jennings, George and Bajo2014). That is, adolescents with atypical RSA patterns (high resting RSA + augmentation or low resting RSA + withdrawal) tended to report greater depression severity than their peers with normative RSA patterns. In both studies, the predictive effects of RSA patterns were incremental to the predictive value of subjects' depression histories.
Although our findings on the predictive value of RSA patterns need to be replicated by others, they are consistent with studies that link individual RSA indices to clinical depression in adults (Kemp et al., Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt2010; Rottenberg, Reference Rottenberg2007), and depression symptoms in children and adolescents (Blom, Olsson, Serlachius, Ericson, & Ingvar, Reference Blom, Olsson, Serlachius, Ericson and Ingvar2010; Gentzler, Santucci, Kovacs, & Fox, Reference Gentzler, Santucci, Kovacs and Fox2009). For instance, in clinically depressed adults, low resting RSA is associated with depression severity (Kemp et al., Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt2010; Hofmann, Schulz, Heering, Muench, & Bufka, Reference Hofmann, Schulz, Heering, Muench and Bufka2010; Rottenberg, Reference Rottenberg2007) and a poor response to treatment (Chambers & Allen, Reference Chambers and Allen2002). Blunted RSA reactivity is also associated with depression (Rottenberg, Clift, Bolden, & Salomon, Reference Rottenberg, Clift, Bolden and Salomon2007) and prognosticates a poor clinical course (Rottenberg, Salomon, Gross, & Gotlib, Reference Rottenberg, Salomon, Gross and Gotlib2005). Subjects amid a major depressive episode are more likely to evidence RSA augmentation than RSA withdrawal in response to interpersonal stressors (Rottenberg et al., Reference Rottenberg, Clift, Bolden and Salomon2007) and blunted RSA augmentation in response to hedonic triggers (atypical response; Cyranowski, Hofkens, Swartz, Salomon, & Gianaros, Reference Cyranowski, Hofkens, Swartz, Salomon and Gianaros2011). Failure to show RSA withdrawal in response to a sad film predicts depression 6 months later (Rottenberg et al., Reference Rottenberg, Salomon, Gross and Gotlib2005). Likewise, atypical RSA reactivity to sadness elicitors and low resting RSA predict depressive symptoms in 6- to 13-year-olds (Gentzler et al., Reference Gentzler, Santucci, Kovacs and Fox2009).
Although meta-analytic reviews support atypical RSA activity (used in isolation) as a risk factor for depression, it is important to note that this link has not been uniformly verified (e.g., Lehofer et al., Reference Lehofer, Moser, Hoehn-Saric, McLeod, Liebmann and Drnorvsek1997; Moser et al., Reference Moser, Lehofer, Hoehn-Saric, McLeod, Hildebrandt and Steinbrenner1998; O'Connor, Allen, & Kaszniak, Reference O'Connor, Allen and Kaszniak2002; Yeragani et al., Reference Yeragani, Pohl, Balon, Ramesh, Glitz and Jung1991). As well, some studies have attributed group differences between depressed and healthy subjects to depressed persons' use of antidepressant medication (e.g., Licht et al., Reference Licht, de Geus, Zitman, Hoogendijk, van Dyck and Penninx2008; Rechlin, Claus, Weis, & Kaschka, Reference Rechlin, Claus, Weis and Kaschka1995). While we believe that the mixed findings may be reconciled through the use of RSA patterns rather than single RSA indices, this proposition needs further empirical support. Therefore, the overall findings suggest that atypical RSA activity and its patterns are predictive of depressive symptoms (Criterion A). However, the relationship between atypical RSA patterns and depression diagnoses has yet to be examined.
State independence (Criterion B)
To our knowledge, no studies have examined whether depressed and healthy populations differ in the prevalence of atypical RSA patterns. However, evidence from our aforementioned study of adults with depression histories provides preliminary support that atypical RSA patterns are state independent. In addition to predicting elevated depression symptoms, atypical RSA patterns predicted a greater probability of a history of child onset depression in adults, of whom 65% were in a remitted state (Yaroslavsky, Rottenberg, et al., Reference Yaroslavsky, Rottenberg and Kovacs2013). Thus, atypical RSA patterns were observed among subjects in the midst of a depressive episode as well as among those in a remitted state.
Heritability (Criterion C)
Heritability is typically examined as shared genetic variance associated with a common genetic influence and, to a lesser extent, via concordance rates of shared characteristics between individuals. While there are no published reports of RSA patterns' heritability, there is promising evidence of heritability of vagal activity from studies of individual RSA indices, particularly in response to environmental stressors. In a series of adolescent and adult twins, Boomsma et al. found that a common genetic factor accounted for 25%–32% of resting state variance and 50%–54% of variance of RSA reactivity in response to frustration tasks (Boomsma, Van Baal, & Orlebeke, Reference Boomsma, Van Baal and Orlebeke1990; De Geus, Kupper, Boomsma, & Snieder, Reference De Geus, Kupper, Boomsma and Snieder2007). Further, the influence of a genetic contribution to RSA reactivity was observed across a variety of laboratory stressors (e.g., mental arithmetic and choice reaction time tasks; Boomsma et al., Reference Boomsma, Van Baal and Orlebeke1990; De Geus et al., Reference De Geus, Kupper, Boomsma and Snieder2007). In a large sample of twins, whose RSA was monitored across 24 hr, 38%–47% of vagal activity was determined by a common genetic influence (Kupper et al., Reference Kupper, Willemsen, Posthuma, De Boer, Boomsma and De Geus2005). The genetic contributions to vagal activity have been supported by other studies of adult and pediatric samples (Singh et al., Reference Singh, Larson, O'Donnell, Tsuji, Evans and Levy1999; Sinnreich, Friedlander, Luria, Sapoznikov, & Kark, Reference Sinnreich, Friedlander, Luria, Sapoznikov and Kark1999; Tuvblad et al., Reference Tuvblad, Isen, Baker, Raine, Lozano and Jacobson2010).
There is also some indication of concordance of vagal reactivity between parents and offspring. In their seminal study, Bornstein and Suess (Reference Bornstein and Suess2000) found that concordance of vagal reactivity between mothers and offspring increased with children's age, while resting RSA showed no concordance. Others have likewise shown no association in baseline vagal activity between mothers and offspring (e.g., Connell, Hughes-Scalise, Klostermann, & Azem, Reference Connell, Hughes-Scalise, Klostermann and Azem2011; Creaven, Skowron, Hughes, Howard, & Loken, Reference Creaven, Skowron, Hughes, Howard and Loken2014). Thus, overall there is preliminary support for the heritability and familial concordance of vagal reactivity (on its own). However, no studies to date have examined whether atypical RSA patterns evidence familial concordance.
Elevated rates among high-risk family members (Criterion D)
Although there is no information on whether rates of atypical RSA patterns are elevated among family members at risk for depression, there is some indication that high-risk family members display atypical RSA activity. For instance, maternal depressive symptoms predict decreased resting RSA in 1-week-old infants (Jones et al., Reference Jones, Field, Fox, Davalos, Lundy and Hart1998) and a decoupling between RSA and infants' hedonic expressions (Pickens & Field, Reference Pickens and Field1995). Maternal depression histories also predict atypical RSA reactivity to both positive and negative mood triggers among school-aged offspring (Ashman, Dawson, & Panagiotides, Reference Ashman, Dawson and Panagiotides2008) and blunted resting RSA trajectories across childhood and adolescence (Gentzler, Rottenberg, Kovacs, George, & Morey, Reference Gentzler, Rottenberg, Kovacs, George and Morey2012). Thus, high-risk samples appear to be characterized by lower resting RSA levels and atypical RSA reactivity. However, because these studies examined the RSA indices independently, rates of atypical RSA patterns among high-risk family members are unknown.
Cosegregation with illness within families (Criterion E)
There is a surprising dearth of studies on whether atypical RSA activity differentiates nondepressed versus depressed family members. No study to our knowledge has examined resting RSA, RSA reactivity, or their patterns in clinical samples and their relatives. However, given the association of RSA indices among healthy individuals (e.g., Boomsma et al., Reference Boomsma, Van Baal and Orlebeke1990; Bornstein & Suess, Reference Bornstein and Suess2000), and the relationship between RSA and depression (e.g., Rottenberg, Reference Rottenberg2007), low resting RSA and atypical RSA reactivity would be expected to be evident among depressed family members.
Summary
Our findings on RSA patterns, and the extant literature on individual RSA indices, support the likelihood that RSA patterns represent endophenotypes. That is, there is evidence that (a) RSA patterns predict depressive symptoms and histories of depressive disorders in healthy controls, previously depressed subjects, and those in the midst of depressive episodes (Criteria A and B); and (b) RSA reactivity to environmental stressors, and to a lesser extent resting RSA levels, are heritable, and RSA reactivity may be concordant between mothers and offspring. This suggests that RSA patterns may also be heritable and concordant among family members (Criterion C).
The Present Investigation
We used two interrelated studies to examine the viability of RSA patterns as depression endophenotypes. In Study 1, we tested four hypotheses:
-
1. relative to normative RSA patterns, atypical RSA patterns will be more prevalent among previously and currently depressed women than among healthy controls;
-
2. atypical RSA patterns will predict high-risk but never depressed juvenile offsprings' depressive symptom trajectories across childhood and adolescence;
-
3. atypical RSA patterns will be concordant between mothers and offspring; and
-
4. such patterns will be more prevalent among high-risk, never depressed youth relative to low-risk peers.
In Study 2, we tested the fifth hypothesis:
-
5. that atypical RSA patterns would be more concordant among siblings with shared depression histories.
The above noted hypotheses sequentially correspond to Gottesman and Gould's (Reference Gottesman and Gould2003) endophenotype Criteria A–E.
Study 1 involved a subsample of women, who had been included in previous analyses of RSA combinations and depression (Yaroslavsky, Bylsma, et al., Reference Yaroslavsky, Bylsma, Rottenberg and Kovacs2013; Yaroslavsky, Rottenberg, et al., Reference Yaroslavsky, Rottenberg and Kovacs2013). This subsample was selected because their offspring also participated in our study. We focused on women in order to reduce heterogeneity associated with sex differences in RSA reactivity (El-Sheikh, Hinnant, & Erath, Reference El-Sheikh, Hinnant and Erath2011; Yaroslavsky, Rottenberg, et al., Reference Yaroslavsky, Rottenberg and Kovacs2013) and because depression is more common among females. Study 2 involved a sample of Hungarian adolescent-sibling pairs in which one or both siblings had a history of depression. This allowed us to examine whether atypical RSA patterns are more prevalent among depressed family members relative to those with no depression histories.
Study 1
Methods
Subjects in this series of experiments were drawn from a larger group who took part in a longitudinal program project on risk factors for juvenile-onset depression. The program project was carried out over a 10-year period and encompassed several studies that investigated physiologic correlates of various emotional states, parent–child interactions, and genetic contributors to depression (see Gentzler et al., Reference Gentzler, Santucci, Kovacs and Fox2009, Reference Gentzler, Rottenberg, Kovacs, George and Morey2012; Kovacs, Rottenberg, & George, Reference Kovacs, Rottenberg and George2009; Miller et al., Reference Miller, Fox, Cohn, Forbes, Sherrill and Kovacs2002; Santucci et al., Reference Santucci, Silk, Shaw, Gentzler, Fox and Kovacs2008). We used data from a sample of 70 mothers and their 100 never-depressed offspring who participated in a protocol designed to elicit physiologic and emotional responses via a variety of tasks (see below). Three offspring were removed from analyses due to their extreme values on RSA indices (Z > 4.7). Of the remaining 97 offspring, 13% percent had at least 1 sibling in the study and 12% had 2 or more siblings.
Proband mothers (n = 27) were those with histories of juvenile-onset depression, while the rest were free of lifetime major psychiatric disorders (control mothers). Proband and control mothers were 28.54 (SD = 3.74) and 30.94 (SD = 5.26) years old, respectively, at the time of their RSA assessment. The two groups did not differ in ethnic distribution, but proband mothers were on average 2 years younger (see Table 1). Proband mothers were 10.28 years old (SD = 3.01) on average at onset of their first depression and had three prior depressive episodes (SD = 2.08) on average. At the time of their assessment, 13 (48%) of the proband mothers were in a depressive episode (current depression), while the rest of the mothers were in remission from depression (remitted depression). Nine proband mothers (33%) were prescribed antidepressant medication, while all control mothers reported no psychotropic medication use.
Table 1. Characteristics of mothers

Note: Proband Mothers, women with histories of depression; BDI, Beck Depression Inventory; RSA, respiratory sinus arrhythmia.
*p < .05. ***p < .001.
At the time of the first assessment, proband (high-risk youth; n = 48) and control offspring (low-risk youth; n = 49) were on average 6.7 years old (SD = 2.37), and 47% were girls. High-risk youth were less likely to be of an African American background and more likely to endorse a biracial or “other” racial category than their low-risk peers. The two offspring groups did not otherwise differ in their demographic characteristics (see Table 2). Offspring were free of major psychiatric disorders and psychotropic medication, with the exception of 2 high-risk and 1 low-risk youth, who were prescribed psychostimulants.
Table 2. Characteristics of offspring

Note. High Risk, offspring of mothers with depression history; Low Risk, offspring of control mothers; CBCL, Child Behavior Checklist; RSA, respiratory sinus arrhythmia.
*p < .05. ***p < .001.
For prospective analyses, a subset of high-risk (N = 42) and low-risk (N = 41) offspring who were aged 7 years or older (the minimum age for completing self-rated scales) provided self-reports of depressive symptoms on up to four occasions. This subset of children was comparable in sex and race distributions to the full sample: high-risk youth did not significantly differ from their low-risk peers in their age, sex, or racial distribution. The subset of children who completed self-rated scales were, on average, 8.24 years old (SD = 1.73) at their first assessment (n = 83), 11.11 years (SD = 2.63) at their second assessment (n = 66), 12.56 years (SD = 2.44) at their third assessment (n = 27), and 14.71years (SD = 0.76) at their fourth assessment (n = 7). Lags between assessments varied, averaging 2.79 year (SD = 2.55) between the first and second assessments, 3.59 years (SD = 2.39) between the second and third assessments, and 5.29 (SD = 0.95) years between the third and fourth assessments. Sixty-four subjects (77%) provided data on two occasions, n = 29 (35%) on three occasions, and n = 7 (8%) completed four assessments. Study participation did not vary as a function of risk status, χ2 (3) = 4.24, p =.24.
Subject recruitment
Procedures for parent recruitment are described in detail elsewhere (see Gentzler et al., Reference Gentzler, Santucci, Kovacs and Fox2009; Miller et al., Reference Miller, Fox, Cohn, Forbes, Sherrill and Kovacs2002; Santucci et al., Reference Santucci, Silk, Shaw, Gentzler, Fox and Kovacs2008). Briefly, proband mothers were recruited from among adults who had participated as children in research studies of depression and anxiety, through clinical sites, or by advertisements in the community. Controls were recruited via the Cole Directory, a cross-reference listing of residential telephone numbers and addresses that is commonly used for business and marketing purposes, as well as through community advertisements and by approaching adults who previously served as controls in pediatric studies. Criteria for study entry included either a verifiable diagnosis of a depressive disorder by the age of 14 years or a history free of major psychiatric disorders. Subjects with major medical conditions were not enrolled in this study.
Diagnosis
Diagnostic procedures for mothers have been described in detail previously (Miller et al., Reference Miller, Fox, Cohn, Forbes, Sherrill and Kovacs2002; Santucci et al., Reference Santucci, Silk, Shaw, Gentzler, Fox and Kovacs2008). All study entry diagnoses were based on information from subjects and second informants (parents or sibling), and were derived by experienced masters' level clinicians via the Structured Clinical Interview of DSM-IV Disorders (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams1995). Clinicians utilized medical and related records to verify age at depression onset, and all diagnoses were finalized via best estimate consensus procedures by pairs of psychiatrists. Offspring diagnoses were ascertained using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn and Moreci1997). Offspring and their mothers were separately interviewed by masters' level clinicians, and final diagnoses were rendered through best estimate consensus procedures by pairs of psychiatrists. Good to adequate interrater reliability was demonstrated across diagnostic interviews (major depressive disorder, κ = 0.92; dysthymic disorder, κ = 0.63).
Rating scales
Offspring's anxiety and depressive symptoms were assessed by the parent-rated Child Behavior Checklist, a validated and reliable measure of child adjustment (Achenbach, Reference Achenbach1991). For children aged 7 years or older, depressive symptoms also were measured using the self-rated Children's Depression Inventory (CDI), a validated and reliable index of depression symptoms in the prior 2 weeks (Kovacs, Reference Kovacs1992). Due to a change in protocol, the Children's Depression Inventory—2 (CDI-2; Kovacs & MHS Staff, Reference Kovacs2011) was used with n = 37 children at their last assessment. The CDI-2 scores were converted to CDI equivalents following standard guidelines (Kovacs & MHS Staff, Reference Kovacs2011). Mothers completed the Beck Depression Inventory, a validated measure of depression severity (Beck, Steer, & Carbin, Reference Beck, Steer and Carbin1988). Mothers also rated the subjective intensity of five discrete emotions (happy, sad, angry, fearful, and disgust) on a 0–8 point Likert-type scale after specific affective stimuli during the experimental protocol (see below). These intensity ratings were used as a manipulation check. Given their ages, offspring did not rate the intensity of their emotions.
Procedures
The data reported in this article were collected as part of a larger electrophysiological protocol targeting emotional reactivity and regulation. Mothers and offspring participated on different days, 1.18 years apart (SD = 1.09) on average. After arrival at the laboratory, mothers received the structured clinical evaluation and completed the symptom questionnaires, along with a questionnaire regarding recent caffeine consumption, smoking, and current medications. Mothers were then connected to equipment that continuously monitored multiple physiological parameters during a protocol that involved a 6-min rest period and experimental tasks (e.g., film clips designed to induce joy and sadness).
Offspring data collection followed a similar process. The children's experimental physiology protocol involved a resting period and several experimental tasks, including short film clips designed to induce various emotions (i.e., joy, fear, and sadness). The present report focuses on mothers' and offspring's RSA during a resting period and while watching a sad film: a clip from The Champ for mothers and a clip from The Lion King for children. Both films depict themes of loss, and they were selected based on their negative mood induction effects (see Gross & Levenson, Reference Gross and Levenson1995; von Leupoldt et al., Reference von Leupoldt, Rohde, Beregova, Thordsen-Sörensen, Nieden and Dahme2007).
Physiological data acquisition and reduction
All subjects were seated upright in a comfortable chair facing a computer monitor. Resting electrocardiogram (ECG) data were collected during a 6-min pretask period for mothers and a 3-min period for offspring during which participants sat quietly and were instructed to open and close their eyes at 30-s intervals. Then, they watched a series of film clips in a fixed order that were intended to elicit discrete emotions (sequence order: neutral, happy, sad, fear, anger, and disgust film clips). For a manipulation check, affect ratings after the sad film were compared to those of the preceding neutral film clip for mothers. Due to their young age, we did not collect affect ratings from offspring.
Standard guidelines were followed in ECG data acquisition and reduction using software and equipment from the James Long Company (Caroga Lake, NY; Berntson et al., Reference Berntson, Bigger, Eckberg, Grossman, Kaufmann and Malik1997). We placed Ag/AgCl ECG electrodes axially on the left and right rib cage, approximately at heart level. The bioamplifier was set for bandpass filtering with frequencies of 0.01 and 1000 Hz. The ECG signal was amplified with a gain of 500, and data were digitized with a sampling rate of 512 Hz (Berntson et al., Reference Berntson, Bigger, Eckberg, Grossman, Kaufmann and Malik1997). R-waves in the ECG signal were automatically identified using a multipass algorithm and manually checked. Ectopic beats were deleted and interpolated. Heart rate variability (HRV) was calculated by detrending the interbeat interval time series that was then tapered with a Hanning window. The high-frequency (HF) power band of HRV (0.20–1.00 Hz for 4- to 5-year-olds, 0.15–0.50 Hz for older children, 0.15–0.40 Hz HF-HRV for adults) was used to estimate cardiac parasympathetic activity, and was calculated through fast Fourier transformation analysis of the study epochs (Berntson et al., Reference Berntson, Bigger, Eckberg, Grossman, Kaufmann and Malik1997). Mothers' 60-s and offspring's 30-s resting HRV epochs were averaged within the resting interval, and these averages and the 3-min sad film epoch were then log-transformed to normalize their distribution. We refer to HF-HRV as RSA, because HF-HRV is the power band of HRV that occurs in the typical range of respiration. RSA reactivity (ΔRSA) was defined as the difference between resting RSA and RSA during the sad film. Negative ΔRSA values indicate vagal augmentation, and positive values indicate vagal withdrawal.
Analyses
All analyses used SAS version 9.3 software (SAS Institute Inc., 2013). Robust standard errors were used where heteroscedasticity was present. Three-level models were fit using Proc Mixed to accommodate multiple offspring within families and multiple assessments nested within offerings when examining the effects of RSA patterns on depression symptom trajectories (Hypothesis 2). Survey logistic procedures were employed to test RSA pattern concordance between mothers and offspring (Hypothesis 3) and the effects of RSA patterns on offspring's depression risk status (Hypothesis 4).
In all analyses, resting RSA was centered at its mean and RSA reactivity remained uncentered, given its meaningful 0 value. As appropriate, we used a mean split to categorize high versus low resting RSA, and RSA reactivity above and below zero as withdrawal and augmentation, respectively. These categories were combined into four RSA patterns: high resting RSA + withdrawal; low resting RSA + withdrawal; high resting RSA + augmentation; and low resting RSA + augmentation. High resting RAS + augmentation and low resting RSA + withdrawal represent atypical RSA patterns, while high resting RSA + withdrawal represents a normative RSA pattern. We made no a priori assumptions about the low resting RSA + augmentation pattern. These categories were used for Hypotheses 3–5 to represent RSA patterns in terms of odds ratios. For Hypotheses 1 and 2, RSA patterns were defined as a second-order interaction between resting RSA and RSA reactivity; significant interaction effects were probed following Aiken and West (Reference Aiken and West1991). The decision to use continuous RSA indices in these analyses maximized the power to detect significant effects.
Missing values ranged from 0% to 12% across variables and were missing completely at random, Little χ2 (188) = 23, p = .33. We first conducted analyses using all available data, and then replicated them with data recovered via multiple imputation (i.e., PROC MI & MI Analyze with the expectation maximization algorithm). The results below represent findings using unimputed data, because analyses using multiple imputation did not alter our major findings.
Results
Descriptive statistics for mothers and offspring in Study 1 (Hypotheses 1–4) are reported in Tables 1 and 2; bivariate associations are presented in Table 3. As expected, mothers reported increased sadness after watching the sad film, M neutral film = 0.21, M sad film = 5.13, t (66) = 17.17, p < .001, which evoked significant vagal withdrawal in mothers and offspring alike, mothers: M = 0.23, SD = 0.64, t (66) = 2.93, p < .01; offspring: M = 0.15, SD = 0.60, t (84) = 2.24, p < .05. Across both offspring risk groups, girls displayed greater RSA withdrawal than boys, M girls = 0.16, M boys = 0.00, t (83) = 2.43, p < .05. Thus, effects of sex were statistically controlled as warranted. Age, race, and psychotropic medication use were not significantly related to the two RSA indices, and they were not considered further in the analyses.
Table 3. Correlations among maternal and offspring characteristics

Note: Offspring sample size was N = 85 to 97, and mother's sample size was n = 67. Sex, High values represent females; Proband, high values represent proband group membership; Anx-Dep, Child Behavior Checklist Anxious Depression Scale; RSA, respiratory sinus arrhythmia during resting baseline; ΔRSA, change score from resting RSA to RSA during the sad film; M_RSA, mother's RSA during resting baseline; M_ΔRSA, mother's change score from resting RSA to RSA during the sad film.
†p < .06. *p < .05. ***p < .001.
Are atypical RSA patterns associated with current and past depression in women?
To address the question, reflecting Hypothesis 1, two logistic regression models were used to regress mothers' depression status (current depression vs. control and remitted depression vs. control) on the first- and second-order effects of resting RSA and RSA reactivity. Depression symptoms were statistically controlled in the model comparing RSA patterns of remitted and control mothers. While in both models the first-order effects of the two RSA indices failed to differentiate currently depressed and remitted mothers from control peers, second-order effects of resting RSA and RSA reactivity robustly predicted depression status, current depression versus control, change in –2 log likelihood (Δ–2LL) (1) = 11.38, p < .001; remitted depression versus control, Δ–2LL (1) = 3.98, p < .05 (see Table 4). As shown in Figure 1, post hoc probes of the interactions revealed increased risk of current depression and remitted depression as a function of high resting RSA and RSA augmentation (atypical RSA pattern) and low resting RSA and RSA withdrawal (atypical RSA pattern). Conversely, high resting RSA in the context of RSA withdrawal (normative pattern) and low resting RSA in the context of RSA augmentation were associated with a reduced probability of being in a depressive episode or having a history of depression. Thus, with regard to being endophenotypes, our results suggest that RSA patterns predict depressive illness (Criterion A) and are state independent, as evidenced by their ability to predict remitted depression status (Criterion B).
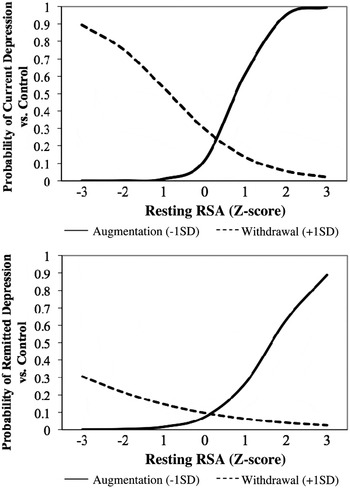
Figure 1. Respiratory sinus arrhythmia (RSA) pattern prediction of mothers' status: (top) current depression or (bottom) remitted depression versus control.
Table 4. Predicting maternal depression status from RSA patterns

Note: Control mothers are the reference category. RSA, respiratory sinus arrhythmia; CI, confidence interval; BDI, Beck Depression Inventory.
*p < .05. **p < .01.
Do atypical RSA patterns among high-risk offspring predict depressive symptom trajectories across childhood and adolescence?
This question, reflecting Hypothesis 2, was addressed by first fitting a series of unconditional linear and quadratic models to determine the best fitting trajectory of youths' depressive symptoms across childhood and adolescence. In these models, the family effect (Level 3) did not significantly explain variability in offspring's depression symptom trajectories, and thus was removed from further analyses. The random intercept, random linear, and quadratic slope model fit the data better than its linear counterpart and was retained for subsequent analyses, linear model: LL = 967.5, Bayesian information criterion = 992.2; quadratic model: LL = 926.5, Bayesian information criterion = 955.3; ΔLL (1) = 41, p < .001. The random quadratic variance component of this model was removed due to its presence causing a nonpositive definite covariance matrix. As displayed in Figure 2, youths' depressive symptoms were best characterized as increasing over time, particularly during the transition from childhood to adolescence. Higher initial levels of depressive symptoms were negatively related to the linear factor (r = –.67), which suggests that high levels of depressive symptoms were related to a slow rate of improvement over time.

Figure 2. Unconditional growth model of depressive symptom trajectories among high- and low-risk children and adolescents. CDI, Children's Depression Inventory.
Next, first-order effects of resting RSA and RSA reactivity were entered as predictors into the model along with sex and offspring's depression risk status. Sex and offspring risk status did not significantly predict depressive symptom trajectories, and they were removed from the model. Consistent with our prior findings, first-order effects of the two RSA indices were not significant. Second-order effects of the two RSA indices were then entered into the model. The combined effects of the two RSA indices predicted depressive symptom trajectory's slope at a trend level and significantly predicted the quadratic growth factor, b slope = 0.68, t (83) = 1.84, p = .07; b quadratic = –0.16, t (83) = 2.72, p < .01. As displayed in Figure 3, normative RSA patterns of RSA withdrawal in the context of high resting RSA, as well as RSA augmentation in the context of low resting RSA, predicted low depressive symptom trajectories. Conversely, atypical patterns (low resting RSA + RSA withdrawal and high resting RSA + RSA augmentation) predicted increased depressive symptoms during adolescence. These findings further support the association between atypical RSA patterns and depression (endophenotype Criterion A).
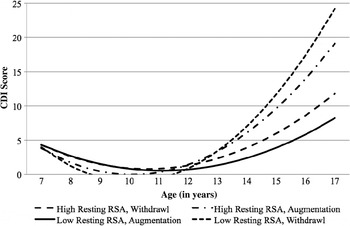
Figure 3. Interaction of resting respiratory sinus arrhythmia and respiratory sinus arrhythmia reactivity (+1 SD/–1 SD) predicting depression symptom trajectories among high- and low-risk children and adolescents. CDI, Children's Depression Inventory; RSA, respiratory sinus arrhythmia.
Are RSA patterns concordant between mothers and offspring?
Reflecting Hypothesis 3, this question was approached by examining bivariate correlations between mothers' and offspring's RSA indices. Partialing out the effects of offspring's sex, we found a significant association between offspring's and mothers' RSA reactivity (r = .35, p = .001), but this was not the case for resting RSA. We followed these analyses using two-level mixed effects models to account for dependence among related offspring's RSA measures. RSA reactivity evidenced a notable association among siblings (intraclass correlation = 0.47, p < .05), while resting RSA did not (intraclass correlation = 0.11, p = .24). Mixed models that regressed offspring resting RSA and RSA reactivity on offspring's sex and maternal RSA indices provided support for the above findings. Specifically, mothers and offspring evidenced significant associations in RSA reactivity (b = 0.33, t = 3.07, p < .01), but not in resting RSA (ns).
Multinomial logistic regression models were then fit to test whether RSA patterns are concordant between mothers and offspring. Consistent with expectation, offspring's RSA patterns were significantly predicted by their mothers' RSA patterns, Wald χ2 (9) = 964.76, p < .001. To clarify this effect, offspring and mothers were classified into dichotomous categories that represented the presence versus absence of each RSA pattern. These analyses revealed strong correspondence between mothers and offspring for the high resting RSA + withdrawal pattern (odds ratio [OR] = 5.66, 95% confidence interval [CI] = 1.94–16.54), and trend level effects for high resting RSA + augmentation (OR = 4.93, 95% CI = 0.96–25.20, p = .06), and low resting RSA + augmentation patterns (OR = 3.04, 95% CI = 0.85–10.90, p = .09). The low resting RSA + withdrawal pattern was not concordant. Thus, our results provide some support for the expectation that normative and atypical RSA patterns are concordant between mothers and offspring (endophenotype Criterion C).
Are rates of atypical RSA patterns elevated among high-risk youths?
To address this question, which reflects Hypothesis 4, offspring risk status (high vs. low) was regressed on offspring's RSA pattern, while controlling for the effects of sex and anxiety/depression symptoms. Consistent with expectations, RSA patterns significantly predicted risk status, incremental to covariates, Wald χ2 (3) = 8.45, p < .05. Relative to the low resting RSA + withdrawal pattern, high resting RSA + withdrawal, and low resting RSA + augmentation patterns, predicted greater probability of membership in the low-risk group (OR high resting RSA+withdrawal = 5.17, 95% CI = 1.48–18.11; OR low resting RSA+augmentation = 6.44, 95% CI = 1.22–33.95). In contrast, subjects with high resting RSA + augmentation, and low resting RSA + withdrawl, did not significantly differ in their risk of familial depression. These findings indicate that atypical RSA patterns are more prevalent among offspring at high familial risk for depression (endophenotype Criterion D).
Discussion
Consistent with Gottesman and Gould's (Reference Gottesman and Gould2003) formulation of endophenotypes, our findings suggest that atypical RSA patterns: predict current depressive episodes and remission status among women with histories of juvenile onset depression and healthy controls (Criteria A and B); predict trajectories of depressive symptoms across childhood and adolescence (Criterion A); are concordant between mothers and their offspring (Criterion C); and are more prevalent among never-depressed youth at high risk for depression (Criterion D). In addition, we found relationships between atypical RSA patterns and depression among mothers and offspring, in conjunction with elevated rates of atypical patterns among high-risk youth, providing indirect support for cosegregation of atypical RSA patterns and depression in families (Criterion E).
The higher rate of atypical RSA patterns among currently and formerly depressed subjects (relative to controls) is consistent with our finding that atypical RSA patterns predict depression histories (Yaroslavsky, Rottenberg, et al., Reference Yaroslavsky, Rottenberg and Kovacs2013). The association of depressive disorders and atypical RSA patterns is also in line with reports of low resting RSA levels and atypical RSA reactivity (in isolation) among depressed subjects (Rottenberg, Reference Rottenberg2007; Rottenberg et al., Reference Rottenberg, Salomon, Gross and Gotlib2005) and low resting RSA and depression severity in clinical populations (Kemp et al., Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt2010; Rottenberg, Reference Rottenberg2007). The concordance between mothers' and offspring's RSA patterns is also consistent with the literature on the heritability of individual RSA indices, particularly with respect to RSA reactivity (Boomsma et al., Reference Boomsma, Van Baal and Orlebeke1990; De Geus et al., Reference De Geus, Kupper, Boomsma and Snieder2007). Consistent with Bornstein and Suess (Reference Bornstein and Suess2000), our investigation of individual RSA indices showed a robust association in mothers' and offspring's reactivity, but not resting RSA levels. In conjunction with the finding of greater prevalence of such patterns among high-risk offspring, our results suggest that atypical RSA patterns may be one mechanism by which mothers transmit risk for depression to their offspring (see Goodman, Reference Goodman2007; Goodman & Gotlib, Reference Goodman and Gotlib1999). Atypical PNS activity has been proposed as a neuroregulatory dysfunction that increases the risk of familial depression transmission (Goodman, Reference Goodman2007). Furthermore, empirical studies show atypical RSA activity among high-risk infants (Pickens & Field, Reference Pickens and Field1995), school-aged children (Ashman et al., Reference Ashman, Dawson and Panagiotides2008), and adolescents (Gentzler et al., Reference Gentzler, Rottenberg, Kovacs, George and Morey2012). Thus, further studies of RSA patterns may be useful for elucidating the mechanisms by which depression risk is transmitted within families. Our finding of elevated depressive symptom trajectories among youth with atypical RSA patterns is in line with reports by other investigators using healthy child samples (Hinnant & El-Sheikh, Reference Hinnant and El-Sheikh2009, Reference Hinnant and El-Sheikh2013).
The results from Study 1 provided indirect support for RSA pattern cosegregation with illness within families by showing associations between atypical RSA patterns and diagnosed depression in mothers, and depressive symptoms in their offspring. However, elevated depressive symptoms cannot be automatically equated with the presence of a depressive disorder. Thus, it is yet to be shown whether atypical RSA patterns cosegregate among family members with histories of depressive disorders. We test this possibility in Study 2, where we compared RSA patterns of sibling pairs in which both versus only one had a history of depression.
Study 2
Methods
In Study 2, we investigated whether atypical RSA patterns cosegregate among adolescent siblings with histories of diagnosed depression (endophenotype Criterion E). We use data from matched sibling pairs from the Program Project, who also participated in a subsequent psychophysiological study (see Kovacs et al., Reference Kovacs, Yaroslavsky, Rottenberg, George, Baji and Benák2014; Rottenberg et al., Reference Rottenberg, Yaroslavsky, Carney, Freedland, George and Baji2014; Tamás et al., Reference Tamás, Kovacs, Gentzler, Tepper, Gádoros and Kiss2007). Subjects were 279 school-age youths (N = 147 proband–sibling pairs; some probands had multiple siblings) in Hungary. This sample was 16 years old, on average (SD = 1.95), 55% male, and 95% Caucasian (5% were multiracial, Roma, or “other” racial category). In each sibling pair, one adolescent was ascertained by virtue of having had pediatric onset depression (proband youth, N = 132). The other sibling in the pair was eventually determined either as also having had a history of depression (affected sibling, N = 36) or having no depression history (unaffected sibling, N = 111). Fifteen proband youth had two siblings in the study (n = 1 affected sibling, n = 14 unaffected siblings). At the time of the study, n = 4 probands and n = 2 siblings were prescribed psychotropic medication (a stimulant, anxiolytic, antidepressant, antihistamine, or antipsychotic).
Subject recruitment and diagnosis
Original recruitment of this sample is detailed elsewhere (see Baji et al., Reference Baji, Lopez-Duran, Kovacs, George, Mayer and Kapornai2009; Kiss et al., Reference Kiss, Gentzler, George, Kapornai, Tamas and Kovacs2007; Kovacs et al., Reference Kovacs, Yaroslavsky, Rottenberg, George, Baji and Benák2014; Tamás et al., Reference Tamás, Kovacs, Gentzler, Tepper, Gádoros and Kiss2007). Briefly, proband youth were recruited from 23 child mental health and guidance facilities across Hungary, along with probands' siblings within 3 years of age. All subjects underwent a stringent assessment procedure that included: a standardized psychiatric diagnostic evaluation using a semistructured interview (each involving the child and a parent informant) by trained interviewers who generated DSM IV diagnoses and independent verification of the diagnoses by pairs of trained child psychiatrists, via “best estimate” consensus (Maziade et al., Reference Maziade, Roy, Fournier, Cliche, Merette and Caron1992). Diagnoses of the probands and siblings in the current study also were subjected to consensus reviews by senior clinicians. Diagnoses were derived by trained clinicians using a DSM-IV based, semistructured diagnostic interview with parents about their offspring and the offspring about themselves (Interview Schedule for Children and Adolescents: Diagnostic version), which has been shown to have good reliability and validity and has been described in detail previously (Baji et al., Reference Baji, Lopez-Duran, Kovacs, George, Mayer and Kapornai2009; Kiss et al., Reference Kiss, Gentzler, George, Kapornai, Tamas and Kovacs2007).
Rating scales
Adolescents completed the CDI-2, a validated and reliable measure of depression symptoms in the prior 2 weeks (Kovacs & MHS Staff, Reference Kovacs2011), which rated the subjective intensity of discreet emotions (blue, sad, happy, enthusiastic, or interested) on a scale of 0 to 7 at various points. Adolescents also completed various other questionnaires (Kovacs et al., Reference Kovacs, Yaroslavsky, Rottenberg, George, Baji and Benák2014). Parents also reported on their child's psychotropic medication use.
Procedures
Data from the Hungarian sample were collected using similar procedures to that in the Study 1 US sample. During the course of an experimental protocol, subjects were hooked up to physiological monitors and completed several stress reactivity (psychology and physical) and positive mood induction tasks. For this article, we used data from the portion of the protocol that included a 3-min paced breathing task and sad mood induction via a 2-min clip from the The Champ (dubbed in Hungarian) described elsewhere (Kovacs et al., Reference Kovacs, Yaroslavsky, Rottenberg, George, Baji and Benák2014). This clip has been extensively used in emotion research with both pediatric and adult samples (e.g., Gross & Levenson, Reference Gross and Levenson1995; Rottenberg, Gross, Wilhelm, Najmi, & Gotlib, Reference Rottenberg, Gross, Wilhelm, Najmi and Gotlib2002), and it was also pilot tested with Hungarian youth (see Kovacs et al., Reference Kovacs, Yaroslavsky, Rottenberg, George, Baji and Benák2014). All adolescents received the same mood induction procedure after a 2- to 5-min intertask rest interval.
Physiological data acquisition and reduction
Resting RSA was assessed during the paced breathing task (respiration rate 12 breaths/min). RSA reactivity was assessed as the change in RSA that resulted from watching the sad film clip. Physiological data were recorded continuously via an ECG using Mindware BioLab software. The ECG signal was acquired according to published guidelines (Bernston et al., 1997) using Ag/AgCl electrodes that were placed in a modified Lead II configuration on the chest. Heart values were sampled online at 1000 Hz using the Mindware Bionex system (MindWare Technologies, Ltd., Gahanna, OH).
RSA was calculated using MindWare HRV 3.0.21 software (MindWare Technologies, Ltd., Gahanna, OH). R-wave markers in the ECG signal were processed with the MAD/MED artifact detection algorithm, and signals were manually inspected and suspected artifacts were corrected (Bernston et al., 1997). The interbeat interval series was resampled in equal intervals, linearly detrended, and tapered using a Hanning window. HRV was calculated using Fast Fourier transformation analysis of the interbeat interval series, with spectral power values determined in milliseconds squared per Hertz (Berntson et al., Reference Berntson, Bigger, Eckberg, Grossman, Kaufmann and Malik1997). Our index of cardiac parasympathetic activity, RSA, was defined as the log transformed HF power band of HRV (0.15–0.04 Hz range; see Berntson et al., Reference Berntson, Bigger, Eckberg, Grossman, Kaufmann and Malik1997). We refer to HF-HRV as RSA, because HF-HRV is the power band of HRV that occurs in the typical range of respiration.
Analyses
All analyses were completed using SAS version 9.3 software (SAS Institute Inc., 2013). Given multiple siblings within some families, we employed Proc Mixed procedures to examine group differences in individual RSA indices, and GLIMMIX procedures to test RSA pattern concordance between proband–sibling pairs. As with Study 1 data, resting RSA was centered at its mean; RSA reactivity remained uncentered, given its meaningful 0 value. A mean split was used to categorize high versus low resting RSA, and RSA reactivity above and below zero, as withdrawal and augmentation, respectively. These categories were combined into four RSA patterns: high resting RSA + withdrawal, low resting RSA + withdrawal, high resting RSA + augmentation, and low resting RSA + augmentation.
Missing values on study variables ranged from 0% to 3% and were missing completely at random, Little χ2 (27) = 18.50, p = .89. Listwise deletion was used in subsequent analyses given its unbiased nature under conditions of low missing data that are missing completely at random (Graham, Reference Graham2009).
Results
Descriptive statistics for proband–sibling pairs are reported in Table 5. All youth reported increased sadness after watching the sad film, proband M paced breathing = 0.54, M sad film = 1.00, t (131) = 4.16, p < .001; affected sibling M paced breathing = 0.72, M sad film = 1.69, t (66) = 3.32, p = .002; unaffected sibling M paced breathing = 0.41, M sad film = 1.42, t (110) = 7.21, p < .001, with siblings reporting greater sadness than probands, t (145) = 3.33, p = .001. The sad film evoked significant vagal withdrawal in proband youth and siblings alike, probands: M = 0.76, SD = 0.79, t (130) = 10.95, p < .001; affected siblings: M = 0.76, SD = 0.74, t (34) = 5.80, p < .001; unaffected siblings: M = 9.93, SD = 0.75, t (110) = 13.14, p < .001. While proband boys displayed a trend for lower resting RSA than proband girls, M boys = 7.07, SD = 1.13, M girls = 7.40, SD = 1.01, t (130) = 1.65, p = .10, siblings' RSA indices did not differ as a function of sex. Nonetheless, sex was covaried in all models. Potential confounding effects of current depressive symptoms were also statistically controlled in all models because we sought to examine RSA patterns' cosegregation with depression histories, rather than their association with depression symptom severity. Age and psychotropic medication use were not significantly related to the two RSA indices, and they were not considered further in the analyses.
Table 5. Characteristics of Hungarian adolescent sample

Note: Affected, history of depression; Unaffected, no history of depression, Other, multiracial, Roma, or other; CDI-2, Children's Depression Inventory (2nd ed.).
*p < .05. **p < .01. ***p < .001.
Do atypical RSA patterns cosegregate with depression within families?
Controlling for sex, bivariate correlations revealed significant associations between probands' and affected siblings' resting RSA (r = .35, p < .05) and RSA reactivity (r = .37, p < .05). Probands' and unaffected siblings' RSA indices were not significantly related. We followed these analyses with two-level mixed effects models to account for multiple proband–sibling pairs. Mixed models that regressed siblings' resting RSA and RSA reactivity on sex and probands' RSA indices supported the findings from the correlation analyses. Specifically, probands and affected siblings evidenced significant associations in resting RSA (b = 0.32, t = 2.05, p < .05) and RSA reactivity (b = 0.40, t = 2.23, p < .05). Probands and unaffected siblings continued to show no significant association in their RSA indices.
Inspection of the 16 possible RSA pattern combinations revealed sparse coverage among the 36 proband–affected sibling pairs (i.e., many cells with 0–1 frequencies). Therefore, RSA patterns were recategorized into optimal and suboptimal patterns to increase the power of our analyses. Based on our findings from Study 1 and prior reports (i.e., Yaroslavsky, Bylsma, et al., Reference Yaroslavsky, Bylsma, Rottenberg and Kovacs2013; Yaroslavsky, Rottenberg, et al., Reference Yaroslavsky, Rottenberg and Kovacs2013), optimal patterns were defined as high resting RSA + withdrawal or low resting RSA + augmentation, while atypical patterns were defined as high resting RSA + augmentation, and low resting RSA + withdrawal combinations.
Logistic regression models were fit to test whether atypical RSA patterns were more concordant across proband youth and affected sibling pairs, relative to proband–unaffected sibling pairs. These models were fit separately for affected and unaffected siblings, given the low power to detect moderation effects of siblings' depression status (affected vs. unaffected) on RSA pattern concordance. In support of our hypothesis, we found that affected sibling–proband pairs shared atypical RSA patterns (t = 2.11, p < .05, OR = 6.46, 95% CI = 1.15–36.47). In contrast, there was no significant association between unaffected siblings' and probands' RSA patterns. Thus, our results provide some support for cosegregation of atypical RSA patterns' among depressed family members (endophenotype Criterion E).
Discussion
In Study 2, we hypothesized that atypical RSA patterns would be more concordant across sibling pairs in which both had histories of diagnosed depression, relative to pairs in which only one sibling had a history of depression. We found that pairs in which both siblings had been affected with depression were more likely to share atypical RSA patterns. We did not find this association across sibling pairs in which only one sibling had a history of depression. The results therefore complement the findings of Study 1 regarding the high rates of atypical RSA patterns among offspring at familial risk for depression, as well as the association between atypical RSA patterns and depressive symptoms in youths. Taken together, our findings provide reasonable support for the expectation that RSA patterns cosegregate with depression within families (endophenotype Criterion E; Gottesman & Gould, Reference Gottesman and Gould2003).
General Discussion
The present article is the first to report on whether atypical patterns of PNS activity during resting states, and in response to negative mood triggers, represent an endophenotype for depression. This topic is timely, given increased efforts to elucidate how genes contribute to physiological and behavioral mechanisms underlying psychiatric disorders (Cuthbert & Insel, Reference Cuthbert and Insel2013; Lenzenweger, Reference Lenzenweger2013). Further, a more mechanistic understanding of psychopathology may inform early identification of groups at risk for a given disorder and thus improve prevention and treatment efforts.
Based on the extant literature and our previous findings, we defined atypical RSA patterns as high resting RSA level combined with an augmentation response, or low resting RSA combined with an RSA withdrawal response to a sad affect trigger (sad film). Given the purported benefits of high resting RSA and findings that RSA withdrawal is a typical response to sadness, high resting RSA levels in conjunction with vagal withdrawal to the sad film was viewed as a normative pattern. We made no a priori assumptions about the role of low resting RSA + augmentation in depression risk.
Does the preponderance of evidence support RSA patterns as endophenotypes for depression?
We believe that the answer is “yes.” Following the criteria proposed by Gottesman and Gould (Reference Gottesman and Gould2003), we have shown that atypical RSA patterns are detectable before the onset of clinical depression, are associated with increased depression risk, predict current depressive episodes, and are present among remitted subjects. Our results also show that RSA patterns are concordant within families, which supports the possibility that such patterns have an intermediary role between genes and their clinical phenotypic manifestations.
If RSA patterns are endophenotypes, then what are their genetic origins?
Although there are likely many genes that influence PNS activity and depression risk, a growing literature shows promising results with respect to polymorphisms of the serotonin transporter gene (5-HTT) linked promoter region (5-HTTLPR). Findings suggest that subjects with short 5-HTT variants show reduced resting RSA levels (Agorastos et al., Reference Agorastos, Kellner, Stiedl, Muhtz, Becktepe and Wiedemann2013; Crişan et al., Reference Crişan, Pană, Vulturar, Heilman, Szekely and Drugă2009; Ellis, Beevers, Hixon, & McGeary, Reference Ellis, Beevers, Hixon and McGeary2011; but for null findings see Vulturar, Chiş, Ungureanu, & Miu, Reference Vulturar, Chiş, Ungureanu and Miu2012), which are linked to activity in ventromedial and amygdalar neural regions. The results from a recent meta-analysis of eight imaging studies support the relationship between RSA activity during emotionally evocative tasks and ventromedial prefrontal cortex and amygala activity (Thayer, Åhs, Fredrikson, Sollers, & Wager, Reference Thayer, Åhs, Fredrikson, Sollers and Wager2012). Taken together with studies that suggest 5-HTTLPR is one susceptibility gene for depression (Caspi et al., Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington2003; Clarke, Flint, Attwood, & Munafò, Reference Clarke, Flint, Attwood and Munafò2010; López-León et al., Reference López-León, Janssens, Ladd, Del-Favero, Claes and Oostra2008), a reasonable argument can be made for 5-HTTLPR to be linked to the heritability of PNS activity and depression risk transmission. Future studies of the relationship among 5-HTTLPR, RSA patterns, and depression would do much to elucidate the mechanisms for depression risk and recurrence.
If atypical RSA patterns are an endophenotype for depression, what are the underlying mechanisms?
The mechanisms of the relationship between RSA patterns and depression are not well understood and cannot be established by the present study. However, we have previously speculated that RSA patterns probably index mechanisms that modulate the intensity of emotional experience and regulatory responding (see Yaroslavsky, Bylsma, et al., Reference Yaroslavsky, Bylsma, Rottenberg and Kovacs2013; Yaroslavsky, Rottenberg, et al., Reference Yaroslavsky, Rottenberg and Kovacs2013). Others have proposed specific relationships among the neural origins of parasympathetic activity, emotional arousal, and attention-mediated self-regulation (e.g., Thayer, Hansen, Saus-Rose, & Johnsen, Reference Thayer, Hansen, Saus-Rose and Johnsen2009; Thayer & Lane, Reference Thayer and Lane2000, Reference Thayer and Lane2009). Empirical studies also have demonstrated a relationship between individual RSA indices and emotion intensity in the laboratory (Dywan, Mathewson, Choma, Rosenfeld, & Segalowitz, Reference Dywan, Mathewson, Choma, Rosenfeld and Segalowitz2008) and in daily life (Fabes & Eisenberg, Reference Fabes and Eisenberg1997). Given the purported benefits of high resting RSA, RSA withdrawal to a negative mood trigger may enable individuals to experience their emotions without being overwhelmed (see Beauchaine, Reference Beauchaine2001). High resting RSA levels have been associated with good executive functioning (Hansen, Johnsen, & Thayer, Reference Hansen, Johnsen and Thayer2003) and attention flexibility (Park, Van Bavel, Vasey, & Thayer, Reference Park, Van Bavel, Vasey and Thayer2012). Therefore, whereas vagal withdrawal in the context of low resting RSA may overwhelm the self-regulation system (e.g., Beauchaine, Gatzke-Kopp, & Mead, Reference Beauchaine, Gatzke-Kopp and Mead2007), appropriate vagal withdrawal in the context of high resting RSA may facilitate flexible mobilization of self-regulation resources. Conversely, those who evidence RSA augmentation, an atypical response to sadness (see Kreibig, Reference Kreibig2010), in the context of high resting RSA may experience difficulty refocusing their attention. For instance, when a desired toy was placed within sight, but out of reach, children who failed to show RSA withdrawal were more likely to focus on the toy (a maladaptive response) than to refocus their attention on the experimenter (Calkins, Reference Calkins1997). We also reported previously that youth with high resting RSA + augmentation patterns benefited less than those with normative RSA patterns from refocusing their attention in the service of mood repair (Yaroslavsky et al., Reference Yaroslavsky, Rottenberg, Bylsma, Jennings, George and Bajo2014). Specifically, whereas adolescents with normative RSA patterns reported less sadness after mood repair via attention refocusing, subjects with high resting RSA + augmentation patterns evidenced little improvement in sad mood (Yaroslavsky et al., Reference Yaroslavsky, Rottenberg, Bylsma, Jennings, George and Bajo2014).
The salubrious effects of the low resting RSA + augmentation patterns are curious at first blush, given that low resting RSA is a purported vulnerability factor for depression (Rottenberg, Reference Rottenberg2007) and RSA augmentation is an atypical RSA response to negative mood triggers (Kreibig, Reference Kreibig2010). However, if low resting RSA is a risk factor for emotional lability (Beauchaine, Reference Beauchaine2001), then increased RSA during an evocative experience may reduce the risk of hyperarousal. While this may serve as a protective factor for depression, reduced hyperarousal may be linked to other problems, including delinquent and externalizing behaviors (El-Sheikh et al., Reference El-Sheikh, Hinnant and Erath2011; Hinnant & El-Sheikh, Reference Hinnant and El-Sheikh2009). Future studies that investigate the mechanisms through which the low resting RSA + augmentation pattern produces its effects would do much to clarify its role in depression risk and adjustment.
It is noteworthy that individual RSA indices were less informative than when they were combined into patterns. Our null findings for individual RSA indices are consistent with a mixed literature on the role of RSA and depression (see Rottenberg, Reference Rottenberg2007). Even when significant, as in the case of the association between RSA reactivity within families, the effect sizes of individual RSA indices were markedly smaller than the effect sizes of RSA patterns (e.g., RSA indices r = .33–.35, RSA patterns r = .36–.63). These findings underscore the complexity of PNS activity and suggest that studying individual RSA indices may provide an incomplete picture of RSA deficiencies in depression.
Our findings should be interpreted in the context of several limitations, including the fact that we did not control for respiration in our analyses. We also did not consider the effects of psychiatric comorbidity or sympathetic nervous system activity on the association between RSA and depression. Our results are also limited by variable time periods between assessments, and a reduced sample size for our longitudinal analyses in Study 1. Replicating the present findings using multimethod assessment approaches and larger samples will be important to elucidate the role of RSA patterns as an endophenotype of depression and in depression risk transmission.
Despite these limitations, the present study has notable strengths. First, we used various well-characterized, initially clinically referred samples. Second, we focused on the pediatric-onset phenotype of depression, thus controlling for age at onset, which is a key contributor to heterogeneity in clinical depression. Third, the use of a high-risk, but not yet affected sample allowed us to look at temporal precedence of parasympathetic activity in depression risk. Fourth, we employed multimethod approaches to systematically examine the relationships at hand. Thus, our results represent an important step toward evaluating atypical parasympathetic nervous system activity as an endophenotype for depression risk and its transmission.










