Introduction
Burrow-nesting petrels are the most abundant seabirds in the Southern Ocean, with a total population in the hundreds of millions of birds (Warham Reference Warham1996). Having evolved as insular birds breeding on remote oceanic islands, they lack behavioural adaptations that allow them to coexist with introduced mammalian predators (Blackburn et al. Reference Blackburn, Cassey, Duncan, Evans and Gaston2004). Since few oceanic islands have escaped invasion, introduced predators (e.g. domestic cats Felis catus L., rats Rattus spp. L. and house mice Mus musculus L.) account for the largest proportion of seabird population declines, more so than incidental bycatch and competition for prey with commercial fisheries (Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008).
House mice were introduced accidentally to sub-Antarctic Marion Island (46°54'S, 37°45'E) in the early 19th century, most probably by sealers or shipwrecks (Watkins & Cooper Reference Watkins and Cooper1986). Domestic cats were taken to the island’s weather station in 1948 to control mice, but they soon turned feral and started eating the island’s seabirds (Rand Reference Rand1954). By the mid-1970s an estimated 2000 cats were killing some 450 000 birds per year, most of which were burrow-nesting petrels (Van Aarde Reference Van Aarde1980). Petrel population densities were reduced more than 20-fold compared to the adjacent, predator-free Prince Edward Island (Schramm Reference Schramm1986), and some small species (e.g. diving petrels and storm petrels) were apparently extirpated (Van Aarde Reference Van Aarde1980, Ryan & Bester Reference Ryan and Bester2008). Fortunately cats were eradicated by 1991 (Bester et al. Reference Bester, Bloomer, van Aarde, Erasmus, van Rensburg, Skinner, Howell and Naude2002), allowing the greatly diminished burrow-nesting petrel numbers to recover.
Initial indications were positive. Following the removal of cats there were marked increases in the breeding success of burrowing petrels, especially great-winged petrels Pterodroma macroptera (Smith), which breed in winter when cat predation pressure was most severe (Cooper & Fourie Reference Cooper and Fourie1991, Cooper et al. Reference Cooper, Marais, Bloomer and Bester1995). However, the post-cat recovery of burrowing petrel numbers on Marion has been much slower than anticipated, especially for smaller species (Dilley et al. Reference Dilley, Schramm and Ryan2017a). Recent evidence from a repeat survey of burrow densities (Dilley et al. Reference Dilley, Schramm and Ryan2017a) and from analyses of prey remains of brown skuas Stercorarius antarcticus (Lesson) (Cerfonteyn & Ryan Reference Cerfonteyn and Ryan2016) both suggest there has been little recovery of burrow-nesting petrel populations at Marion. At least nine species of burrow-nesting petrels breed on Marion Island (Ryan & Bester Reference Ryan and Bester2008) and while the effects of cat predation were well documented up to the early 1990s (Schramm Reference Schramm1983, Fugler et al. Reference Fugler, Hunter, Newton and Steele1987, Van Rensburg & Bester Reference Van Rensburg and Bester1988, Newton & Fugler Reference Newton and Fugler1989, Cooper & Fourie Reference Cooper and Fourie1991, Cooper et al. Reference Cooper, Marais, Bloomer and Bester1995), recent estimates of petrel breeding success are lacking.
Since 2015, the dramatic increase in mouse predation on albatrosses at Marion Island has been of particular concern (Dilley et al. Reference Dilley, Schoombie, Schoombie and Ryan2016). The hundreds of thousands of petrels that breed in burrows and lava caves are also likely to be attacked by mice, yet to date there has been little direct evidence of mouse predation on burrowing petrel chicks, probably at least in part because attacks on petrels nesting in underground burrows are much harder to detect that those on albatross chicks. Fugler et al. (Reference Fugler, Hunter, Newton and Steele1987) suspected that mice predated ‘some eggs and small chicks’ of blue petrels Halobaena caerulea (Gmelin) at Long Ridge in 1982 when they found ‘one chick carcass had deep wounds on the back of the neck, probably made by a mouse’ (p. 106). On Gough Island mice have been shown to be very efficient predators of burrow-nesting petrel chicks (Wanless et al. Reference Wanless, Ratcliffe, Angel, Bowie, Cita, Hilton, Kritzinger, Ryan and Slabber2012, Dilley et al. Reference Dilley, Davies, Bond and Ryan2015), and there is circumstantial evidence that mice impact breeding success and distribution of storm petrels on Steeple Jason Island (Bolton et al. Reference Bolton, Stanbury, Baylis and Cuthbert2014), but the extent of mouse predation on burrow-nesting petrels on Marion Island is unknown.
Here, we report the breeding success of four species of burrowing petrels over one to five breeding seasons and assess the extent of mouse predation using video surveillance inside burrow chambers. Reasons for nest failures are summarized with a particular focus on the frequency of chick mortalities in the first 1–2 weeks after hatching. We hypothesize that i) mice are suppressing the post-cat recovery of petrel populations through depredation of petrel eggs and chicks, and ii) petrel species that breed in winter are more severely affected by mouse predation than summer breeders, similar to the pattern observed on Gough Island, because mice face a greater challenge to obtain food in winter than in summer.
Methods
Fieldwork was conducted from April 2012 to March 2017 at Marion Island (290 km2), south-west Indian Ocean. Four species of burrowing petrels were monitored for one to five seasons: blue petrels (one season), white-chinned petrels Procellaria aequinoctialis L. (two seasons), grey petrels P. cinerea (Gmelin) (five seasons) and great-winged petrels (five seasons). Study nests were individually marked with numbered PVC poles and regular nest checks made with a burrowscope (custom-made burrowscope with a high-resolution conical pinhole camera, light-emitting diode (LED) torch (200 lumens) and an 18×21 cm colour monitor) to record breeding success. The bright torch allowed sufficient image quality to monitor chicks for mouse wounds. Infrared video cameras (details below) were installed at a subsample of burrows to record activity inside the nest chambers. Access to nest chambers was facilitated by digging hatches over the entrance burrow ~0.3 m away from the nest chamber. These access hatches were cut to snugly accommodate a five litre plastic tub, which was filled with the vegetated ‘plug’ removed to cut the hatch. The tub with its live vegetation plug could then be removed and reinserted with minimal disturbance, crucially not revealing the location of the nest to brown skuas.
Breeding success of blue petrels was estimated at three study sites (Fig. 1), which represent the main blue petrel breeding habitats (Dilley et al. Reference Dilley, Davies, Schramm, Connan and Ryan2017b): i) Acaena slopes at Macaroni Bay (46°53.432'S, 37°52.493'E), where the creeping stems of Blechnum penna-marina (Poir.) ferns and Acaena magellanica (Lam.) Vahl creepers form large soft mats of vegetation on well-drained soils, ii) Leptinella plains at Swartkop (46°55.380'S, 37°35.799'E), where there are extensive low herb fields of Leptinella plumosa (Hook.f.) and Crassula moschata Forst. with occasional large sprawling cushion plants Azorella selago Hook.f. on coastal slopes and flat areas with frequent sea spray, and iii) tussock slopes at Long Ridge (46°50.841'S, 37°49.098'E) dominated by tussock grass Poa cookii Hook.f., tufts of the sedge Uncinia compacta A.Rich. and introduced grasses Poa annua Cham. & Schltdl. and Agrostis stolonifera L. At each site, 50 burrows containing incubating birds were individually marked and fitted with access hatches to view the nest chamber (see above). Study nests were selected at the end of September 2012 when birds had already started laying, thus early egg failures and accurate laying dates were not recorded. Study nests were monitored for one breeding season (2012/13, n=150 breeding attempts) from early–mid-incubation until chicks fledged. At Macaroni Bay, nests were checked every 2 days from mid-incubation until chicks were 3 weeks old and weekly thereafter. At Long Ridge, nests were checked every 2 weeks from early incubation, but weekly at hatching. At Swartkop, nests were checked every 3 weeks from early incubation.

Fig. 1 Study area in the north-east corner of Marion Island, showing the locations of the burrow-nesting petrel study areas. The insert shows the location of the Prince Edward Islands, with Prince Edward Island 22 km to the north-east of Marion Island.
White-chinned petrels study burrows (Fig. 1) were located on coastal slopes dominated by Blechnum penna-marina ferns and patches of Acaena magellanica near the station (Base) and inland of Gentoo Lake (46°52.649'S, 37°51.572'E), and burrows for monitoring with nest cameras were located down-slope from the helicopter hanger (46°52.523'S, 37°51.436'E). Freshly renovated burrows were selected for the study and access hatches were fitted to 50 burrows in late October 2012, prior to laying when birds were on their pre-laying exodus. Eggs were laid in 37 of these burrows, so a further 13 burrows were selected after laying to make up 50 study burrows. Study burrows were monitored every 7–10 days from laying until chicks fledged over two breeding seasons (50 breeding attempts in 2012/13 and 41 in 2013/14), with more frequent checks (3–5 days) from hatching until chicks were 2 weeks old.
Grey petrels are scarce on Marion Island, where they nest singly or in small groups in burrows or in well concealed caves (Fig. 1). Most breeding caves are among large grey lava boulders (e.g. inland from Duikers Point, 46°52.041'S, 37°51.397'E), but nests were also found in black lava caves. Extensive searches of all possible burrows and caves found 20 nest sites (11 in caves, nine in burrows) within an ~300 ha area around the station (Fig. 1) in the early winters (April–May) of 2012–16. Useful clues to an active nest site were feathers lying near the entrance and fresh faecal stripes, often on a small steep slope covered by Blechnum where birds display at night. Grey petrels were responsive to call backs which were used to identify the occupants of active looking burrows (see Dilley et al. Reference Dilley, Schramm and Ryan2017a). Study burrows were monitored every 7–10 days from laying until chicks fledged over five breeding seasons (57 breeding attempts, 11±2 (standard deviation, SD) per year), with more frequent checks (1–5 days) from hatching until chicks were 2 weeks old.
Great-winged petrels study burrows were located along the inland slopes at Nellie Humps (46°52.934'S, 37°51.365'E, Fig. 1), an area of undulating hummocks with well-drained soils dominated by Blechnum penna-marina ferns. An additional five burrows were selected down-slope from the helicopter hanger for monitoring with nest cameras. In 2012, 15 recently renovated burrows where fitted with an access hatch and checked every 2–5 days from 20 May to 20 June to monitor laying dates. Eggs were laid in nine of these burrows, with a further 48 occupied burrows selected after laying to make 57 study burrows. Study burrows were monitored weekly from laying until chicks fledged over five breeding seasons (276 breeding attempts, 55±2 per year), with more frequent checks (every 2–5 days) over the laying, hatching and small chick stages.
Filming nests with video surveillance cameras
Twelve small infrared cameras linked to digital video recorders were customized to film activity inside nest chambers. Each camera (B/W low light mini camera, code E-25B-B36, 1/3'' CCD) had a 2.1 mm wide angle board lens, covering 120°, accompanied by a ring of 12 infrared LEDs. Inspection hatches were dug through the roof of the burrow passage to gain access to the nest chamber. Each camera was housed in 40 mm PVC piping to keep it dry and to prevent mouse damage, secured to a metal angle-iron pole and positioned 20–30 cm away from the incubating bird.
Eight of these cameras were deployed in burrows on coastal Blechnum slopes within 200 m of the helicopter hanger, which allowed the cameras to be linked to the station by video cables. These cameras were motion activated and connected to a video surveillance system (SuperDVR software) which enabled a live feed, with footage recorded onto a computer. These long-term burrowcams were used to monitor complete breeding cycles and were installed in active white-chinned petrel burrows (16 breeding cycles filmed over the five year study period) in summer and moved to active great-winged (12) and grey petrel (two) burrows in winter (see Table I for details). Cameras were either installed into the burrow chamber before laying or at mid-incubation, when the disturbance of installation was less likely to cause the occupants to abandon their nest. Camera installation took<10 minutes and did not result in any immediate nest failures. Since we suspected the mice would depredate newly hatched and newly independent chicks, it was important to have the cameras in situ before hatching.
Table I Number of burrow chambers filmed using permanent and mobile infrared burrowcams for four species of burrow-nesting petrels at Marion Island from 2012–17.

a Mean±standard deviation.
The remaining four cameras were moved among burrows and sites to monitor small chicks<2 weeks old (the time when chicks are most vulnerable to mouse predation; see Dilley et al. Reference Dilley, Davies, Bond and Ryan2015). Each motion activated camera connected to an independent MemoCam (Video Domain Technologies; powered by 50 Ah 12 v battery; charged manually/solar; data storage micro SD). These mobile burrowcams were used to monitor inside 16 grey, seven great-winged and two blue petrel nest chambers over the study period (Table I).
Data analysis
For all species, hatching success was calculated as the proportion of eggs that produced live chicks; this was a maximum estimate as not all eggs were monitored from laying. To account for this, we calculated daily rates of egg survival for each species over each season using the nest survival model in MARK (version 8.x; White & Burnham Reference White and Burnham1999). We estimated the corrected hatching success as the daily egg survival raised to the power of the length of the incubation period (Rotella Reference Rotella2009). Blue petrel incubation length (49.0±2.0 days, n=7) was taken from Fugler et al. (Reference Fugler, Hunter, Newton and Steele1987); data on incubation lengths for white-chinned petrels (59.5±1.9, n=6), grey petrels (56.6±1.5 days, n=3) and great-winged petrels (55.6±4.2 days, n=6) were collected by BJD in 2012/13 (FitzPatrick Institute, unpublished data). This method of estimating egg survival assumes that daily nest survival is similar across the incubation period within a study site (Mayfield Reference Mayfield1975). Since all nests were followed from egg stages, fledging success was calculated as the proportion of hatched chicks that survived to fledge. The overall breeding success was calculated as the product of the estimates of hatching success and fledging success. Skuas predated 12 burrows by digging out the inspection hatches (2% of breeding attempts at burrows with inspection hatches over the study period: two white-chinned and ten great-winged petrel burrows). The installation of access hatches might have increased the risk of skuas digging up these burrows, thus these breeding attempts were excluded from analyses.
The video files recorded a date and time stamp which enabled us to record a detailed sequence of activity for each filmed nest, including hatching date, frequency of mouse visits/attacks, age of the chick when it was first left alone and the date/time of death for chicks that died before fledging. Video footage from the 2012–13 seasons was manually reviewed to calculate the visitation rate of mice in burrows with chicks 1–14 days old. To quantify the visitation rate, we counted each time a mouse entered the frame as a single mouse visit. This doubtless resulted in multiple records of the same mouse, but it provided an objective criterion to quantify visitation rates. When multiple mice were in the burrow at one time, each mouse counted as a separate visit. All footage was analysed by one person (BJD), eliminating individual observer effects. Consequently, the method provided an index of visitation rates that could be compared between seasons and species.
Seasonal and inter-species differences between frequency of mouse visits to burrows in 2012 were tested using Kruskal–Wallis tests with P<0.05 as the cut-off for significance. Means are presented±SD unless stated otherwise. Breeding years refer to seasons (i.e. 2012 for the 2012–13 summer breeding season).
Results
Breeding success
Breeding success of blue petrels in the three study colonies in 2012 was 61±6% (Tables II & III), more than double the breeding success in the 1980s (Fig. 2). No direct evidence of mouse predation (chicks with mouse wounds) was found; however, 20–44% of failed eggs had mouse incisor marks on freshly broken egg shells, and small chick carcasses were scavenged by mice at all three sites (Table III). Predation by skuas accounted for 50% (5/10) of the chick mortalities at Macaroni Bay, where the loose soil and low woody Acaena shrub provided little defence against burrow excavation by skuas. At Swartkop (Leptinella) and Long Ridge (Poa) there was a similar skua presence to the Macaroni Bay colony, but the proportion of failures due to skuas was lower (20% of chick failures at both sites), possibly due to the compact soil and dense summer growth of Poa and Leptinella vegetation which seemed to provide better protection.

Fig. 2 Breeding success of four species of burrow-nesting petrels at Marion Island from 1979–2016. Cat eradication efforts started in the 1970s and progressed through multiple phases until all cats were removed from the island in 1991 (vertical line). Circled data points indicate study areas in cat-free enclosures (Van Rensburg & Bester Reference Van Rensburg and Bester1988; other data sources: Schramm Reference Schramm1983, Fugler et al. Reference Fugler, Hunter, Newton and Steele1987, Newton & Fugler Reference Newton and Fugler1989, Cooper & Fourie Reference Cooper and Fourie1991, Cooper et al. Reference Cooper, Marais, Bloomer and Bester1995, FitzPatrick Institute, unpublished data).
Table II Breeding attempts (number per year (mean±SD)) and overall breeding success (%; mean±SD (range)) for four species of burrow-nesting petrels monitored from one to five seasons at Marion Island.

a At three locations in 2012.
SD=standard deviation.
Table III Breeding success and probable causes of egg and chick mortality for four species of burrow-nesting petrels monitored at Marion Island from 2012–17.

Data are presented as n, unless otherwise stated.
Blue petrels were monitored for one season only (2012) at three different locations: aMacaroni Bay (Acaena), bLong Ridge (Poa) and cSwartkop (Leptinella).
Breeding success of white-chinned petrels was 63% in 2012 and 55% in 2013 (Tables II & III). No direct evidence of mouse predation was found during nest checks or recorded in the camera-monitored burrows, but in 2013, small chicks were found dead with mouse wounds on two occasions.
Breeding success of grey petrels averaged 34±21% (range 0–56%, n=57 monitored breeding attempts) over the five breeding seasons (Tables II & III). Most chick mortalities occurred in the first week after hatching and in the last three study years all chick mortalities were very small chicks and were almost certainly due to mouse predation. At Duikers Caves in 2012, two large chicks died when almost fully feathered. One of the dead chicks was too deep in a narrow cave to retrieve, but burrow-camera footage revealed no visible wounds or obvious mouse activity around the carcass. The other chick was retrieved and a post-mortem revealed no mouse predation wounds, very little body fat and an empty stomach. We suspect that these chicks died of starvation.
Breeding success of great-winged petrels averaged 52±7% (range 41–62%, n=276 monitored breeding attempts) over the five breeding seasons (Tables II & III). Chick mortality was highest in the first week after hatching and small chicks were found dead with mouse wounds in all five seasons.
Mouse predation, frequency of mouse visits and temporary egg neglect
Mouse activity in the nest chambers of two blue petrel burrows were recorded from early–mid-incubation until both chicks fledged. Blue petrel chicks rarely reacted to a mouse entering the nest chamber and only very occasionally did a mouse make brief contact with the chick. Mice appeared to scavenge around the nest bowl. Egg neglect was recorded in one of the filmed burrows when the parent left its egg unattended for 49 hours (egg age 32 days). Mice did not visit the burrow during this time and the chick hatched 15 days later. Egg neglect was also recorded for two nests in the Macaroni Bay study colony in early incubation (8–10 days after laying) when, over a sequence of nest checks every other day, the eggs were recorded as being incubated, to being left unattended and cold to the touch, to being incubated again. Both eggs hatched successfully. It is possible that other eggs were also temporarily neglected, but were eaten by mice before the adult returned (see Table III ‘Eaten with mouse teeth marks in shell’).
Sixteen white-chinned petrel breeding cycles were monitored with burrow cameras from 2012–17 (Table I). None of the chick mortalities were due to mouse predation. Mice were observed in all filmed nest chambers relatively infrequently (compared to winter-breeding species, Fig. 3) and the only direct contacts observed were of mice licking the chicks’ down, presumably to glean spilt oil and food after a chick was fed. Chicks appeared to be fairly tolerant of this intrusion. They would occasionally sit up and bill snap; however, no defensive vomiting was recorded.
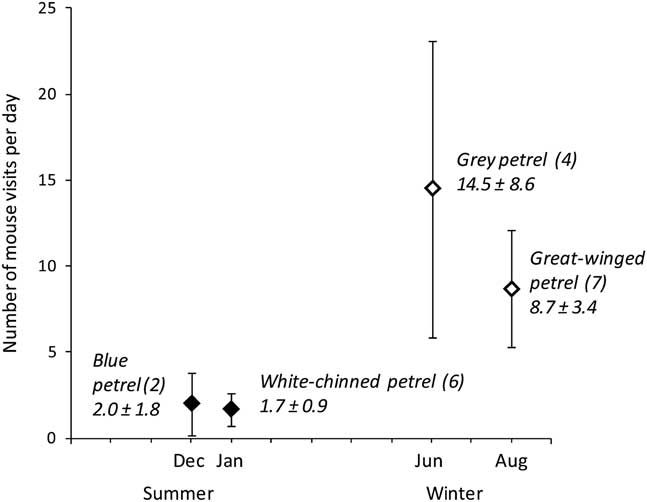
Fig. 3 Relationship between the average daily visitation rates of mice in burrowing petrel study burrows during the first week after chicks hatched in the summer/winter of 2012–13 at Marion Island. Data from burrows monitored with infrared video cameras (see methods for details and visitation analyses); numbers in parentheses indicate number of monitored nests per species. Black diamonds indicate summer breeders and white diamonds indicate winter breeders. Data represent mean±standard error.
Two complete grey petrel breeding cycles were recorded in a burrow near the helicopter hanger, with no mouse predation recorded in either year. Video footage showed that incubating birds often left their burrows for a short period (< 10 minutes), usually in the early evening and more frequently in the week after hatching. In 2012 a female abandoned her newly laid egg in a cave at Nellie Humps and the egg was eaten by mice before the male arrived 2 days later. An additional 16 grey petrel nests were filmed from hatching for 1–41 days; three of these chicks were attacked and killed by mice (Table I). Mouse visitation rates in 2012 when chicks were< 7 days old were the highest recorded (Fig. 3; 14.5±8.6 (standard error, s.e.) per day, range 1–74 per day, n=4 nests, no significant difference in visitation rates between grey petrel burrows, Kruskal–Wallis, H4,15=8.85, P=0.904). At three of the nests with the highest visitation rates, mice harassed the small chicks to such an extent that the chicks vomited oil and repeatedly shuffled around on their nest mound. Two of these chicks were dead the following morning and it is very likely that mice were the cause, yet conclusive evidence was not recorded on video as the camera wires had been chewed by mice. The other chick survived the first 2 weeks unwounded, but mice continued to frequently visit the burrow, especially when the chick was being fed by a parent (Fig. 4). This chick died at 12 weeks old and the freshly mouse scavenged carcass was found on the nest, but the cause of death was not confirmed. However, in 2015 video footage was obtained of a small chick (age<5 days) being attacked and killed while still being brood-guarded by its parent in Duikers Cave. This was the first conclusive video evidence of mouse predation on burrowing petrel chicks at Marion Island (see https://youtu.be/Og1d6a2cmXQ).

Fig. 4 Images from infrared video footage of a grey petrel nest in a cave showing mice apparently licking oils spilt during parental feeds off the chicks’ downy feathers (a.) even in the presence of the parent (b.) (photos by B.J. Dilley).
No mouse predation was recorded during 12 complete monitored breeding cycles of great-winged petrels in burrows near the helicopter hanger (Table I). Incubating adults often left their burrows for short periods, especially shortly after laying, and in one case a mouse attempted unsuccessfully to eat a neglected egg. Mouse visitation rates at burrows with chicks<7 days old were high (Fig. 3; 8.7±3.4 (s.e.), range 1–39 per day, n=7 nests) compared to summer-breeding species (average<2 visits per day, range 0–9), but lower than visits to grey petrel burrows earlier in winter when mouse densities are higher (see discussion). In 2012, video recordings from mobile burrowcams showed mice aggressively and repeatedly harassing small chicks on four occasions, causing the chicks to shuffle around on their nest mounds to face the intruding mice while bill snapping and sitting upright. All four chicks survived and on closer inspection none had mouse injuries. On 20 July 2015, a small newly independent chick (< 5 days old) was filmed being attacked by two mice in a burrow at Nellie Humps (Fig. 5). The chick was dead within 24 hours of being attacked (see https://youtu.be/D9vPoFsjvgs).

Fig. 5 Two-day-old great-winged petrel chick attacked and killed by two mice within hours of being left alone by its parent after the brood-guard phase on 20 July 2015 at Nellie Humps, Marion Island (photo by S. Schoombie).
In summary, winter breeders had lower breeding success than summer breeders (Table II), with most chick fatalities of small chicks<14 days old (Table III). Mice were filmed attacking and killing chicks of both winter-breeding species: grey (3/18 nests filmed; 17%) and great-winged petrels (1/19; 5%). These are the first confirmed records on video of fatal mouse attacks on burrow-nesting petrel chicks at Marion Island. Mouse predation was suspected previously, when small chicks were found dead with fresh wounds typical of those inflicted by mice (open wounds mainly to the back, rump or head; Dilley et al. Reference Dilley, Davies, Bond and Ryan2015). Winter breeders were worse affected by mouse predation than summer breeders, and this was related to higher mouse visitation rates to petrel burrows in winter. In 2012, mouse visitation rates to burrow chambers containing chicks<7 days old were significantly higher (Kruskal–Wallis, H2,29=67.34, P<0.001; Fig. 3) for winter breeders (10.9±12.8, 1–74 visits per day, n=11 burrows) than summer breeders (1.8±2.5, 0–9, n=8).
Discussion
Predation on petrel chicks
While there is mounting evidence of an increase in mouse attacks on surface-nesting albatross chicks at Marion since the early 2000s (Jones & Ryan Reference Jones and Ryan2010, Dilley et al. Reference Dilley, Schoombie, Schoombie and Ryan2016), few direct records of mouse interactions with burrow-nesting petrels existed because of the technical difficulties of observing inside burrows. This study illustrates how mouse predation impacts the breeding success of burrow-nesting petrels at Marion Island. As expected, winter-breeding petrels were affected to a greater extent than those species that breed in summer. The magnitude of the impact on the breeding success of at least grey petrels probably is sufficient to limit population growth and explains why grey petrels show no evidence of a population recovery since cats were eradicated from Marion Island in 1990 (Dilley et al. Reference Dilley, Schramm and Ryan2017a).
Small chicks of winter-breeding grey and great-winged petrels were filmed being attacked and killed by mice. Chicks were dead within hours of being attacked and carcasses were usually consumed completely, leaving little evidence as to the reason for the nest failure. This could explain why so few chicks injured by mice have been found during routine nest checks relative to the high proportion of small chick fatalities, whereby many chicks ‘disappear’ between nest checks. Summer-breeding white-chinned and blue petrels appear to be less affected, with few small chick fatalities in the first weeks after hatching (Table III) and lower mouse visitation rates inside burrows compared to winter-breeding species. During the summer months at Gough Island, mice are less desperate for food, but as winter sets in mice have limited food resources (Cuthbert et al. Reference Cuthbert, Wanless, Angel, Burle, Hilton, Louw, Visser, Wilson and Ryan2016) and have resorted to eating seabird chicks (Dilley et al. Reference Dilley, Davies, Schramm, Connan and Ryan2017b). A similar process probably occurs at Marion Island. In 1992, Avenant & Smith (Reference Avenant and Smith2003) estimated the per capita food availability for mice (macroinvertebrate biomass per mouse) to be ~3.4 kg ha-1 in biotic habitats (mostly coastal areas where the vegetation is heavily influenced by seals and seabirds; Gremmen & Smith Reference Gremmen and Smith2008) and 3.6 kg ha-1 in mire habitats (boggy areas, ranging from wet to dry mires; Gremmen & Smith Reference Gremmen and Smith2008) in early summer, but in early winter the per capita food availability was<10% of the summer estimates (0.4 kg ha-1 and 0.2 kg ha-1, respectively). Grey petrel chicks hatching in early winter had the highest level of mortality of small chicks, at a time when mouse densities are still fairly high but food availability is low, resulting in the lowest seasonal food availability per capita for mice. In the last three study years, all of the grey petrel chicks that died were<7 days old and it is very likely that death was due to mouse predation. Great-winged petrel chicks hatch 1–2 months later than grey petrels, when mouse numbers have already fallen, explaining the better breeding performance of great-winged petrels.
There are no estimates of adult petrel survival rates on Marion, but their breeding success increased immediately following the removal of cats (Fig. 2) and remains at moderate levels which suggests that petrel populations have the potential to recover. However, the recent repeat survey of burrow densities showed only a modest recovery of most burrow-nesting petrel populations since cats were eradicated 25 years ago, with no evidence of an increase in grey petrels (Dilley et al. Reference Dilley, Schramm and Ryan2017a). Grey petrels are drowned accidentally on longlines, which might also contribute to their failure to recover after cats were removed from Marion Island. However, the closely related white-chinned petrel is killed in much larger numbers by fisheries in the region (e.g. Petersen et al. Reference Petersen, Honig, Ryan and Underhill2009), and yet its population has shown the fastest growth following the removal of cats (Dilley et al. Reference Dilley, Schramm and Ryan2017a). Across all petrels, the changes in burrow density (Dilley et al. Reference Dilley, Schramm and Ryan2017a) and breeding success results (this study) show a similar pattern; summer breeders have higher breeding success and recover faster than winter breeders, suggesting there is a common factor suppressing the recovery of winter-breeding petrels.
The breeding success estimates for blue and white-chinned petrels are within the ranges reported elsewhere. We did not find any live blue petrel chicks with mouse injuries, but most of the chick mortalities we recorded were very small chicks, similar to those found on Gough Island, where mice are significant predators of petrel chicks (Wanless et al. Reference Wanless, Ratcliffe, Angel, Bowie, Cita, Hilton, Kritzinger, Ryan and Slabber2012, Dilley et al. Reference Dilley, Davies, Bond and Ryan2015). The low breeding success of blue petrels in the early 1980s (Fig. 2) was largely caused by cat predation because at that stage the cat control programme was in its early phases and it was estimated that there was a 70% increase in cat predation on blue petrels from 1975–82 (Van Rensburg Reference Van Rensburg1985). Once cats were eradicated, blue petrel breeding success improved (Fig. 2) and although too few data exist since 1991 to show any long-term trend, the levels of breeding success on Marion in 2012–13 appear to be within or even above the range reported for blue petrels at Mayes Island, Kerguelen Archipelago, where mice are also the sole introduced mammal (Chastel et al. Reference Chastel, Weimerskirch and Jouventin1995). Chastel et al. (Reference Chastel, Weimerskirch and Jouventin1995) reported that breeding success varied significantly from 1986–94 (26–65%) and hatching failure (52%) accounted for 80% of the total breeding failures, primarily due to egg desertion, especially in years when birds showed poor body condition at the start of the breeding season. From 2012–14, breeding success of white-chinned petrels was similar to that recorded from 1997–2002, following the cat eradication on Marion, when breeding success averaged 61% (42–79%, n=26–53 study nests per year, Fig. 2). These values are within the range reported from other sub-Antarctic breeding sites: at Bird Island (free of introduced predators), South Georgia, breeding success varied from 12–54% at two different study sites (n=72 and 40 burrows) in 1985 (Hall Reference Hall1987) and was consistent from 1996–98 at 44% despite interannual variation in the availability/abundance of Antarctic krill Euphausia superba (Dana) (Berrow & Croxall Reference Berrow and Croxall1999); and at Ile de la Possession, Crozet archipelago, where black rats Rattus rattus (L.) are known to depredate seabird chicks, breeding success was 55–79% at sites where rats were poisoned and 30–61% at control sites (Jouventin et al. Reference Jouventin, Bried and Micol2003).
Although grey petrels are locally common on nearby Prince Edward Island (Ryan & Bester Reference Ryan and Bester2008), they are scarce on Marion with a very low nest density. Grey petrel chicks are particularly vulnerable to mouse predation since they hatch in early winter when mice have few alternative food sources (McClelland et al. Reference McClelland, Altwegg, van Aarde, Ferreira, Burger and Chown2017; Fig. 6). Grey petrels were considered ‘not common’ on Marion at the start of the cat era in 1952 (Rand Reference Rand1954), but their numbers were depleted over four decades of cat predation. It is unclear why the two large chicks where abandoned at Duikers Caves in 2012; it is possible that the adults were killed at sea. One banded pair has not been resighted and the nest site has since been used by a different pair. The average breeding success over this study period (34±21%) was similar to grey petrels on Gough Island (34%, Dilley et al. Reference Dilley, Davies, Bond and Ryan2015), where mice also prey on chicks. This suggests that mice are a major source of breeding failure for this species on Marion Island, which has shown the least evidence of recovering since cats were eradicated (Dilley et al. Reference Dilley, Schramm and Ryan2017a).

Fig. 6 The estimated change in winter invertebrate biomass (kg ha-1) in mire, slope and biotic habitats on Marion Island (sensu Gremmen & Smith Reference Gremmen and Smith2008) in 1976, 1996 and 2006 (adapted from McClelland et al. Reference McClelland, Altwegg, van Aarde, Ferreira, Burger and Chown2017).
The breeding success of great-winged petrels improved dramatically following the eradication of cats (Cooper & Fourie Reference Cooper and Fourie1991) and remains at moderate levels (Fig. 2). However, like grey petrels, most breeding failures occur as a result of chick mortality in the first week after hatching (Table III), likely to be in large part due to mouse predation. On rodent-free Whale Island, New Zealand, closely related grey-faced petrels Pterodroma [macroptera] gouldi (Hutton) achieved 65% breeding success in 2000 and the population has apparently more than doubled since Norway rats Rattus norvegicus (Berkenhout) and rabbits Oryctolagus cuniculus (L.) were eradicated in 1985–87 (Imber et al. Reference Imber, Harrison, Wood and Cotter2003).
Temporary egg desertion
Temporary egg desertion has been documented for many burrow-nesting procellariiforms, and eggs may still hatch despite being neglected for up to two days (Ancel et al. Reference Ancel, Petter and Groscolas1998). However, mice have been recorded to eat unattended eggs within hours (Campos & Granadeiro Reference Campos and Granadeiro1999, Dilley et al. Reference Dilley, Davies, Bond and Ryan2015). The two temporary abandonments of blue petrel eggs recorded 8–10 days after laying were probably females unable to incubate until relieved by their partners. The reason for the 49 hour egg abandonment in another nest only two weeks before it hatched is less clear, but blue petrels are known to leave their egg unattended temporarily throughout the incubation cycle (Ancel et al. Reference Ancel, Petter and Groscolas1998). Although in these cases the unattended eggs were not attacked by mice, we know this occurs from evidence of incisor marks in broken shells. It is unclear how large an egg mice can successfully gnaw into. On Gough Island, mice are able to gnaw into the eggs of great shearwaters Ardenna gravis (O’Reilly), which average 80×52 mm (Dilley et al. Reference Dilley, Davies, Bond and Ryan2015), and probably grey petrels, which average 82×55 mm (unpublished data). However, mice on Gough are larger than mice on Marion Island (Cuthbert et al. Reference Cuthbert, Wanless, Angel, Burle, Hilton, Louw, Visser, Wilson and Ryan2016), thus Marion mice might not be able to access such large eggs. Elsewhere, Imber (Reference Imber1976) reported that that Norway rats ‘ate many abandoned eggs’ (p. 58) that had been temporarily abandoned by grey-faced petrels for 1–6 days on Whale Island, New Zealand, and Campos & Granadeiro (Reference Campos and Granadeiro1999) reported that mice ate almost half of the white-faced storm petrel Pelagodroma marina (Latham) eggs on Selvagem Grande Island, with most being eaten within 24 hours of being left unattended.
When did mice start depredating seabird chicks?
It is unclear when mice started attacking seabird chicks at Marion Island as the timeline is complicated by the presence of cats as the superpredators from 1949–91, but it is likely to have occurred at least since the early 1980s. Mice have been present on Marion since the early 1800s and biological researchers have been monitoring some seabird species year-round since 1965 and more intensively since the 1980s and 1990s (Cooper et al. Reference Cooper, Brooke, Burger, Crawford, Hunter and Williams2001). Surface-nesting seabirds such as albatrosses are well studied, as these species are readily observed and are therefore easier to monitor; the first wandering albatross Diomedea exulans L. chick injured by mice was found in 2003 (Jones & Ryan Reference Jones and Ryan2010). There are few early records of burrow-nesting petrel populations, but the destructive impacts of cat predation were well documented (Van Aarde Reference Van Aarde1980). Michael Schramm (personal communication 2017) found no evidence of mouse predation on live or dead burrow-nesting petrel chicks during his intensive monitoring of 137 Pterodroma burrows over 14 months in 1979–80 (Schramm Reference Schramm1983), but Fugler et al. (Reference Fugler, Hunter, Newton and Steele1987) found evidence of blue petrel chicks injured by mice at Long Ridge in 1982. Mouse biomass on Marion increased from 1990–2008 (Ferreira et al. Reference Ferreira, van Aarde and Wassenaar2006, McClelland et al. Reference McClelland, Altwegg, van Aarde, Ferreira, Burger and Chown2017), yet invertebrate biomass declined > 80% since the late 1970s (McClelland et al. Reference McClelland, Altwegg, van Aarde, Ferreira, Burger and Chown2017), driven in part by a warmer, drier climate (Le Roux & McGeoch Reference Le Roux and McGeoch2008) and the combined impacts of invasive species disrupting the ecosystem functioning (Chown & Smith Reference Chown and Smith1993). Since 2015, there has been a definite increase in the frequency of mice utilizing surface-nesting seabird chicks as an additional food source (Dilley et al. Reference Dilley, Schoombie, Schoombie and Ryan2016) and if invertebrate biomass continues to decline, the impact of mouse predation on Marion’s seabird chicks may become increasingly significant.
We conclude that mice are suppressing the recovery of burrow-nesting petrel populations, especially those that breed in winter, through depredation of petrel eggs and chicks. The widespread increase in mouse predations on albatross chicks at Marion in 2015 is cause for concern and these results show burrow-nesting petrels are also at risk, providing further motive for the eradication of mice from Marion Island.
Acknowledgements
The Department of Environmental Affairs provided logistical support through the South African National Antarctic Programme (SANAP). Financial support was received from the University of Cape Town and the National Research Foundation (SANAP). We thank Innocent Mthembu, the late Nompilo Radebe, Rory Meyer, Fred Fourie, Makabongwe Sigqala and Mariette Wheeler for technical and field support; and Nigel Butcher for developing the MemoCams. We thank Graham Parker and an anonymous referee for constructive suggestions on an earlier draft.
Author contributions
BJD, SS, KS, DD, VP, AO, JS, CWB, TC-K and PGR conducted the fieldwork. BJD analysed the data and wrote the draft. PGR supervised the research and advised on manuscript preparation.
Data deposit
All data are stored with the Niven Library (http://www.fitzpatrick.uct.ac.za/fitz/nivenlibrary/about) at the University of Cape Town.












