Introduction
Inbreeding increases homozygosity, increasing the expression of recessive alleles. This increase in the expression of recessive alleles can expose rare alleles to selection, potentially unmasking variation that facilitates adaptation to new environments (Knowles et al., Reference Knowles, Levy, McNellis, Greene and Futuyma1999; Reed et al., Reference Reed, Lowe, Briscoe and Frankham2003). Commonly, however, these recessive alleles are deleterious and are maintained in populations because their detrimental effects are masked from selection in heterozygous individuals (Carr & Dudash, Reference Carr and Dudash2003). Increased homozygosity and the resulting increased expression of deleterious recessives leads to inbreeding depression – a reduction in the fitness of inbred relative to outbred offspring (Charlesworth & Charlesworth, Reference Charlesworth and Charlesworth1987). Inbreeding depression is well documented across a variety of taxa (Crnokrak & Roff, Reference Crnokrak and Roff1999), including many insects (Roff, Reference Roff2002; Henter, Reference Henter2003; Luna & Hawkins, Reference Luna and Hawkins2004; Saccheri et al., Reference Saccheri, Lloyd, Helyar and Brakefield2005; Fox & Scheibly, Reference Fox, Scheibly, Wallin, Hitchcock, Stillwell and Smith2006), but the degree to which inbreeding depression varies among populations within species, and among traits within populations, is poorly studied in insects other than Drosophila (Fox & Scheibly, Reference Fox, Scheibly, Wallin, Hitchcock, Stillwell and Smith2006).
The seed-feeding beetle, Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae) is a cosmopolitan pest of stored pulses (Fabaceae), especially those of the genus Vigna (such as cowpea, mung and azuki bean). Populations of beetles in seed stores are likely to be founded by a small number of individuals, after which the population rapidly expands, such that population bottlenecks and inbreeding are likely significant features of the biology of this beetle in nature (Tran & Credland, Reference Tran and Credland1995). Because of its ease of laboratory rearing, C. maculatus, and its congener C. chinensis (Linnaeus), have been widely used as models for behavioural, life history and genetic studies (Bieri & Kawecki, Reference Bieri and Kawecki2003; Fox et al., Reference Fox, Czesak and Wallin2004b; Messina, Reference Messina2004a, Reference Messinab; Arnqvist et al., Reference Arnqvist, Nilsson and Katvala2005; Vamosi, Reference Vamosi2005; Yamane & Miyatake, Reference Yamane and Miyatake2005). Most of these studies use beetles from a small number of laboratory colonies that are shared among laboratories. For example, recent work by Messina, Fox and colleagues primarily use colonies from South India (SI) and Burkina Faso (BF).
Here we examine inbreeding effects on the proportion of egg development, egg hatch, hatch-to-adult survival, and larval development time in these two populations of C. maculatus (BF and SI) that are commonly used for ecological and evolutionary experiments. Both populations were established with 100–200 field-collected adults and have been maintained in the laboratory for >100 generations (albeit at large population sizes). They have adapted to laboratory rearing conditions (Messina & Karren, Reference Messina and Karren2003; Messina, Reference Messina2004a, Reference Messinab) and have potentially been subject to substantial loss of genetic variation. We also measure in-breeding depression in a population of the non-pest seed-feeding beetle, Stator limbatus Horn (also Coleoptera: Chrysomelidae), that was recently brought into the laboratory from the field, to ask whether the inbreeding depression observed in these populations of C. maculatus is of similar magnitude to the inbreeding depression observed in another beetle species studied under similar laboratory conditions.
Materials and methods
The biology of C. maculatus
The life cycle of C. maculatus revolves around seeds. Females cement their eggs to the surface of host seeds (Messina, Reference Messina1991). When eggs hatch, first instar larvae burrow into the seed under the egg. Larval development and pupation are completed within a single seed – larvae do not move among seeds and are thus restricted to the seed chosen by their mother. Beetles emerge as reproductively mature adults and require neither food nor water as adults before mating and laying eggs.
The South India (SI) population was collected in 1979 from infested pods of mung bean, Vigna radiata (L.) Wilczek, and the closely related black gram, Vigna mungo (L.) Hepper, in Tirunelveli, India (Mitchell, Reference Mitchell1991). The Burkina Faso (BF) population was collected in 1989 from infested pods of cowpea, V. unguiculata (L.) Walp., in Ouagadougou, Burkina Faso (Messina, Reference Messina1993). These two populations differ in body size, lifetime fecundity, patterns of egg dispersion, oviposition preference, and adult longevity (Fox et al., Reference Fox, Bush, Roff and Wallin2004a, Reference Messinab; Messina, Reference Messina2004b). Both populations were maintained in laboratory growth chambers on seeds of V. radiata (SI) or V. unguiculata (BF) at >1000 adults per generation for >100 generations (BF) or >200 generations (SI) prior to this experiment.
The biology of S. limbatus
Stator limbatus is a seed-feeding beetle native to the southwestern United States and distributed through dry forests and deserts all the way south to northern South America (Morse & Farrell, Reference Morse and Farrell2005a, Reference Messinab). Its entire life cycle takes place on or near host seeds. Eggs are glued to the surface of host seeds and larvae complete development inside a single seed, emerging after pupation as an adult. Adults from most populations reproduce using larval resources; they require neither food nor water, making them a very practical laboratory model. Stator limbatus uses >70 host species throughout its large geographic range. For these experiments beetles were raised on seeds of Albizia julibrissin Durazz. Albizia julibrissin is not a native host but is invasive in the United States and readily colonized by beetles in the field. It is a better host for beetles (i.e. lower mortality) than many of their native hosts. We use A. julibrissin for many laboratory experiments because we can purchase large supplies of seeds from horticultural suppliers.
The study population of S. limbatus used here was collected along Mount Lemmon Highway in Oracle, Pinal Country, Arizona, USA (32.61° N 110.77° W) from >20 Acacia greggii trees as larvae inside of seeds. Beetles emerging from these field-collected seeds were used to establish a laboratory colony (>200 beetles) that was maintained for six generations in the laboratory at >100 families per generation at 26–28°C, light:dark 15:9, prior to this experiment.
Experimental design
The experimental design is illustrated in fig. 1. To measure inbreeding depression a ‘block’ design (Roff, Reference Roff1998) was used. Each block was created by randomly pairing two families chosen from an outbred population. From each family two female and two males were randomly chosen to become parents. These two families were then crossed as shown in fig. 1, creating two inbred and two outbred families per block. The advantage of this design is that it assures that inbred families are created from the same set of alleles as are the outbred families to which they are compared (Fox, Reference Fox2005).

Fig. 1. The block design used to measure inbreeding depression. Each block is created by crossing beetles from two unrelated families, creating two outbred matings (reciprocal crosses between the two families) and two inbred matings (crosses between full-sibs within each family). Outbreds and inbreds within each block thus have, on average, the same set of alleles but differ in degree of homozygosity due to the mating treatment.
Callosobruchus maculatus
Pairs were confined in a 35 mm Petri dish with 35 seeds of mung, V. radiata. Dishes were checked for eggs twice per day for two days. Larvae (∼30 offspring per family) were allowed to develop at one egg per seed (excess eggs were scraped from the seed) and one seed per dish inside a temperature and photoperiod controlled growth chamber at 26°C (±1°C), light:dark 15:9. Dishes were checked twice per day for adult beetles that emerged from a seed.
Egg-to-emergence development time and egg and larval survival were scored for all offspring. All of the eggs were classified to one of four fates; those that failed to develop, developed but did not hatch (a developing larva/embryo was visible inside the clear egg), hatched but did not emerge as an adult, or emerged as an adult.
In total we collected 9113 eggs that yielded 8023 adult beetles in 74 blocks, each with two inbred and two outbred families of ∼30 offspring each (32 blocks for the BF population and 42 blocks for the SI population).
Stator limbatus
Stator limbatus were handled the same as C. maculatus except that pairs were confined in a 35 mm Petri dish with 10 seeds of silk tree, Albizia julibrissin. Because fecundity of S. limbatus females is much lower than fecundity of C. maculatus family sizes are necessarily smaller. We thus created a larger number of blocks. In total we collected 4974 eggs from which we raised 3195 offspring to adult from 167 blocks.
Analyses
Blocks are the lowest level of independence in this design and thus block means were used in all analyses. All block means were calculated first by averaging across offspring within a family and then by averaging across families within the block and treatment. For mortality data, each block contains two means, one for each treatment. For development time, each block contains four means, one for each sex×treatment combination (inbred male offspring, outbred males, inbred females and outbred females).
Development time data fit assumptions of standard general linear models and are thus analysed using analysis of variance on block means. Mortality data are proportions and do not meet the assumptions of analysis of variance. We thus used a paired non-parametric analysis to test for differences in mortality between inbred and outbred beetles within each population. To test for differences between populations of C. maculatus in inbreeding depression we calculated a measure of the proportional reduction in survival due to inbreeding depression, δ=(Meanoutbred− Meaninbred)/Meanoutbred, calculated separately for each family, then used a Mann-Whitney U-test to test for differences in δ between populations.
Results
Callosobruchus maculatus
Both populations of C. maculatus suffered substantial inbreeding depression at all stages of development. Eggs laid by sib-mated pairs were less likely to develop (χ21>23, P<0.001 for both populations) and less likely to hatch (given that they developed; χ21>19, P<0.001 for both populations) compared to eggs laid by outbred pairs (fig. 2). Inbred larvae were less likely to survive to emergence (given that an egg hatched; fig. 2; χ21>24, P<0.001 for both populations). Combining all sources of mortality, 98% (BF) and 96% (SI) of eggs produced by outbred pairs produced an adult offspring, whereas only 82% (BF) and 76% (SI) of eggs produced by sib-mated pairs produced an adult offspring (fig. 2). There was no evidence that inbreeding depression differed between the populations for any of the periods of mortality.

Fig. 2. The effect of inbreeding on the proportion of eggs that develop, egg hatch, hatch-to-emergence survival, and the proportion of all eggs that gave rise to an emerged adult offspring for two populations of Callosobruchus maculatus (from Burkina Faso (a) and South India (b)) and one population of Stator limbatus (c). Means are calculated first by averaging across families in a block, then across blocks (●, outbred; ○, inbred).
In both the BF and SI populations, inbred beetles took more than one day (∼5%) longer to develop from egg to emergence than did outbred beetles (fig. 3, table 1; F 1, 73=118, P<0.001). Though the two populations differed in development time (F 1, 73=242, P<0.001), there was no significant difference between the populations in the degree of inbreeding depression (population×treatment interaction; F 1, 73=1.4, P=0.23). Likewise, there was no significant difference between males and females in the effect of inbreeding on development time (sex×treatment interaction; F 1, 73=0.2, P=0.67).

Fig. 3. The effect of inbreeding (sib-mating) on egg-to-emergence development time for two populations of Callosobruchus maculatus (from Burkina Faso (a) and South India (b)) and one population of Stator limbatus (c). Means are calculated first by averaging both families in a block, then across blocks. Note that the large standard errors for S. limbatus reflects substantial variation in development time among blocks (●, outbred; ○, inbred).
Table 1. The magnitude of inbreeding depression in Callosobruchus maculatus and Stator limbatus.
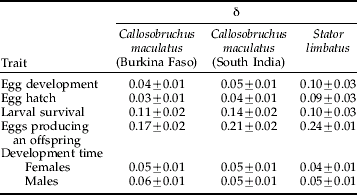
δ=(Meanoutbred−Meaninbred)/Meanoutbred (the proportional decrease in fitness of inbreds relative to outbreds) except for development time for which δ=(Meaninbred−Meanoutbred)/Meanoutbred (the proportional increase in development time of inbreds relative to outbreds). δ is calculated separately for each block then averaged across blocks. δ has an expectation of 0 when there is no inbreeding depression, and can vary from positive to negative depending on whether inbreds have lower or higher performance than outbreds, respectively.
Stator limbatus
Stator limbatus suffered significant inbreeding depression at all stages of the life cycle, as observed in C. maculatus. Eggs from sib-mated pairs were less likely to develop (χ21=45, P<0.001), less likely to hatch (χ21=120, P<0.001), and larvae from these eggs were less likely to survive to emergence (χ21=97, P<0.001; fig. 2). Overall, inbreeding resulted in a substantial reduction in the proportion of eggs that produced an adult offspring from 84% to only 58% (χ21=114, P<0.001; fig. 2).
Development time was more than one day (∼4%) longer in inbred than in outbred beetles (fig. 3, table 1; F 1, 166=82.3, P<0.001). Development time was marginally longer in males than in females (marginally non-significant sex effect, F 1, 166=3.4, P=0.06) but there was no difference between the sexes in the effect of inbreeding on development time (no sex×treatment interaction; F 1, 166=0.07, P<0.78).
Discussion
Rearing animals in captivity can lead to rapid evolution – animals frequently adapt to their rearing conditions (Gilligan & Frankham, Reference Gilligan and Frankham2003; Frankham, Reference Frankham2005) and both small population size (including population bottlenecks) and inbreeding are often more common in captivity than in nature. This can lead to the erosion of genetic variation (Gilligan et al., Reference Gilligan, Briscoe and Frankham2005) and a general deterioration in fitness due to inbreeding depression (Woodworth et al., Reference Woodworth, Montgomery, Briscoe and Frankham2002). This has important implications for conservation biology and also for the use of long-term laboratory colonies for experiments in ecology, evolution and behaviour. Recent studies with C. maculatus have shown that populations do adapt quickly to their laboratory rearing conditions (Messina & Karren, Reference Messina and Karren2003; Messina, Reference Messina2004a, Reference Messinab). However, despite our C. maculatus populations being in captivity for >100 generations (BF) or >200 generations (SI) we detected substantial inbreeding depression in both populations at all stages of development. The observed inbreeding depression was similar in magnitude to the inbreeding depression observed in our S. limbatus population that had recently been brought into the laboratory from nature. This suggests that both C. maculatus populations harbour fairly high genetic loads; i.e. they contain a large number of deleterious recessive alleles. By extension, both populations appear to be highly genetically variable, consistent with previous studies showing that these populations contain substantial amounts of selectable genetic variation (Messina, Reference Messina1993; Kawecki, Reference Kawecki1995; Fox et al., Reference Fox, Bush, Roff and Wallin2004a) and can respond quickly to natural selection (Messina & Karren, Reference Messina and Karren2003; Messina, Reference Messina2004a, Reference Messinab).
The inbreeding depression observed here for C. maculatus is of similar magnitude to that observed in another study using a different population of this same beetle (Tran & Credland, Reference Tran and Credland1995). They found greater inbreeding depression on C. maculatus egg hatch (δ=0.14 average across lines) than was found in the present study (δ∼0.04) but inbreeding depression on total larval survival (the number of eggs producing an adult) was slightly lower in Tran & Credland (δ=0.13 averaged across replicates) than in the current study (0.17–0.21). Intriguingly, Tran & Credland (Reference Tran and Credland1995) found a gender-difference in inbreeding depression on adult body size – inbred females were smaller than outbred females, but there was no difference in body size between inbred and outbred males. In our experiment we found no evidence of gender differences in inbreeding depression for development time for either species but, unfortunately, we did not measure adult body size. However, in a companion study to the one reported here (Fox et al., Reference Fox, Scheibly, Wallin, Hitchcock, Stillwell and Smith2006) substantial inbreeding depression was found on adult lifespan of females, but not adult lifespan of males, in both the SI and BF populations (δ=0.11 to 0.13 for the SI and BF females, respectively; δ=−0.01 to 0.01 for males). Gender differences in inbreeding depression have been reported rarely in the literature (Saccheri et al., Reference Saccheri, Lloyd, Helyar and Brakefield2005) but imply gender differences in the genetic architecture underlying body size and adult lifespan of C. maculatus.
We found similar effects of inbreeding between S. limbatus and C. maculatus on both the proportion of eggs producing an offspring and development time (see table 1). However, the magnitude of inbreeding depression measured here for S. limbatus was generally lower than the magnitude of inbreeding depression observed for S. limbatus in a previous study (Fox & Scheibly, Reference Fox and Scheibly2006) in which the magnitude of inbreeding depression was compared among three populations, including this same Oracle population of S. limbatus, after just two generations in the laboratory. The magnitude of inbreeding depression on development time in that study (δ=0.05–0.06) was similar to that observed here (0.04–0.05) (Fox & Scheibly, Reference Fox and Scheibly2006). However, the previous study detected much greater inbreeding depression on both egg hatch and larval survival for this same S. limbatus population, resulting in an overall proportional reduction in offspring production, δ, of 0.41±0.03 in that study vs. 0.24±0.01 in the present study. This difference may simply reflect environmental differences between the two laboratory studies – different seeds were used for the two experiments and rearing environment is well documented to affect inbreeding depression (Kristensen et al., Reference Kristensen, Dahlgaard and Loeschcke2003; Armbruster & Reed, Reference Armbruster and Reed2005). Alternatively, the difference may reflect rapid purging of deleterious alleles in the first few generations of laboratory rearing. In a companion study to this, we (C.W. Fox & K.L. Scheibly) are measuring the rate at which deleterious alleles are purged from inbred populations of S. limbatus. We expect inbreeding to lead to lead to rapid evolution of population mean fitness and a dramatic reduction in inbreeding depression as deleterious recessive alleles are purged from the population.
Acknowledgements
The authors thank Frank Messina for providing the SI and BF colonies used in this experiment, A. Amarillo-Suárez and R.C. Stillwell for help collecting S. limbatus, and M.E. Czesak, F.J. Messina, and R.C. Stillwell for comments on previous versions of the manuscript. This work was funded in part by NSF DEB-0271929 to CWF. This paper is publication 06-08-090 of the Kentucky Agricultural Experiment Station.


