Published online by Cambridge University Press: 29 April 2005
The effect of Sanguinicola inermis on serum antibody and complement activity in Cyprinus carpio was assessed using an ELISA and haemolytic assays. Possible immune evasion strategies were assessed using immunodetection of host proteins on the surface of the parasite. Carp acclimatized to 20 or 25 °C were infected by exposure to 500 cercariae or injected intraperitoneally with 150 cercariae, and serum monitored over a 63-day period. In cercariae-injected carp, irrespective of time and temperature, a significant increase occurred in complement activity being greatest at 25 °C. In addition, fish exposed to the cercariae of S. inermis and maintained at 20 °C the level of complement activity was significantly higher after 5 weeks compared to controls. At 20 °C intraperitoneal injections of parasites increased serum antibody levels which peaked after 7 days. In contrast, at 25 °C, antibody levels were maintained over 63 days. Exposure of fish to infection did not appear to stimulate antibody production. Immunofluorescence studies revealed ‘host-like’ molecules on the surface of the cercarial body exposed to carp serum and adult flukes obtained directly from the fish or cultured for 24 h in L15 medium. The possible role of ‘host-like’ molecules in immune evasion is discussed and the response at different temperatures is related to infection dynamics.
Previous studies on the immunological interactions between Cyprinus carpio and the blood fluke Sanguinicola inermis have revealed that infection induces an intense cellular reaction. This is manifested in a severe pathology caused by cercariae penetrating the skin, migrations of post-cercarial juvenile adult stages and eggs that become entrapped in host tissue (Lee, 1990). This response involves eosinophils, macrophages and neutrophils which encapsulate and destroy parasite eggs in granulomatous lesions (Richards et al. 1994a). In addition, infection is also associated with an alteration in the cellular composition of the immune organs in carp (Richards et al. 1994b). In vitro studies (Richards et al. 1996a,b,c) have also revealed that adult and cercarial stages of S. inermis induce proliferation of carp lymphocytes and migration of carp leucocytes, although leucocyte attachment to the parasite stages is minimal. Previous studies have also noted that the level and nature of the immune response of carp is determined by a range of parameters including the duration of infection (Richards et al. 1994a,b) and environmental conditions such as temperature (Richards et al. 1996a) and pollution (Schuwerack et al. 2001, 2003; Hoole et al. 2003). The role of humoral factors such as complement and antibody in the immune response of carp to this parasite has not been elucidated.
The complement cascade fulfils multiple roles within the immune system of teleosts including non-specific and antibody-specific virucidal, bactericidal and parasiticidal activity (Yano, 1992). Although there have been several investigations on the association between complement and viral and bacterial pathogens in fish, there has, in contrast, been a limited number of studies on the parasiticidal activity of teleost complement. The majority of investigations have been carried out on protozoan parasites. For example, the genus Cryptobia were killed in vivo and in vitro by activation of the alternative pathway of the complement system of refractory fish species (Bower & Woo, 1977; Wehnert & Woo, 1980), whilst the classical pathway of complement activation, in association with specific antibodies, has been implicated in the killing of Cryptobia salmositica in rainbow trout (Jones & Woo, 1987) and sockeye salmon (Oncorhynchus nerka) (Bower & Evelyn, 1988). Recent studies by Buchmann, Lindenstrom & Sigh (1999) have also suggested that non-specific factors including complement could play an important role in the host response against the pathogenic ciliate Ichthyophthirius multifiliis and that this response may be affected by the presence of other parasite species e.g. the monogenean Gyrodactylus derjavini. In contrast, there have been a limited number of investigations of the interaction between the fish complement system and metazoan parasites. Studies by Whyte, Chappell & Secombes (1989, 1990) revealed that the immune protection mechanism in rainbow trout against the eye-fluke Diplostomum spathaceum involved both the alternative and classical pathways of complement activation. In studies by Buchmann (1998) it was also noted that the lethal effect of plasma from Oncorhynchus mykiss on the monogenean Gyrodactylus derjavini was mediated through the binding of complement factor C3 to carbohydrate-rich parasite structures. In addition to the lytic properties of complement, biologically active peptides, which play important roles in the inflammatory processes, are produced during the activation of the complement cascade. Several authors have speculated that complement may be involved in the inflammatory response of cyprinids against metazoan parasites perhaps associated with leucocyte chemoattraction and opsonization (e.g. Hoole & Arme, 1986; Taylor & Hoole, 1993; Richards et al. 1996b,c).
There have been several studies that have also highlighted the involvement of antibodies in the host/parasite relationship in fish (see Hoole, 1997). Antibodies have been implicated in the immune response to a range of parasitic infection such as protozoa (e.g. Cobb, Levy & Noga, 1998; Ardelli & Woo, 2002; Xu & Klesius, 2003), flukes (e.g. Bortz et al. 1984; Aaltonen, Valtonen & Jokinen, 1997), nematodes (e.g. Coscia & Oreste, 1998, 2000) and tapeworms (e.g. Kennedy & Walker, 1969; Sharp, Pike & Secombes, 1989). There has also been intensive speculation on how humoral factors in the immune response of fish are involved in protection. Such mechanisms as effects on parasite fecundity (Grayson et al. 1995), binding to glandular secretions and surface structures (Sharp et al. 1989; Williams & Hoole, 1995), mediation of leucocyte adherence (Hoole & Arme, 1986; Whyte et al. 1990), induction of parasite migration out of the fish (Clark & Dickerson, 1997) and mediation of complement-induced lysis (Saeij, De Vries & Wiegertjes, 2003) have been proposed. In contrast, the mechanisms by which fish helminth parasites evade and/or suppress the immune response has not been extensively considered although Hoole & Arme (1983) have speculated that host proteins may be acquired on the surface of the plerocercoid of Ligula intestinalis and used to evade the immune response in its cyprinid hosts.
Despite an intense cellular reaction in C. carpio to primary infections, of S. inermis, fully mature flukes do occur in the heart and associated efferent vessels and commence egg production approximately 30 days post-infection at 20 °C (Kirk & Lewis, 1993). In contrast, recent investigations carried out by Roberts and reported by Hoole et al. (2003) have revealed that there is a significant decrease in flukes recovered in challenge infections compared with primary infections. In the UK, infection of fish with S. inermis is seasonal (Lee, 1990) with temperature appearing to play an important role in the development of the parasite in the intermediate and definitive hosts as well as affecting transmission periods and the host/parasite interactions. In this paper the association between Sanguinicola inermis and the complement and antibody component of the immune response in Cyprinus carpio has thus been investigated at different temperatures with the aim of elucidating the role of the humoral component of the immune response of carp to this parasite and the mechanisms by which it might evade these immune responses.
Sanguinicola inermis-free carp, 5–10 cm in length, obtained from Tern Fisheries, Market Drayton, UK were acclimatized to either 20 °C or 25 °C for 1 month prior to use. Fish were held in dechlorinated tap water in a 12 h light[ratio ]12 h dark lighting regime and fed on a commercial carp diet (Mazuri Zoo Foods, UK).
Lymnaea peregra collected from the margins of a lake in the West Midlands, UK during May and September were maintained using the protocol of Kirk & Lewis (1992) in aerated filtered pond water at 20 °C. Snails were fed Bemax snail diet and washed lettuce leaves every 3 days and screened for cercarial emergence which took place between 16.00 and 22.00 h. Cercariae were collected and used to infect or inject carp experimentally within 1 h of being released from the intermediate host (Richards et al. 1994a).
Groups of 7–8 carp acclimatized to 20 °C or 25 °C were either exposed to or injected with cercariae of S. inermis. Infection was only carried out at 20 °C by exposing individual fish to 500 cercariae in 400 ml of water at 20 °C for 1 h as described by Kirk & Lewis (1992). Injection protocols on fish maintained at 20 °C and 25 °C comprised a single intra-peritoneal injection of 150 live cercariae in 100 μl of sterile phosphate buffered saline (PBS). Controls consisted of sham-infected fish, which were maintained in identical conditions without exposure to the parasite, or carp injected with PBS alone. Carp were maintained at their acclimatization temperature and blood was collected by caudal puncture from fish on day 7 post-injection (p.ij.) or post-exposure (p.i.), and then at weekly intervals up to 49 or 63 days p.i./p.ij. for determination of serum levels of complement or antibody respectively. Following overnight storage at 4 °C and centrifugation at 1000 g for 10 min serum was collected and stored at −80 °C.
Carp anti-sheep heat-inactivated erythrocyte serum (hiCaSE) was produced using standard protocols. Briefly, 500 μl of washed sheep erythrocytes suspended in PBS (2×108 cells/ml) were injected intraperitoneally into each of 3 carp (200–300 g) held at 25 °C. A booster injection was given after 32 days and 15 days post-booster the carp were bled by caudal puncture. Pooled serum samples were heat-inactivated (20 min at 60 °C) and stored at −20 °C.
The protocol used for the production of sensitized sheep erythrocytes (SE) was adapted from that described by Yano (1992). Briefly, washed sheep erythrocytes (1×109 cells/ml in Hanks Balanced Salt Solution, HBSS) exposed to a range of dilutions of hiCaSE (1[ratio ]50 to 1[ratio ]1600) and a standard concentration of carp serum (1[ratio ]75), were incubated with mixing at 25 °C for 30 min. The cells were then washed twice in HBSS by centrifugation at 500 g. A concentration of 1[ratio ]200 of hiCaSE was adopted which produced the greatest sensitisation of the erythrocytes as indicted by the highest level of haemolysis. Suspensions of sensitized sheep erythrocytes were adjusted to 5×108 cells/ml, stored at 4 °C and used within 1 day of production.
Experimental serum was diluted to 260 μl with HBSS (1[ratio ]98) and placed in a well of a 96-well microplate (ICN Biomedicals). Then 40 μl of sensitized sheep erythrocyte suspension was added to each well and the plate incubated at 25 °C for 1 h with continual shaking. Prior to incubation all reagents were held on ice to retard the action of complement. Following incubation, the microtitre plates were centrifuged at 350 g for 3 min and 200 μl of the cell-free supernatant transferred to another microplate and analysed spectrophotometrically at 540 nm. The percentage of haemolysis induced by the serum was determined by comparison with the optical density reading obtained with 100% haemolysis induced by distilled water
An ELISA was developed to investigate the presence and levels of anti-S. inermis antibodies in the serum of treated and control carp held at 20 °C and 25 °C. S. inermis cercarial homogenate was prepared from live cercariae collected within 6 h of shedding from Lymnaea peregra. Cercariae were washed 3 times in distilled water by centrifugation at 1800 g for 5 min, re-suspended in 0·5 ml of distilled water and disrupted by sonication. Protein determination was carried out using a Bio-Rad protein assay kit using human gamma globulin as a standard. Wells of a 96-well microtitre plate (ICN Biomedicals) were coated overnight at 4 °C with cercarial antigens using 100 μl of cercarial homogenate at 5 μg/ml. Vacant protein binding sites were blocked using 200 μl of 1% dried milk powder. Unfortunately due the limited amount of parasite antigen available it was necessary to pool serum from the fish at each time-point. Then 100 μl of this serum at a dilution range of 1[ratio ]4 to 1[ratio ]2560 in PBST (PBS containing 0·1% Tween 20) was added to the antigen-coated wells and incubated at 30 °C for 1 h. Bound carp IgM was detected using a monoclonal antibody raised against the heavy chain of carp IgM (WCI12, Secombes, van Groningen & Egberts, 1983; Koumans-van Diepen et al. 1995) and an anti-mouse Ig peroxidase-labelled secondary antibody (AMIg/P, Sigma). Following preliminary experiments to optimize the above, 100 μl of WCI12 monoclonal at a 1[ratio ]300 dilution with PBST was added to each well after the excess carp serum had been removed and the plate washed 3 times with PBST. After a further wash, 100 μl of AMIg/P was added to each well, incubated for 1 h at 30 °C and, after washing, 100 μl of OPD substrate was added and incubated for 15 min at 30 °C. The reaction was stopped by the addition of 50 μl of 2N sulphuric acid per well and the absorbance read at 490 nm in an Anthos Labtec plate reader. Controls were provided by substitution of certain steps i.e. cercarial antigen, dried milk powder blocking, carp serum, WCI12 monoclonal antibody and/or AMIg/P with PBS.
Live flukes, recovered from the heart and associated efferent vessels of carp infected 35 days previously, were divided into 2 groups. One group was washed 3 times in PBS and fixed by the addition of 4% paraformaldehyde in phosphate buffered saline (PBS) for 1 h at room temperature (approximately 20 °C). The second group was incubated at 20 °C in Leibovitz (L-15) culture medium (Sigma) for 24 h (Richards et al. 1996c) prior to being washed and fixed as above. After washing, the vacant protein binding sites were blocked overnight at 4 °C with 3% Bovine Serum Albumin (BSA) in PBS. Flukes were then incubated with polyclonal sheep anti-carp IgM (ACIgM) at a dilution of 1[ratio ]100 (PBS, 1% BSA) and washed again before incubation with anti-sheep IgG/fluorescein isothiocyanate (FITC) conjugate (Sigma) at a dilution of 1[ratio ]40 (PBS, 1% BSA) in the dark. Controls were provided by omission of the polyclonal antibody or labelled secondary antibody.
In addition, live flukes, fixed, washed and blocked as above were incubated with the more specific monoclonal antibody WCI12 which interacts with heavy chain of carp immunoglobulin (Secombes et al. 1983; Koumans-van Diepen et al. 1995) at a dilution of 1[ratio ]100 (PBS, 1% BSA). Flukes were then washed and treated with anti-mouse IgG antibodies directly conjugated to FITC (AMIg/FITC) at a dilution of 1[ratio ]40 (PBS, 1% BSA). Controls in which either the monoclonal antibody WCI12 or AMIg/FITC were omitted were also included. All parasites were washed, mounted in Citifluor (Citifluor Ltd, City University, London) and viewed under an ultraviolet microscope. Washes were performed 3 times and incubations were conducted at 30 °C for 1 h, unless stated otherwise.
Live cercariae, concentrated to an approximate density of 1000 per ml by centrifugation (1800 g, 1 min) were fixed by the addition of 500 μl of 4% paraformaldehyde in PBS for 1 h at room temperature. Cercariae were allowed to precipitate on ice for 15 min, washed and then incubated in 3% BSA in PBS. Pooled serum, which had been obtained by caudal puncture from carp that had been exposed to 500 cercariae of S. inermis 8 days previously was added at a dilution of 1[ratio ]100 (PBS plus 1% BSA, 0·1% Triton X-100) and the cercariae further incubated and then processed as for adult flukes using ACIgM. Controls consisted of serum from uninfected fish and the omission of the polyclonal antibody.
Data obtained were tested for normality and subjected to parametric analysis using a two-way ANOVA (General Linear Model, Minitab version 11.2). Interactions between pairs of means were further analysed using the Tukey multivariate range test. In the complement assay since the percentage haemolysis obtained was low, CH50 could not be determined and an arcsine transformation was carried out on the data prior to statistical analysis to equalize variances.
Analysis of the combined data for each treatment group (Figs 1 and 2), irrespective of the time-point, revealed that significant differences occurred between sham-injected and cercariae-injected fish held at 20 °C (P<0·001) and 25 °C (P<0·05). In addition, when fish held at 20 °C were compared with those held at 25 °C there were also significant differences between the two sham- injected groups (P<0·05) and the two groups injected with cercariae (P<0·05). However, further analysis revealed that there was no significant difference at individual time-points examined in fish injected with cercariae or indeed, when compared with the respective time-matched sham-injected controls.

Fig. 1. The kinetics of complement activity in the serum of carp injected with 150 live cercariae of Sangiunicola inermis ([lozf ]) or PBS sham-injected controls (□) and maintained at 20 °C (n=7, mean±S.E.).

Fig. 2. The kinetics of complement activity in the serum of carp injected with 150 live cercariae ([lozf ]) or PBS sham-injected controls (□) and maintained at 25 °C (n=7, mean±S.E.).
Fish that were either uninfected or infected by exposure to 500 cercariae and maintained at 20 °C displayed a slightly different pattern of complement activity over time than fish that had been injected with cercariae (Fig. 3). The level of complement in fish exposed to the parasite gradually increased to a peak at 5 weeks p.i. and then decreased to control levels by week 7. Despite there being no overall significant difference between control and fish exposed to S. inermis, results for infected fish at week 5 were significantly higher (P<0·05) than activity levels for the control group at weeks 1 and 5 and the infected group at week 2. Similarly, no significant differences were detected between fish infected by exposure to cercariae and those injected with cercariae.

Fig. 3. The kinetics of complement activity in the serum of control (□) and infected carp, exposed to 500 cercariae ([lozf ]) maintained at 20 °C (n=8, mean±S.E.). Points sharing the same letters are significantly different (P<0·05).
Serum collected from carp maintained at 20 °C that had been injected intra-peritoneally with 150 live S. inermis had increased antibody levels compared to PBS injected control fish (Fig. 4A). These antibody levels were highest 7 days p.ij. (i.e. approximately 1·9× control levels) and gradually declined to control levels at 56 days p.ij. Further analysis of the antibody levels over the range of dilutions of carp serum assayed revealed that over the time period, 7–14 days p.ij., higher levels of parasite-specific antibodies occurred in the serum from parasite injected fish than the respective sham-injected controls (i.e. 1·9× greater at 7 days; 1·5× greater at 14 days than their respective controls). In contrast, in carp maintained at 25 °C although there was an increase in antibody levels at 7 days p.ij. (i.e. 1·9× greater than control) this was maintained over the 63 days of the experiment (Fig. 4B). When considering the percentage increase compared to controls over the initial 7–14 day period the antibody response was greater in fish maintained at 25 °C (i.e. 7 days 73·2%, 14 days 40·7%) compared to those kept at 20 °C (i.e. 7 days 27·7%, 14 days 14·7%). Analysis of serum obtained from fish maintained at 25 °C and exposed to 500 cercariae revealed the absence of any detectable antibodies against cercarial antigens over a 63 day infection period (Fig. 4C).

Fig. 4. Primary antibody response of carp exposed to cercariae of Sanguinicola inermis. Fish injected with live cercariae (I) or sham injected controls (S) and maintained at 20 °C (A) and 25 °C (B). (C) Fish maintained at 20 °C and exposed to 500 cercariae (I) compared to untreated control fish (Control). Controls from 7-day and 14-day post-injection fish were combined (7.14).
Molecules recognized by the polyclonal ACIgM antibody were located on the tegumental surface of flukes either fixed immediately after removal from their host or cultured in L-15 medium for 24 h prior to processing (Fig. 5A and B). Fluorescence was distributed evenly over the surface of flukes and was particularly associated with numerous lobular projections on the tegument. All control treatments proved negative for specific immunofluorescence.

Fig. 5. Immunofluorescent labelling of molecules recognized by the polyclonal ACIgM on the surface of adult stage of Sanguinicola inermis. (A) Fluke fixed immediately after removal from Cyprinus carpio. (B) Fluke fixed after 24 h culture in L-15 medium. Note fluorescence localized at periphery of fluke in both instances (arrows).
Immunofluorescence was, however, not observed associated with the tegumental surface of freshly recovered adult flukes 35 days post-infection exposed to the monoclonal anti-carp IgM (WCI12) or in the control groups (Fig. 6A and B).

Fig. 6. Exposure of adult Sanguinicola inermis to monoclonal antibody WCI12 (A) with anti-mouse IgG/fluorescein isothiocyanate (FITC) conjugate, and (B) without anti-mouse IgG/fluorescein isothiocyanate (FITC). Note similar level of autofluorescence and absence of intense fluorescence at periphery of flukes.
Immunofluorescence, detected by using ACIgM, was observed on cercariae incubated with infected serum (Fig. 7) and was only associated with the cercarial body. Specific fluorescence was absent on the cercarial tail, the control treatments and on cercariae incubated with serum obtained from uninfected carp.

Fig. 7. Immunofluorescent labelling of molecules recognized by the polyclonal ACIgM in cercaria of Sanguinicola inermis incubated in serum obtained from Cyprinus carpio infected with 500 cercariae for 8 days. Note fluoresence localized on periphery (arrow) of cercarial body (c). Fluorescence absent from tail region.
The present results revealed that S. inermis, in addition to inducing a cellular reaction in C. carpio, is also associated with an humoral response which incorporates both non-specific i.e. complement and specific i.e. antibody components. Previous studies on complement activity of fish have revealed that the activation of complement components by infectious agents reduces the levels in the serum, a process termed consumption by Sakai (1992). For example, in Oncorhynchus mykiss the spontaneous activity of complement has been shown to decrease significantly within 6 days following experimental infections with virulent strains of Aeromonas salmonicida and Vibrio anguillarum (Sakai, 1983). Likewise, Thomas & Woo (1989) recorded a long-term reduction in complement activity of O. mykiss infected with Cryptobia salmositica. Although a reduction in complement activity has also been associated with protozoan and metazoan infections in mammals (Leid, 1988), the present studies have revealed that in Cyprinus carpio experimentally infected with S. inermis the levels of serum complement activity increased. Fish injected with live cercariae and maintained at either 20 °C or 25 °C for 7 weeks displayed significantly higher overall levels of haemolytic activity than sham-injected controls, suggesting that infection induced higher complement levels. Although complement activity was greatest in injected fish at 25 °C, an increase in activity compared to the respective sham control was greatest at 20 °C. This may indicate that the effect of the parasite on complement levels is more pronounced at this temperature. Previous studies by Richards et al. (1996a) have also indicted that at 20 °C cercariae of S. inermis are able to stimulate proliferation of pronephric lymphocytes of carp; however, this response is absent at 10 °C. The absence of any significant difference between experimental and control fish at any time-point studied here at either 20 °C or 25 °C is probably due to the high level of intraspecific variation observed. However, there was a trend for higher complement levels in fish injected with cercariae. These peaked after 3 weeks p.ij. and then fell to control values by week 7 for fish maintained at 20 °C and week 5 for those at 25 °C, although at the latter temperature a further increase occurred at 7 weeks p.ij. A similar enhanced complement activity was observed by Agu, Farrell & Soulsby (1981) in golden hamsters (Mesocricetus auratus) experimentally infected with Leishmania donovani over a 9-week period. Interestingly, carp experimentally infected by exposure to cercariae of S. inermis exhibit significantly higher numbers of splenic and pronephric macrophages, between 5 and 9 weeks p.i., than uninfected controls (Richards et al. 1994b). It is possible therefore that increased levels of complement activity induced in carp injected with S. inermis resulted from increases in macrophages in the spleen and pronephros. Recent extensive studies have been carried out by Nakao and co-workers (Kato et al. 2003; Nakao et al. 2003b,c; Nakao, Uemura & Yano, 2003) on the structure and action of different complement components in Cyprinus carpio and have also revealed that complement is associated with pronephric granulocytes and macrophages (Nakao et al. 2003a) and peripheral lymphocytes (Nakao et al. 2004).
An increase in the complement activity in the serum of fish injected with or exposed to cercariae of S.inermis compared to their respective sham controls could alternatively be due to a reduction in the consumption of complement components by S. inermis. This would be particularly pronounced if the production of these components is either unaffected or increased by exposure to the parasite. In mammalian/trematode systems, for example the blood fluke Schistosoma mansoni, cercarial and schistosomula stages are potent activators of the alternative pathway (Machado et al. 1975; Santoro et al. 1979). In contrast, mechanically transformed schistosomula lose their ability to activate and consume complement via the alternative pathway (Marikovsky et al. 1986). Similarly, adult schistosomes are largely refractory to complement activity (Fishelson, 1989). Whether a similar mechanism of activation/evasion occurs within the carp/S. inermis model is unknown. However, in experimental infections several adult worms survive and produce eggs within the heart and blood vessels of C. carpio (Kirk & Lewis, 1992).
Carp infected by exposure to cercariae of S. inermis and maintained at 20 °C showed a significant increase in complement levels in the infected group from week 2 to a peak in week 5 after which time activity decreased to control levels by week 7. A peak in activity levels at week 5 may be related to the life-cycle of the blood fluke, as the adult parasite, which commences egg production approximately 30 days p.i. at 20 °C (Kirk & Lewis, 1993). Work by Santoro et al. (1980) demonstrated a direct correlation between elevated complement levels in S. mansoni-infected patients and parasite egg numbers. Richards et al. (1994a) recorded neutrophils infiltrating areas around the eggs of S. inermis which had become trapped in the pronephros of infected carp. These authors suggested that infiltration by these cells was possibly due to parasite-derived chemotactic factors; however, they did not rule out the role of complement in this response. In addition to differences in complement activity between infected and control fish, significantly higher levels of complement were recorded in carp maintained at 25 °C compared to fish maintained at 20 °C. The present results therefore corroborate previous studies (e.g. Matsuyama et al. 1988; Carlson, Baker & Fuller, 1995) which suggest that, in fish, complement activity is directly correlated with temperature and raises interesting issues relating to the seasonal transmission and occurrence of S. inermis in UK waters. Previous studies have revealed that temperature may play an important role in the interactions between S. inermis and its hosts. Adults can over-winter in carp in the wild (Lee, 1990) and mature after 2–3 months in carp maintained at 15–18 °C (Sommerville & Iqbal, 1991) and in less than 1 month in fish kept at 20 °C. Studies by Lee (1990) also showed that in the UK infection of carp primarily took place in the late summer/ autumn when cercarial emergence was at its peak. Any cercarial emergence occurring in the spring was thought to have arisen from over-wintering infected snails.
The effect of temperature on the immune response of carp to S. inermis is also revealed when considering antibody levels associated with the injection of parasites. Antibody levels were greater and were maintained at higher levels for a longer period in carp kept at 25 °C compared to fish maintained at 20 °C. Within the physiological range of a particular teleost species it is widely accepted that higher environmental temperatures enhance the magnitude and timing of antibody production (e.g. Rijkers, Frederix-Wolters & van Muiswinkel, 1980; Secombes et al. 1991). It is of interest that previous studies by Richards et al. (1996a) have also revealed that carp lymphocytes cultured with S. inermis extracts were also temperature sensitive i.e. proliferation of pronephric lymphocytes stimulated with sonicated S. inermis cercariae and adult flukes was greater at 20 °C compared to 10 °C. It would thus appear that environmental temperature may be an important parameter in the interaction between S. inermis and its carp host. In contrast to the above, there was little evidence of antibodies being produced in carp infected with cercariae of S. inermis. The dichotomy of the response to injected vs. exposure routes of infection has important implications regarding the route and presentation of parasite antigens, a phenomenon that also been observed by Hoglund & Thuvander (1990) who noted that whilst O. mykiss infected with live cercariae of Diplostomum spathaceum did not produce an antibody response, fish injected with frozen cercariae, diplostomulae or metacercariae, displayed significantly higher levels of specific antibody than control fish. In addition, Nie & Hoole (1999) also noted that whilst carp injected with extracts of Bothriocephalus acheilognathi produced a significant antibody response, antibody levels in naturally infected fish were comparable to non-infected controls. The inability to detect antibodies in carp experimentally infected with S. inermis may indicate that they are rapidly bound to the surface of the parasite or alternatively the antibody levels are reduced as part of an immune evasion/suppression strategy employed by the parasite.
The use of the indirect immunofluorescence technique has revealed that molecules recognized by the polyclonal antibody (ACIgM) are associated with the surface of the adult S. inermis both immediately after removal of the parasites from the host and after 24 h in L-15 culture medium. Previous unpublished Western blot analysis has demonstrated that the polyclonal antibody used during this work binds to 8 protein bands in carp serum, including the heavy chain of IgM. The positive reaction of the cercarial body and its absence from the tail of S. inermis when exposed to infected carp serum and the polyclonal antibody suggests that the cercarial body and the subsequent adult fluke may share similar surface molecules. However, the apparent absence of IgM on the surface of the flukes, as indicated by the absence of surface fluorescence on flukes exposed to the monoclonal antibody (WCI12) which recognizes the heavy chain of carp IgM (WCI12, Secombes et al. 1983; Koumans-van Diepen et al. 1995), possibly suggests that the surface molecules may not be antigenic but may have a protective role.
Various parasites, for example Schistosoma mansoni (Sher, Hall & Vadas, 1978), Brugia pahangi (Premaratne, Parkhouse & Denham, 1989) and Wuchereria bancrofti (Kar et al. 1993) have the ability to acquire or mimic host molecules that are incorporated into their tegument. These host molecules are thought to disguise the parasite against immune attack. Immune evasion strategies are not exclusively employed by mammalian parasites and while research is very limited, such avoidance techniques have been proposed for parasites infecting fish. In a fish/cestode model Hoole & Arme (1983) proposed that the plerocercoid of Ligula intestinalis acquired proteins from its host cyprinid and later Williams & Hoole (1995) detected roach (Rutilus rutilus) molecules, presumed to be antibodies, on the tegumental surface of this cestode. It is unknown whether the presence of ‘host-like’ molecules on the surface of S. inermis is utilised in any immune evasion strategies. However, the presence of these molecules on the surface of the flukes that have been cultured for 24 h does suggest they may also be parasite derived. Sanguinicola inermis must adopt some mechanism(s) of evading the immune response as flukes survive for up to 42 days in the host's circulatory system (Lee, 1990). However, this protection appears to be limited since the present results indicate it is not as effective against challenge infections, particularly at 8 months p.i. (Hoole et al. 2003).
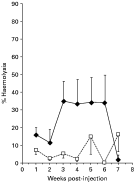
Fig. 1. The kinetics of complement activity in the serum of carp injected with 150 live cercariae of Sangiunicola inermis ([lozf ]) or PBS sham-injected controls (□) and maintained at 20 °C (n=7, mean±S.E.).
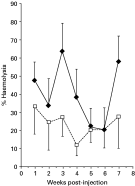
Fig. 2. The kinetics of complement activity in the serum of carp injected with 150 live cercariae ([lozf ]) or PBS sham-injected controls (□) and maintained at 25 °C (n=7, mean±S.E.).
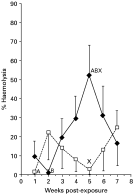
Fig. 3. The kinetics of complement activity in the serum of control (□) and infected carp, exposed to 500 cercariae ([lozf ]) maintained at 20 °C (n=8, mean±S.E.). Points sharing the same letters are significantly different (P<0·05).

Fig. 4. Primary antibody response of carp exposed to cercariae of Sanguinicola inermis. Fish injected with live cercariae (I) or sham injected controls (S) and maintained at 20 °C (A) and 25 °C (B). (C) Fish maintained at 20 °C and exposed to 500 cercariae (I) compared to untreated control fish (Control). Controls from 7-day and 14-day post-injection fish were combined (7.14).

Fig. 5. Immunofluorescent labelling of molecules recognized by the polyclonal ACIgM on the surface of adult stage of Sanguinicola inermis. (A) Fluke fixed immediately after removal from Cyprinus carpio. (B) Fluke fixed after 24 h culture in L-15 medium. Note fluorescence localized at periphery of fluke in both instances (arrows).

Fig. 6. Exposure of adult Sanguinicola inermis to monoclonal antibody WCI12 (A) with anti-mouse IgG/fluorescein isothiocyanate (FITC) conjugate, and (B) without anti-mouse IgG/fluorescein isothiocyanate (FITC). Note similar level of autofluorescence and absence of intense fluorescence at periphery of flukes.

Fig. 7. Immunofluorescent labelling of molecules recognized by the polyclonal ACIgM in cercaria of Sanguinicola inermis incubated in serum obtained from Cyprinus carpio infected with 500 cercariae for 8 days. Note fluoresence localized on periphery (arrow) of cercarial body (c). Fluorescence absent from tail region.