Oxidative stress reflects an imbalance between reactive oxygen species and a biological antioxidant system. It can cause toxic effects against all the human tissues and important biomacromolecules such as proteins, lipids and DNA. It is believed that various diseases are connected tightly to oxidative stress (Lau et al. Reference Lau, Shukitt-Hale and Joseph2005). Thus, food-derived antioxidant peptides that can be consumed in diet have attracted increasing attention due to the growing consumer preference for naturally derived products and the increased concern over the quality and safety in food industry.
Many researches and our previous study revealed that peptides obtained from natural food such as soybean (Beermann et al. Reference Beermann, Euler, Herzberg and Stahl2009), eggs (Sakanaka & Tachibana, Reference Sakanaka and Tachibana2006), rice bran (Parrado et al. Reference Parrado, Miramontes, Jover, Gutierrez, Collantes de Teran and Bautista2006), and whey protein (Zhang et al. Reference Zhang, Ling, Sun, Zhang, Yu, Kamau and Lu2012) have antioxidant activities. However, the antioxidant activities of these peptides, even the peptides from the same source, were significantly different from each other using the same evaluation system. For example, the antioxidant activities of enzymatic whey protein peptides on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals differed from 19·68 to 84·78 (our unpublished data). It is said that hydrophobicity is generally considered as advantageous regarding antioxidant properties, because hydrophobic antioxidants are more active in emulsions than their hydrophilic homologues. Laguerre's et al. (Reference Laguerre, Wrutniak-Cabello, Chabi, Giraldo, Lecomte, Villeneuve and Cabello2011) study implied that there was a dependency, though nonlinear, between hydrophobicity and antioxidant capacity of phenolipids. Amarowicz et al. (Reference Amarowicz, Karamac and Wanasundara1997) also found that hydrophobic components from flax extract showed stronger antioxidant effect than hydrophilic fractions. Thus, this study tried to explore the connection between hydrophobicity and antioxidant abilities of peptides derived from whey protein hydrolysates (WPHs).
Whey is a by-product of the cheese-making process. It is a rich and varied mixture of globular proteins possessing a wide range of nutritional and functional properties. In this study, WPHs were fractionized according to their hydrophobicity. And H2O2-induced rat pheochromocytoma line 12 (PC12) oxidative model was established to assess the antioxidative stress activity.
Materials and methods
Materials
Heat-stable ALACEN392 whey protein concentrate was a product of Fonterra (Auckland, New Zealand). Pepsin (EC. 3.4.23.1, 1 : 10 000 U) and trypsin enzymes (EC. 3.4.21.4, 1 : 2500 U) were purchased from Sangon Biotech (shanghai) Co., Ltd. PC12 cells were purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) and foetal bovine serum were obtained from Gibco (Grand Island, NY, USA). Total antioxidant capacity (T-AOC), superoxide dismutase (SOD) activity, catalase (CAT) activity, malondialdehyde (MDA), and lactate dehydrogenase (LHD) Assay Kits were sourced from Institute of Biological Engineering of Nanjing Jian-cheng (Nanjing, China). Propidium iodide (PI) and Hoechst 33342 were purchased from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade and provided by commercial suppliers.
Preparation and fractionation of whey protein hydrolysates
The preparation of WPHs involved the method described by Zhang et al. (Reference Zhang, Ling, Sun, Zhang, Yu, Kamau and Lu2012) with pepsin and trypsin. After preparation, WPHs were absorbed by macroporous adsorption resin DA201-C and eluted by three various concentrations of ethanol (20, 40 and 60%), the graded elution fractions obtained were named M20, M40 and M60, respectively.
HPLC molecular weight profile and amino acid composition
Molecular weight distribution (MWD) of WPHs was determined using an HPLC system (Agilent 1100®, CA, USA). A total of 17 amino acids were determined using oxidative acid hydrolysis for cysteine/cystine and methionine and conventional acid hydrolysis for the others.
The degree of hydrophobicity
The degree of hydrophobicity is calculated according to the Q rule established by Ney (Reference Ney1971). Ney's Q value is the average free energy for the transfer of the amino acid side chains from ethanol to water. Ney proposed the Q value as a means to theoretically measure hydrophobicity by the equation: Q=ΣΔf/n, where Q=average hydrophobicity of a peptide, ΣΔf=the sum of free energy for the transfer of amino acid side chains from ethanol to water in cal/mol for each residue, and n=the number of amino acid residues.
Determination of the antioxidative activity
The reducing ability of WPHs is determined by potassium ferricyanide method, using the method described by Peng et al. (Reference Peng, Xiong and Kong2009) and Zhu et al. (Reference Zhu, Zhou and Qian2006). The free radical DPPH scavenging activity was determined by the method of by Wu et al. (Reference Wu, Shiau, Chen and Chiou2003).The suppression rate of lipid peroxidation is determined by thiobarbituric acid (TBA) assay (Osawa & Namiki, Reference Osawa and Namiki1985).
Cell morphological assay
PC12 cells were plated in cell culture plates at 3×105 cells per well according to the method described by Lu et al. (Reference Lu, Qian, Sun, Zhou, Chen, He, Zhang and Wu2010). To produce oxidative stress, H2O2 was freshly prepared from a 30% stock solution prior to each experiment. Cells in WPHs-group were pretreated with 100 or 200 μg/ml WPHs for 2 h, following co-incubated with 100 μmol/l H2O2 for another 24 h. The H2O2-group cells were only treated with 100 μmol/l H2O2 for 24 h, while the control-group cells were cultured with the same medium without H2O2 or WPHs. At the end of the experiment, all the cells were photographed by phase-contrast microscopy (Nikon).
Cells viability assay
The cell viability was determined by MTT (3-(4,5-dimehylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide) method as described by Zhao et al. (Reference Zhao, Zou, Lin, Shi and Zhu2007). Briefly, MTT was dissolved in phosphate buffer saline (PBS) solution at a concentration of 5 mg/ml, and cells (5×103 cells/well) seeded in 96-well plates. After exposure to different concentration of esculin and 50 mm Dopamine for 24 h, 10 ml MTT solution was added to each well and incubated for additional 4 h. Then, the medium was aspirated off. To achieve solubilization of the formazan crystal formed in viable cells, 200 ml dimethylsulphoxide was added to each well. The absorbance was read at 570 nm.
Nuclear staining for assessment of apoptosis
PC12 cells were stained with two fluorescent dyes, PI and Hoechst 33342 (Lieberthal et al. Reference Lieberthal, Menza and Levine1998). Each group of cells was photographed twice (Olympus Optical., Japan magnification, ×400). Apoptotic cells were counted for five independent microscopic fields for each group (Giovannini et al. Reference Giovannini, Sanchez, Straface, Scazzocchio, Silano and De Vincenzi2000).
T-AOC, SOD, CAT, MDA and LDH assays
To analyse T-AOC, SOD, CAT, and MDA activities, the cells were washed with PBS twice and lysed on ice for 30 min and then centrifuged at 12 000 g for 5 min at 4 °C. Protein content was measured using a bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China). In order to analyse the level of LDH activity, the medium was collected. The activities of T-AOC, SOD, CAT, MDA and LDH were tested by Assay Kits according to the manufacturers’ instructions.
Statistical analysis
Results were expressed as mean±sd of at least three independent experiments. Multiple groups’ comparisons were evaluated by Duncan's multiple range test, and performed in SPSS (SPSS 18.0, USA). Differences between the means were determined and were considered statistically significant when P<0·05.
Results and discussion
Fractionation of WPHs and Amino acid composition of each component
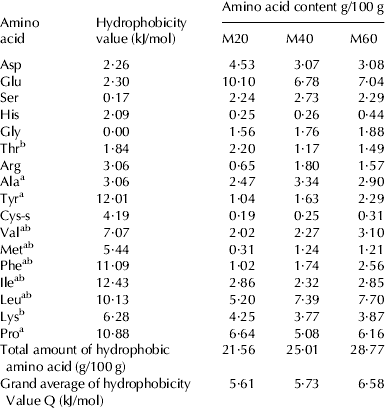
The amino acid composition and the degree of hydrophobicity of M20, M40 and M60 were analysed. As shown in Table 1, component of hydrophobic amino acids Tyr, Val, Phe, Leu and hydrophobicity Q value showed a trend of increase from M20 to M60. Many researchers have confirmed that some kinds of amino acid residues, such as Pro, Tyr, Val, Ile, and Leu were possibly responsible for the high antioxidant effects (Chen et al. Reference Chen, Muramoto, Yamauchi and Nokihara1996; Zhu et al. Reference Zhu, Chen, Tang and Xiong2008; Alemán et al. Reference Alemán, Giménez, Pérez-Santin, Gómez-Guillén and Montero2011). As an antioxidant needs to be stable enough to donate electron to stabilize and neutralize free radicals (Patil et al. Reference Patil, Phatak, Chandra and Lobo2010), these hydrophobic amino acids could act as qualified electron donors to stabilize free radicals and break free radical chain reaction.
Table 1. Amino acid content of WPHs (g/100 g protein)

Note: aHydrophobic amino acid
bEssential amino acid
Antioxidant ability of WPHs in vitro
DPPH radical scavenging capacity, suppression rate of lipid peroxidation and reducing power of WPHs were tested to evaluate antioxidant abilities in vitro from different perspectives. Based on the data in Table 2, three components of WPHs displayed reasonably good antioxidant ability. Moreover, with the increase of hydrophobicity, antioxidant ability of WPHs improved significantly (P<0·05).
Table 2. Antioxidant ability of WPHs

Note: A, B and C represent the significant difference among three samples, P<0·05
Porter et al. (Reference Porter, Black and Drolet1989) first formulated a polar paradox hypothesis that regarding oil-in-water emulsion, hydrophobic antioxidants are more active than their hydrophilic homologues and there was a linear dependency between the antioxidant capacity and the hydrophobicity. Frankel et al. (Reference Frankel, Huang, Kanner and German1994) explained that this phenomenon might be because hydrophobic antioxidants tend to concentrate at the interfacial membrane where the oxidation is supposed to occur. Laguerre et al. (Reference Laguerre, Giraldo, Lecomte, Figueroa-Espinoza, Barea, Weiss, Decker and Villeneuve2010) confirmed that there was indeed a dependency between the antioxidant capacity and the hydrophobicity though in a nonlinear trend. Our results showed that the more hydrophobic amino acids WPHs contained, the better antioxidant ability they presented.
WPHs increased intracellular antioxidase system load
Monitoring the change of antioxidase system could help us to understand the antioxidative activities in living cells. The T-AOC reflects the ability of the body to remove free radicals, which may be one of the most effective defences of a living body against various diseases. The CAT level reflects catalase activity, which decompose H2O2 into water and oxygen. SOD catalyses the neutralization of superoxide anion to H2O2.
Compared with control-group, T-AOC, CAT, SOD levels of H2O2-group declined significantly (P<0·05) (Table 3) owing to H2O2-induced oxidative damage of. living cells. While pretreatment with (M20, M40 and M60), all the three indexes increased significantly (P<0·05) compared with the H2O2-group. These results suggested that WPHs could increase the clearance rate of H2O2 and protect the cells against oxidative injury. Among the three WPHs, M60 exhibited the highest protective effect.
Table 3. The effect of WPHs on T-AOC, SOD, CAT, MDA and LDH on H2O2-induced PC12 cells (X±sd)

Note: a, b and c represent the significant difference among samples, P<0·05
While MDA reflects the degree of lipid peroxidation in cells and LDH suggests the level of cell injury. The MDA and LDH levels of H2O2-group increased (P<0·05) compared with that of the control group (Table 3), indicating that the lipid system had been damaged. However, the MDA and LDH levels of the WPHs group decreased significantly compared with the H2O2-group. The results indicated that WPHs could reduce the invasion of reactive oxygen and inhibit lipid peroxidation, thus protect the cell membrane to a certain extent. And the ability of inhibiting oxidative damage was enhanced from M20 to M60 in turn.
Morphological alterations and cell viability of PC12 Cells induced by H2O2
It is believed that H2O2 could cause apparent damage and result in cell morphological alterations (Li et al. Reference Li, Zhao, Ji, Song, Dong, Lo, Cheung, Zhu and Tsim2003). Different concentrations of H2O2 were used to induce dose-dependent oxidative damage in PC12 cells and 200 μmol/l H2O2 was median lethal dose (data not show). Normal cells are multipolar, have elongated shapes, and grow attached to a substrate (Fig. 1Aa). Morphological change was observed under inverted microscope after 200 μmol/l H2O2 affect cells. PC12 cells are spherical in shape and in suspension without attaching to a surface (Fig. 1Ab). WPHs exerted a protective effect against H2O2-induced morphological change (Fig. 1Ac–e). The data were consistent with quantitative data on cell viability (Fig. 1B). These results indicated that WPHs can protect PC12 cells against H2O2-induced cytotoxicity and significantly improve the morphology of H2O2-injured cells.

Fig. 1. The effect against H2O2-induced oxidative stress of PC12 cells morphology and viability. (A) Effect of Graded elution fractions of WPHs on H2O2-induced morphological alterations in PC12 cells (a Control, b Model (200 μmol/l H2O2), c M20+200 μmol/l H2O2, d M40+200 μmol/l H2O2, e M60+200 μmol/l H2O2). bar=20 μm. (B) Graded elution samples of WPHs inhibit the reduction of cell viability induced by H2O2 in PC12 cells (a, b, c, and d represent the significant difference among samples, P<0·05).
Attention should be concentrated on the increasing protective effect of graded elution samples of WPHs. A better cell morphology and a significantly 19·3% increase in cell viability were presented (P<0·05) while PC12 cells were cultured with M60. It is interesting to find that highly hydrophobic samples of WPHs achieved a more effective protecting ability against oxidative stress.
Nuclear staining for assessment of apoptosis
The effects of WHPs on H2O2-induced apoptosis in PC12 cells are shown in Fig. 2. The PC12 cells nuclei of control group exhibited diffuse homogeneous blue fluorescence (Fig. 2Aa). When exposed to 200 μmol/l H2O2, PC12 cells showed the condensation of chromatin, and the appearance of brilliant condense blue fluorescence in nuclei, compared with the control group (Fig. 2Ab). However, when the cells were pre-incubated with 200 μg/ml WPHs (M20, M40 and M60), H2O2-induced cell apoptosis was significantly attenuated. The nuclear staining count assay indicated that compared with control group, up to 42·6±1·6% of cells incubated with 200 μmol/l H2O2 for 24 h apoptosis and that pretreatment with WPHs (M20, M40 and M60) reduced 13·4, 20·7 and 28·6% of apoptotic cells respectively (P<0·05) (Fig. 2B).

Fig. 2. Graded elution samples of WPHs protected PC12 cells against H2O2-induced apoptosis. (A) Morphological apoptosis was determined by staining with Hoechst 33342 and PI. Arrowheads indicate apoptosis cells. (a Control, b Model (200 μmol/l H2O2), c M20+200 μmol/l H2O2, d M40+200 μmol/l H2O2, e M60+200 μmol/l H2O2). bar=50 μm. (B) Apoptosis ratio was determined by counting under fluorescence microscope after PC12 cells were stained with Hoechst 33342 and PI. (a, b, c, d, and e represent the significant difference among samples, P<0·05).
Excessive reactive oxygen species (ROS) could promote oxidative reaction and ultimately leads to apoptotic or necrotic cell death. Many studies have shown that nuclear condensation and DNA fragmentation are closely related to H2O2-induced neuronal cell death (Zhou et al. Reference Zhou, Zeng, Kong and Sun2008; Zhao et al. Reference Zhao, Zhang, Yu, Sun, Lin, Tan and Lu2011). Our results indicated that WPHs quenched excess free radicals and prevented DNA from being attacked by ROS. There was a positive correlation between the ability of inhibiting apoptosis and hydrophobicity of WPHs.
These results illustrated that WPHs exerted significant protection on PC12 cells against H2O2-induced cell apoptosis. This might be one of the important antioxidant abilities WPHs represented. What's more, highly hydrophobic samples of WPHs showed a more effective anti-apoptosis ability.
Conclusion
Oxidative stress damaged PC12 cells were protected by WPHs. In terms of chemical antioxidant ability and cultured cell model systems, WPHs improved antioxidant ability, maintained cell viability, and inhibited apoptosis by increasing clearance rate of H2O2 and inhibiting cell membrane's lipid peroxidation. These results indicate the potential benefits of WPHs as valuable food antioxidative additives. And more notably, hydrophobicity enhanced the protective effect of WPHs against oxidative stress.
This study was supported by the National Natural Science Foundation of China (No. 31071491), ‘Twelfth 5-year’ National Key Technology R&D Program of China (2012BAD33B05), the 863 project (2013AA102207), and the Natural Science Foundation of Jiangsu Province (No. BK2010156).