Introduction
Cartilage is a resilient load-bearing tissue and provides a smooth surface with low friction that can withstand and distribute high tensile stresses (Ref. Reference Hendriks, Riesle and van Blitterswijk1). Articular cartilage has the capability to increase joint congruence, protect the subchondral bone from high stresses and reduce friction at the edge of long bones (Refs Reference Mano and Reis2, Reference Swieszkowski3). Cartilage defects have become increasingly ubiquitous in clinical medicine, and they are mainly caused by trauma, tumour removal, osteoarthritis (OA) and rheumatoid arthritis (Refs Reference Reddi, Becerra and Andrades4, Reference Bovee5, Reference Cully6, Reference Block7, Reference Firestein8). For instance, patients with OA suffer from worsening pain, tenderness and mobility because of the progressive degeneration of the joint. OA can eventually lead to full-thickness focal chondral defects and represents a huge socioeconomic burden to the society (Refs Reference Benders9, Reference Chung and Burdick10). However, cartilage tissues have very limited regenerative ability because of the lack of vascular networks, low cellularity and proliferation rate of chondrocytes, as well as the formation of protease inhibitors and growth inhibitor (Refs Reference Ouzzine, Venkatesan and Fournel-Gigleux11, Reference Ateshian12, Reference Temenoff and Mikos13). Natural repair of full-thickness cartilage defects results in fibrocartilage formation with function and mechanical force inferior to the original hyaline cartilage, and further deterioration can occur (Refs Reference Benders9, Reference Chung and Burdick10, Reference Ochi14). Although many clinical treatments have been developed for cartilage repair, there are still lack of effective treatments and the long-term prognosis is not good (Refs Reference Benders9, Reference Panseri15, Reference Gomoll16, Reference Handorf and Li17).
Tissue engineering has emerged as an interdisciplinary field merged by life sciences, physical sciences and engineering, and holds great promise in repairing various tissues or organs (Refs Reference He, Xiao and Jiang18, Reference Lutolf and Hubbell19, Reference Place, Evans and Stevens20, Reference Hosseinkhani, Hosseinkhani and Kobayashi21). It is well known that tissue engineering has the ability not only to rebuild the structure of damaged tissues, but also to recover their biological functions (Refs Reference Freed22, Reference Dvir23, Reference Tuan24). Enormous studies have demonstrated the capability of tissue engineering in repairing damaged tissues or organs (e.g. cartilage (Refs Reference Benders9, Reference Ebihara25), bone (Refs Reference Benders9, Reference Kokemueller26, Reference Hosseinkhani27), nerve (Refs Reference He, Xiao and Jiang18, Reference Kehoe, Zhang and Boyd28), heart (Refs Reference Segers and Lee29, Reference Dolgin30) and bladder (Refs Reference Drewa, Adamowicz and Sharma31, Reference Kropp and Zwischenberger32)). Material scaffold is considered to be one of the important and key elements of cartilage tissue engineering (Ref. Reference Benders9). Peptide nanofibre scaffolds are regarded as an ideal candidate material for enhancing cartilage regeneration (Refs Reference Kopesky33, Reference Kisiday34, Reference Shah35). Their fabrication is based on the principle of molecular self-assembly, which relies on chemical complementarity and structural compatibility (Refs Reference He, Xiao and Jiang18, Reference Zhang36). Peptide self-assembly into supramolecular architectures are supported by numerous non-covalent intermolecular interactions (e.g. hydrogen bonds, ionic interactions, electrostatic interactions, hydrophobic interactions, van der Waals forces and water-mediated hydrogen bonds) (Refs Reference Zhang36, Reference Loo, Zhang and Hauser37). Although these intermolecular interactions are relatively weak in isolation, collectively they can exert strong molecular forces to initiate peptide self-assembly and improve the stability of supramolecular structures (Refs Reference Zhang36, Reference Zhao and Zhang38, Reference Hartgerink, Beniash and Stupp39, Reference Hosseinkhani, Hong and Yu40).
There have been many peptides used to construct three-dimensional (3D) biomaterial scaffolds and they are grossly divided into three categories (i.e. β-sheet peptides, α-helical peptides and collagen-mimetic peptides) (Refs Reference He, Xiao and Jiang18, Reference Hosseinkhani, Hong and Yu40, Reference Cui, Webber and Stupp41, Reference Matson and Stupp42). It is widely accepted that β-sheet peptides play a dominant role in the design and fabrication of peptide-based biomaterials (Refs Reference He, Xiao and Jiang18, Reference Cui, Webber and Stupp41, Reference Stephanopoulos, Ortony and Stupp43). They include ionic self-complementary peptides (Refs Reference Zhang44, Reference Gelain, Horii and Zhang45), peptide amphiphile (PA) (Refs Reference Hartgerink, Beniash and Stupp39, Reference Hartgerink, Beniash and Stupp46, Reference Hosseinkhani47), self-assembling β-hairpins peptides (Refs Reference Schneider48, Reference Pochan49), β-sheet tapes (Refs Reference Collier and Messersmith50, Reference Jung51), ABA-block copolymer (Refs Reference Dong52, Reference Aulisa, Dong and Hartgerink53), various dipeptide and fluorenylmethoxycarbonyl (Fmoc)-conjugates (Refs Reference Zhao, Ma and Xu54, Reference Gazit55, Reference Ulijn and Smith56). Modification with peptide epitopes (i.e. functional motifs) and controlled release of molecular signals (e.g. growth factors, cytokines and chemokines) are capable to increase the similarity of peptide nanostructures to natural extracellular matrices (ECMs), and to significantly improve the bioactivity of peptide nanofibre scaffolds for cell function and tissue regeneration (Refs Reference He, Xiao and Jiang18, Reference Cui, Webber and Stupp41).
In the β-sheet system, self-assembly peptides have been extensively developed to form β-sheet secondary structure and 3D nanofibre scaffolds for various biomedical applications (Refs Reference Matson and Stupp42, Reference Gelain, Horii and Zhang45, Reference Hosseinkhani, Kobayashi and Tabata57, Reference Hosseinkhani, Hosseinkhani and Khademhosseini58). For instance, RADA16-I peptide nanofibre scaffolds are able to facilitate neurite outgrowth, heart and bone regeneration as well as wound healing (Refs Reference Holmes59, Reference Schneider, Garlick and Egles60, Reference Guo61, Reference Yoshimi62), whereas nanofibre scaffolds derived from KLDL12 peptides are beneficial to chondrocyte culture and cartilage regeneration (Refs Reference Kisiday63, Reference Liu64). IKVAV-PA hydrogels have the capability to inhibit nerve scar formation, and promote robust regeneration of nerve fibres (Refs Reference Tysseling-Mattiace65, Reference Okada66). Furthermore, peptide scaffolds formed by rP11–4 peptides and Fmoc-RGD peptides can augment the cell function of dermal fibroblasts (Refs Reference Kyle67, Reference Zhou68). This review will highlight the potential of peptide nanofibre scaffolds in cartilage tissue engineering.
Clinical treatments for cartilage repair
There have been many important treatments for cartilage defects, and they include debridement (Refs Reference Jackson and Dieterichs69, Reference Shannon70), cell-based therapies such as autologous chondrocyte implantation (ACI) (Refs Reference Kon71, Reference Marlovits72) and matrix-induced chondrocyte implantation (MACI) (Refs Reference Kon71, Reference Zheng73), replacement of the damaged tissues (e.g. by mosaicplasty (Ref. Reference Hangody74), autograft and allograft transplantation (Refs Reference Raub75, Reference Bugbee, Cavallo and Giannini76)), as well as recruitment of mesenchymal stromal cells by microfracture (Ref. Reference Steadman, Rodkey and Briggs77). Cartilage autografts are regarded as the golden standard for treating cartilage defects, but are limited by the shortage of donor grafts and the donor site morbidity (Refs Reference Clair, Johnson and Howard78, Reference Ahmed and Hincke79). Although other treatments provide fairly acceptable clinical results, repaired cartilage tissues have mechanical property and function inferior to natural hyaline cartilage and patients with these treatments do not have a good long-term prognosis (Refs Reference Panseri15, Reference Gomoll16, Reference Ebihara25, Reference Huey, Hu and Athanasiou80, Reference Mithoefer81, Reference Dhinsa and Adesida82).
Cartilage tissue engineering has become an increasingly important approach to optimise the functional restoration of damaged tissues, and focuses on the optimal combination of material scaffolds, cells and signal molecules (Ref. Reference Vinatier83). Material scaffolds used in cartilage tissue engineering can be grossly classified into three groups: (Reference Hendriks, Riesle and van Blitterswijk1) natural materials such as collagen (Ref. Reference Chen84), gelatin (Ref. Reference Shin, Olsen and Khademhosseini85), fibrin (Ref. Reference Ahmed86), alginate (Ref. Reference Schmidt, Jeong and Kong87), agarose (Ref. Reference Bougault88), chitosan (Ref. Reference Jayakumar89) and hyaluronan (Ref. Reference Chen90); (Reference Mano and Reis2) synthetic materials such as polylactic acid (PLA) (Ref. Reference Oshima91), poly(glycolic acid) (PGA) (Ref. Reference Mano and Reis2), poly(lactic-co-glycolic) acid (PLGA) (Ref. Reference Nair and Laurencin92) and polycaprolactone (PCL) (Ref. Reference Jeong, Zhang and Hollister93); as well as (Reference Swieszkowski3) their composites such as PGA/fibrin (Ref. Reference Endres94), collagen type II and RGD peptide-modified PLA/PLGA (Ref. Reference Hsu95). Natural materials have good biocompatibility and biodegradation and are able to mimic certain aspects of native ECMs. They have favourable influence on cell functions (e.g. cell proliferation, migration and differentiation) and ECMs formation (Ref. Reference Lee and Shin96). However, these materials are limited by immunogenecity, difficulty in processing, pathogens transmission and relatively weak mechanical force (Ref. Reference Panseri15, Reference Lee and Shin96). Synthetic materials have the advantage of relatively easy production as well as the ability to control dissolution and degradation (Ref. Reference Capito and Spector97). However, these synthetic materials have relatively low bioactivity for cell function and tissue regeneration. Meanwhile, they may elicit inflammatory responses (Ref. Reference Getgood98). Thus, there is a great need to develop novel material scaffolds for cartilage tissue engineering.
Peptide-based biomaterials
Peptides are regarded as versatile building blocks for fabricating supramolecular architectures, and peptide-adopted secondary structures (e.g. β-sheet, α-helix and collagen-like triple helix) contribute favourably to the design and fabrication of self-assembling biomaterials with hierarchical 3D macromolecular architectures (e.g. synthetic membranes, multilamellar structures, amphiphilic micelles, tubules and fibrillar networks), nanoscale features and tunable physical properties (Refs Reference He, Xiao and Jiang18, Reference Zhang36, Reference Loo, Zhang and Hauser37, Reference Hosseinkhani, Hong and Yu40, Reference Hartgerink, Zubarev and Stupp99). Peptide self-assembly is highly specific and the stability of peptide structures is reinforced by enormous noncovalent intermolecular interactions (Refs Reference He, Xiao and Jiang18, Reference Zhang36). The interactions between adjacent peptides can be manipulated through engineering the amino acid sequences and secondary structures of peptides (Refs Reference Loo, Zhang and Hauser37, Reference Zhao and Zhang38). Chemical design versatility of peptides and specific secondary structures provide the feasibility to tailor the structural features of peptide scaffolds (Refs Reference Zhang36, Reference Zhao and Zhang38, Reference Cui, Webber and Stupp41). This review would focus on β-sheet peptides-formed nanofibre scaffolds that have showed extraordinary ability to promote cartilage regeneration.
Ionic self-complementary peptides
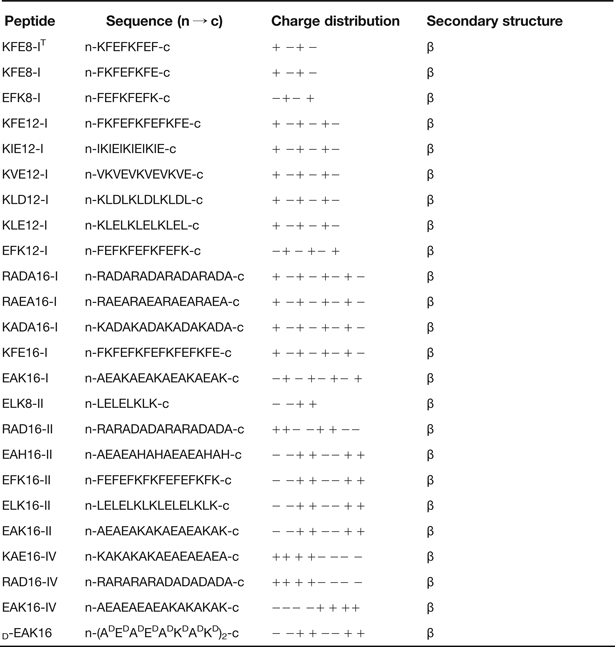
In the early 1990s, a natural protein motif Zuotin in a yeast protein was serendipitously found to have the ability to form nanofibres and 3D nanostructure (Ref. Reference Zhang44). It is the first member of ionic self-complementary peptides and has the peptide sequence AEAEAKAKAEAEAKAK (i.e. EAK16-II) (Refs Reference Zhang44, Reference Yanlian100). Ionic self-complementary peptides are comprised of periodic repeats of hydrophobic sides (e.g. alanine, valine, leucine, isoleucine and phenylalanine) and hydrophilic sides such as positively charged amino acid (e.g. lysine, arginine and histidine) and negatively charged amino acids (e.g. aspartic acids and glutamic acids) (Refs Reference He, Xiao and Jiang18, Reference Zhang36, Reference Yanlian100). The complementary ionic sides are categorized into several moduli (e.g. modulus I, II, III, IV and mixed moduli: modulus I, − + − + − + − +; modulus II, − − + + − − + +; modulus III, − − − + + +; and modulus IV, − − − − + + + +) based on the hydrophilic surfaces of the molecules (Refs Reference Zhang36, Reference Yanlian100, Reference Hauser and Zhang101). In addition, the design of charge orientation in reverse orientations can produce entirely different molecules with distinct molecular behaviours (Refs Reference Zhang36, Reference Zhao and Zhang38). There have been many ionic self-complementary peptides that are under extensive studies (Table 1) (Refs Reference Zhang102, Reference Caplan103, Reference Chen104, Reference Luo, Zhao and Zhang105). During peptide self-assembly, the hydrophobic charged amino acids yield overlapping hydrophobic interactions, whereas positive and negative charges of adjacent peptides pack together through intermolecular ionic interactions in a checkerboard-like manner (Refs Reference Yanlian100, Reference Yokoi, Kinoshita and Zhang106). Assembling environment (e.g. ion strength, temperature, pH of the solution and denaturing agents) is found to have no significant influence on ionic self-complementary peptides-formed nanostructures (Refs Reference Luo, Zhao and Zhang105, Reference Gelain, Horii and Zhang107, Reference Luo, Wang and Zhang108). For instance, after the incubation of d-EAK16 peptides at 100°C for 4 h, the secondary structures of peptides have the propensity to undergo a transition from β-sheet to α-helix, indicating that β-sheet structure is unstable when meeting dramatically temperature change. After continuous incubation for 2 days, almost all peptides can form nanofibres (Fig. 1a) (Ref. Reference Luo, Zhao and Zhang105). In the solution with pH value ranging from 3.7 to 10.6, β-sheet structures formed by d-EAK16 peptides are relatively stable, and only in the solution with pH value below 1.0 and above 12.8, their stability can be reduced (Fig. 1b) (Ref. Reference Luo, Zhao and Zhang105). Some denaturing agents (e.g. 1% SDS, 8.1 m urea and 7.1 m Guanidine.HCl) are used as an interference to peptide self-assembly, but no notable changes of d-EAK16 peptide-formed structures are observed from atomic force microscopy (AFM) images (Fig. 1c) (Ref. Reference Luo, Zhao and Zhang105). Although many factors (e.g. peptide sequences, secondary structures, assembling environment and assembly kinetics) have been identified to have some impact on peptide self-assembly, the detailed mechanisms mediating peptide self-assembly into nanofibre scaffolds are still not clear (Refs Reference He, Xiao and Jiang18, Reference Zhang36, Reference Loo, Zhang and Hauser37, Reference Hosseinkhani, Hong and Yu40, Reference Yokoi, Kinoshita and Zhang106). Additionally, it is found that proteases can degrade l-form peptide bonds but cannot degrade d-form peptide bonds, suggesting that d-amino acids may contribute to the better stability of peptide bonds than l-amino acids (Refs Reference Zhang44, Reference Luo, Zhao and Zhang105). Nanofibre scaffolds formed by d-form peptide or even the combination of l-form and d-form peptides are likely to have some special biomedical applications (Ref. Reference He, Xiao and Jiang18).

Figure 1. AFM images of d-EAK16 peptide nanofibres in different assembling environment: (a) d-EAK16 peptide solution is incubated at 25°C for 4 h and then PBS is added for self-assembling overnight (left). d-EAK16 peptide solution is incubated at 100°C for 4 h and PBS is added for self-assembling overnight (middle). d-EAK16 peptide solution is incubated at 100°C for 4 h and PBS is added to stimulate self-assembly for two nights (right). (b) d-EAK16 peptides (100 µm) are displayed under different pH conditions. At pH 1, globular structures are formed (left). At pH 7, nanofibres are formed (middle). At pH 10.6, both globular structures and nanofibres are formed (right). (c) d-EAK16 peptides (1 mg/ml, 0.1%) are incubated with different denaturation agents: 1% SDS (left), 8.1 m urea (middle) and 7.1 m Guanidine.HCl (right) (Ref. Reference Luo, Zhao and Zhang105). Adapted and reprinted with permission from (Ref. Reference Luo, Zhao and Zhang105).
Table 1. The members of self-assembling ionic-complementary peptides (Refs Reference Zhang102, Reference Caplan103, Reference Chen104, Reference Luo, Zhao and Zhang105).

Peptide amphiphile
The chemical structure of a representative PA molecule has four key structural units including the hydrophobic domain (e.g. a long alkyl tail), a short peptide sequence capable to form intermolecular hydrogen bonding, charged amino acids for the design of pH and salt-responsive nanostructures, as well as the hydrophobic alkyl tail (e.g. peptide epitopes) for the interactions with cells or proteins (Fig. 2a) (Refs Reference Cui, Webber and Stupp41, Reference Stephanopoulos, Ortony and Stupp43, Reference Hartgerink, Beniash and Stupp46). The peptide sequences immediately adjacent to the hydrophobic segment benefit to form intermolecular hydrogen bonding (Refs Reference Hartgerink, Beniash and Stupp39, Reference Hartgerink, Beniash and Stupp46). For the assembly of PAs in water, there are three major energy contributions: hydrophobic interactions of the alkyl tails, hydrogen bonding among the middle peptide segments and electrostatic repulsions between the charged amino acids (Refs Reference Cui, Webber and Stupp41, Reference Stephanopoulos, Ortony and Stupp43). Their delicate balance determines the size, shape and interfacial curvature of final assemblies. The sheet-like structure of a group of PAs rather than the tapered shape of a single PA can be assembled together through intermolecular hydrogen bonding and subsequently form nanofibres as well as 3D biomaterial scaffolds (Refs Reference Cui, Webber and Stupp41, Reference Matson, Zha and Stupp109). Functional modification of PAs using peptide epitopes (e.g. RGD and IKVAV) is beneficial to cell behaviours (e.g. adhesion, proliferation and differentiation) (Refs Reference Matson and Stupp42, Reference Hosseinkhani110, Reference Hosseinkhani, Hosseinkhani and Kobayashi111). These nanofibre scaffolds can act as carriers for signal molecules and hydrophobic drugs crucial for cell function and tissue regeneration (Ref. Reference Cui, Webber and Stupp41).

Figure 2. Various peptides-formed nanofibre structures: (a) molecular model of an IKVAV-containing PA, their self-assembly into nanofibres, as well as scanning electron microscopy (SEM) image of IKVAV nanofibres after adding cell media (DMEM) to PA aqueous solution (Ref. Reference Cui, Webber and Stupp41). (b) Q11 peptides are stimulated to self-assemble by the addition of salts, pH change, or the passage of time, and consequently they result in self-assembled matrix. Quick-freeze deep etch (QFDE) TEM images shows that Q11 peptides are assembled into a highly entangled network with fibrils of ~20 nm in diameter and ~100 nm between entanglement points (Ref. Reference Collier and Messersmith50). (c) Chemical structure Fmoc-FF dipeptide and Cryo-SEM image of Fmoc-FF/K (1:1) nanofibrous hydrogel (Ref. Reference Jayawarna113). Adapted and reprinted with permission from (Refs Reference Cui, Webber and Stupp41, Reference Collier and Messersmith50, Reference Jayawarna113).
Other β-sheet peptides
In one of the β-sheet tapes, Q11 peptides are found to have the ability to form nanofibre hydrogels (Fig. 2b) (Ref. Reference Collier and Messersmith50). They can also be modified with peptide epitopes that are beneficial to cell differentiation and attachment (Ref. Reference Jung112). Dipeptides modified with an N-terminal Fmoc moiety are developed to form nanofibres and hydrogels. Hydrophobic interaction, π–π stacking effects and intermolecular β-sheet hydrogen bonds facilitate the supramolecular association and substantially improve structural stability (Ref. Reference Zhao, Ma and Xu54). For instance, fluorenylmethoxycarbonyl-diphenylalanine (Fmoc-FF) can yield stable hydrogels in physiological condition and show extraordinary cytocompatibility with chondrocytes. Combining Fmoc-FF dipeptide with Fmoc-modified amino acids (e.g. lysine, glutamic acid, arginine and serine) is intended to optimise gel stiffness and possibly increase bioactivity (Fig. 2c) (Ref. Reference Jayawarna113). In the β-sheet system, there also have been other peptide-based nanofibre scaffolds derived from self-assembling β-hairpins peptides and ABA-block copolymer (Refs Reference Schneider48, Reference Dong52).
Rational design of peptide nanofibre scaffolds resembling ECMs
Natural ECMs
Cartilage ECMs are comprised of various collagens, proteoglycans and glycosaminoglycans (GAGs) in which multiple bioactive factors (e.g. growth factors and functional peptides) are incorporated (Refs Reference Benders9, Reference Chen114). Natural tissue regeneration is mediated by highly complex temporal and spatial coordination of diverse cell–matrix and cell–cell interactions which are regulated by: (Reference Hendriks, Riesle and van Blitterswijk1) insoluble hydrated macromolecules (e.g. collagens, elastin, laminin and fibronectin), (Reference Mano and Reis2) soluble macromolecules (e.g. growth factors, chemokines and cytokines) and (Reference Swieszkowski3) proteins on the surfaces of neighbouring cells (Refs Reference Lutolf and Hubbell19, Reference Place, Evans and Stevens20, Reference Friedl115). In addition, an external stimulus (e.g. biomechanical triggers) also has an influence on the reciprocal interaction between cells and ECMs (Ref. Reference Nelson and Bissell116). The ultimate decision of cell function and ECM production is a coordinated response of cells to these ECM effectors (Ref. Reference Lutolf and Hubbell19). It is very difficult and important to manipulate the right quantity of molecular signals for corresponding cells to elicit cell function at the right time (Refs Reference Hynes117, Reference Baumann118).
Increasing the similarity to natural ECMs
Rather than directly reconstructing tissue or organs ex vivo, an emerging design philosophy for tissue engineering scaffolds is to develop smart biomaterials to induce the body's innate powers of organisation and self-repair (Ref. Reference Place, Evans and Stevens20). It is very important to obtain the symbiosis of material scaffolds, cells and signal molecules (Refs Reference He, Xiao and Jiang18, Reference Lutolf and Hubbell19). The design and fabrication of peptide nanofibre scaffolds for biomedical applications should focus on mimicking the microstructure and regulatory mechanisms of natural ECMs. The diameter of nanofibres and pore size of peptide nanofibre scaffolds are at nanometer scale (Refs Reference He, Xiao and Jiang18, Reference Cui, Webber and Stupp41, Reference Stephanopoulos, Ortony and Stupp43). For instance, ionic self-complementary peptide-formed scaffolds have nanofibres with ~10–20 nm in diameter and pore size ranging from ~5 to 200 nm (Refs Reference Dvir23, Reference Zhang36). These nanofibre networks and pore sizes are analogous to those found in natural ECMs, and they have favourable influence on cell infiltration and dwell, the delivery of oxygen and soluble signal molecules, as well as waste product removal (Refs Reference Luo, Wang and Zhang108, Reference Branco119, Reference Koutsopoulos120). Furthermore, stable hydrogels can be formed and contain extremely high water content, more than 99% in water (5–10 mg/ml, w/v) (Refs Reference Hauser and Zhang101, Reference Gelain, Horii and Zhang107).
Two significant approaches to increase the natural ECM mimicking of peptide nanofibre scaffolds are highlighted: (Reference Hendriks, Riesle and van Blitterswijk1) modification with functional motifs and (Reference Mano and Reis2) controlled release of molecular signals (Fig. 3) (Refs Reference He, Xiao and Jiang18, Reference Matson and Stupp42). There have been many functional motifs (e.g. RGD (Ref. Reference Woerly121), IKVAV (Ref. Reference Graf122) and YIGSR (Ref. Reference Jucker, Kleinman and Ingram123)) that are developed to modify biomaterial scaffolds. The tripeptide sequence RGD found in fibronectin and other ECM proteins can facilitate cell differentiation and migration through binding to α5β1 integrin receptor (Ref. Reference Ruoslahti and Pierschbacher124). One study reveals that lower RGD concentrations are found to assist in the maintenance of chondrocyte number and phenotype, as well as the increase in ECM contents compared with higher RGD concentrations (Ref. Reference Smith Callahan125). In addition, peptide PHSRNG6RGD can be fabricated through combining peptide RGD and PHSRN with the aim of increasing the similarity to functional structures of fibronectin, and result in significantly increased cell binding (Ref. Reference Potter, Kalil and Kao126). IKVAV sequences found in laminin are able to reinforce cell adhesion, migration and angiogenesis (Ref. Reference He, Xiao and Jiang18). For instance, peptide RADA16 solution and peptide RADA16–IKVAV solution are combined to form IKVAVmx hydrogel scaffolds for culturing neural stem cells, leading to substantially improved cellular proliferation, differentiation and migration when compared with pure RADA16 hydrogels (Ref. Reference Zhang127).

Figure 3. Schematic illustration of two important approaches to design self-assembling peptide nanofibre scaffolds: there have been many self-assembling peptide sequences that can be modified with functional motifs (e.g. RGD and IKVAV). These self-assembling peptides have the ability to form stable 3D nanofibre structures with functional motifs. The density of these motifs can be changed through mixing self-assembling peptides and peptides with functional motifs in different ratios. In addition, it is feasible to store, retain and release various signal molecules (e.g. growth factors) within such 3D nanofibre scaffolds. These released growth factors and functional motifs can significantly induce cell function and tissue regeneration via binding to their corresponding receptors.
In many cases, only very tiny quantities of signal molecules are required to elicit biological response, and some cellular processes demand several signalling pathways mediated by many signal molecules (Refs Reference Boontheekul and Mooney128, Reference Richardson129). Controlled release of signal molecules within peptide nanofibre scaffolds is both feasible and important to mediate cell–cell and cell–matrix interactions, and significantly promote cell function and tissue regeneration (Refs Reference Lutolf and Hubbell19, Reference Hosseinkhani, Kobayashi and Tabata57, Reference Hosseinkhani, Hosseinkhani and Khademhosseini58, Reference Hosseinkhani, Kobayashi and Tabata130, Reference Hosseinkhani131, Reference Gelain, Unsworth and Zhang132). For instance, RADA16-I peptide nanofibre scaffolds can serve as good substrates to release various functional proteins, e.g. lysozyme, trypsin inhibitor, BSA, IgG, basic-fibroblast growth factor (βFGF), vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF), and these released functional proteins can maintain their original protein conformation and functionality based on the secondary and tertiary structure analyses and biological assays (Refs Reference Koutsopoulos120, Reference Gelain, Unsworth and Zhang132). It is found that physical hindrances can prevent proteins mobility, and interactions between proteins and nanofibres can be affected by charges (Ref. Reference Gelain, Unsworth and Zhang132). In vivo studies of animal model, the released bone morphogenetic protein 2 (BMP2) from self-assembled PA nanofibre scaffolds notably result in homogeneous ectopic bone formation in the back subcutis of rats (Ref. Reference Hosseinkhani133), while the released bFGF from PA nanofibre hydrogels leads to remarkable angiogenesis in the subcutaneous tissue of mice (Ref. Reference Hosseinkhani110). In addition, the local concentration of growth factors can be increased via the electrostatic interactions with heparan sulphate proteoglycans. There is possibility to manipulate the concentration of growth factors through modifying material scaffolds to mimic the heparan sulphate-binding groups (Refs Reference Gelain, Unsworth and Zhang132, Reference Freeman, Kedem and Cohen134). For example, modifying an alginate hydrogel through sulphating the uronic acids in the saccharide backbone can be used to mimic the heparin/heparan sulphate-binding groups. Sustained release of angiogenic and pro-survival factors within these modified hydrogels significantly increase the capability to promote pre-vascularisation of engineered cardiac patches and repair infarcted heart (Refs Reference Freeman, Kedem and Cohen134, Reference Dvir135).
Cartilage regeneration using peptide nanofibre scaffolds
Peptide nanofibre scaffolds are formed by natural amino acids, and have the properties of biological self-recognition, good biocompatibility as well as nontoxic degradation products (Ref. Reference Zhang36). Their microstructures highly mimic the natural ECM and have appropriate porosity for cell infiltration and growth (Refs Reference Zhang36, Reference Cui, Webber and Stupp41). In addition, modification with functional motifs and controlled release of molecular signals have been under extensive studies to increase the similarity of peptide nanofibre scaffolds to the microstructure and regulatory mechanisms of natural ECMs (Refs Reference He, Xiao and Jiang18, Reference Zhao and Zhang38, Reference Hosseinkhani, Hong and Yu40, Reference Cui, Webber and Stupp41). This review would focus on the capability of peptide nanofibre scaffolds to induce cartilage repair from the aspects of cell culture in vitro and tissue regeneration in vivo.
In vitro cell culture
Many peptide nanofibre scaffolds have demonstrated the ability to induce cellular activities (e.g. proliferation, differentiation, adhesion and migration) as well as ECM production by cell culture tests in vitro (Refs Reference Kisiday63, Reference Liu64, Reference Jayawarna113). FEFEFKFK peptides-formed nanofibre scaffolds are used to culture chondrocytes, resulting in good cellular viability and proliferation, cell morphology retention as well as type II collagen deposition (Fig. 4a–c) (Ref. Reference Mujeeb136). Likewise, nanofibre scaffolds derived from KLDL12 and RADA16-I peptides are beneficial to culture chondrocytes and the formation of cartilage-like ECMs enriched in GAG, proteoglycans and type II collagen (Refs Reference Kisiday63, Reference Liu64). Bone marrow stromal cells (BMSCs) cultured in RAD16-I peptide hydrogels lead to the formation of sulphated GAG, type II and type I collagens (Fig. 4d–f) (Ref. Reference Kopesky33). In addition, Fmoc-FF/S and Fmoc-FF/D hydrogels can be used to culture bovine chondrocytes, demonstrating good cell function and morphology retention (Ref. Reference Jayawarna113).
Controlled release of growth factors has been widely used for cell culture within peptide nanofibre scaffolds. For instance, KLDL12 peptide hydrogels can serve as the substrate to incorporate and release transforming growth factor β1 (TGF-β1). At day 21, cumulative release of TGF-β1 from peptide hydrogels is 32–44% as opposed to 62% of released TGF-β1 from peptide hydrogels with encapsulated BMSCs, and it is possibly associated with cell-mediated TGF-β1 degradation. Sustained release of TGF-β1 is revealed to stimulate chondrogenesis of young equine BMSCs (Ref. Reference Kopesky137). Furthermore, KLDL12 peptide hydrogels are used to seed bone marrow mesenchymal stem cells (BM-MSCs) and the released TGF-β1 from peptide hydrogels can significantly increase cell function, as well as the synthesis of proteoglycan and type II collagen (Fig. 4g and h) (Ref. Reference Kisiday34). Additionally, it is well known that dexamethasone (Dex) has pro-anabolic and anticatabolic effects on cartilage tissue engineering. However, in one study, RADA16 peptide hydrogels comprising TGF-β1 and Dex are utilised to culture young bovine and adult human BMSCs. The results demonstrate that the released Dex has the capability to reduce aggrecanase activity within peptide hydrogels for both young bovine and adult human BMSCs (Ref. Reference Florine138).

Figure 4. Promotion to cell culture in vitro: chondrocytes seeded on FEFEFKFK peptide nanofiber scaffolds contribute to good cell viability as demonstrated by (a) confocal microscopy z-stack image viewed along the z-axis (viable cells in green and the nuclei of dead cells in red) and cell morphology retention as demonstrated by (b) optical microscopy image showing the classic rounded morphology of chondrocytes in the gel matrix after 25 days culture and by (c) environmental scanning electron microscopy (ESEM) micrograph displaying a single-cell attached to the gel (Ref. Reference Mujeeb136). BMSCs cultured in RAD16-I peptide scaffolds result in extensive ECM production, as evidenced by (d) toluidine blue staining for sulphated GAG, immunohistochemistry images of (e) collagen type II and (f) type I (Ref. Reference Kopesky33). Sustained release of TGF-β1 from KLDL12 peptide hydrogels comprising BM-MSCs is beneficial to the extensive accumulation of (g) proteoglycan identified by toluidine blue staining and (h) type II collagen identified by immunohistochemical staining (Ref. Reference Kisiday34). (i) Chondrocyte-seeded KLDL12 peptide hydrogels substantially facilitate proteoglycan synthesis as showed by toluidine blue staining after 39 days of alternate day dynamic compression (Ref. Reference Kisiday144). Adapted and reprinted with permission from (Refs Reference Kopesky33, Reference Kisiday34, Reference Mujeeb136, Reference Kisiday144).
Various types of cells have been studied to serve as cell source and result in different ECM production within peptide nanofibre scaffolds. BMSCs-incorporated KLDL12 peptide hydrogels lead to form cartilage-like neotissue with longer core protein and chondroitin sulphate chains, as well as higher mechanical stiffness than that produced by chondrocytes (Ref. Reference Kopesky139). Compared to MSCs as a cell source, chondrocytes can produce ECMs with superior mechanical properties when using RADA16-I peptide hydrogels for cell culture (Ref. Reference Erickson140). In addition, for identifying the influence of GAG on chondrogenic differentiation, sulphonate, carboxylate and hydroxyl groups are incorporated on self-assembled peptide nanofibres. These functional peptide nanofibre scaffolds contribute favourably to rapid aggregation of TDC5 cells in the insulin-free medium, cartilage-like nodules formation, sulphated GAGs deposition, as well as significant gene expressions of type II collagen and aggrecan indicative of chondrogenic differentiation (Ref. Reference Ustun141).
Culturing mouse embryonic fibroblasts (MEFs) within peptide nanofibre hydrogels is affected by different elastic modulus values. At low elastic modulus values (approximately 0.1 kPa), both chondrogenic inductor BMP4 and its antagonist Noggin are detected, while at higher elastic modulus values (approximately 5 kPa), Noggin rather than BMP4 is detected, resulting in the inhibition of chondrogenesis. This study reveals that mechanical stimulus has important influence on cell function (Ref. Reference Fernandez-Muinos142). In another study, human dermal fibroblasts are cultured within self-assembling peptide hydrogels with standard chondrogenic medium. During the first day of culture, the 3D constructs undergoes a substantial contraction process and consequently form a small compact structure, resulting in a chondrocyte-like construct based on the elevated expression of GAG, proteoglycan aggrecan and type II collagen (Ref. Reference Bussmann143). Furthermore, dynamic compression is found to significantly increase proteoglycan synthesis within chondrocyte-seeded KLDL12 peptide hydrogels (Fig. 4i) (Ref. Reference Kisiday144). It is very important to control mechanical stimulus for chondrogenesis and ECM production, and much effort is needed to elucidate the mechanisms regarding the influence of mechanical stimulus on chondrogenesis.
In vivo tissue regeneration
Controlled release of growth factors from peptide nanofibre scaffolds has been used in vivo experiments. RADA16-I peptide hydrogels containing chondrocytes and TGF-β3 are transplanted into the cartilage defects of bovine model, resulting in extensive synthesis of GAG and type-II collagen, as well as good integration with native cartilage tissue and the formation of mechanically stable interface (Fig. 5a and b) (Ref. Reference Maher145). Chondrogenic factors (i.e. TGF-β1, insulin-like growth factor-1 (IGF-1) and Dex) are combined with KLDL12 peptide hydrogels to treat the full-thickness and critically sized defects of rabbit cartilage, contributing to significant formation of aggrecan and type II collagen (Ref. Reference Miller146). In addition, TGF-binding PAs (TGFBPA: HSNGLPLGGGSEEEAAAVVV(K)-CO(CH2)10CH3) are designed with binding epitopes to TGF-β1 and can effectively reduce the passive release of TGF-β1. In vitro experiments reveal that these peptide scaffolds comprising TGF-β1 are capable to induce the chondrogenic differentiation of human MSCs. TGF-binding PA nanofibre scaffolds comprising TGF-β1 can substantially promote the regeneration of articular cartilage in the full-thickness chondral defects treated with microfracture in the trochlea of adult rabbits in the presence of bone marrow MSCs, indicating the importance of growth factor release and functional modification within peptide nanofibre scaffolds for cartilage repair (Fig. 5c–e) (Ref. Reference Shah35).

Figure 5. Promotion to cartilage regeneration in vivo: Alcian blue–eosin stained sections of the interface between the inner core and outer ring articular cartilage components at culture day 21, (a) RADA16-I hydrogel containing both TGF-β3 and chondrocytes showing robust cartilage formation in intimate contact with the native cartilage components compared to the treatment of (b) hydrogel-containing chondrocytes only (Ref. Reference Maher145). Articular cartilage defects after 12 weeks postop treated with 10%TGFBPA + 100 ng/ml TGF-β1 (100TGF), showed by macroscopic views (c), histological evaluation of sample sections: (d) safranin-O staining for GAGs and (e) type II collagen staining (Ref. Reference Shah35). Adapted and reprinted with permission from (Refs Reference Shah35, Reference Maher145).
Conclusion and future prospective
Self-assembling peptide nanofibre scaffolds have become increasingly important biomaterials for cartilage tissue engineering. Their nanofibre networks highly mimic natural ECMs and these similarities can be increased by modification with functional motifs and controlled release of signal molecules. However, the mechanical force of peptide nanofibre scaffolds is relatively weak and it is very difficult to use such biomaterial scaffolds to repair the defects of loading-bearing cartilages.
It is generally accepted that the design of synthetic biomaterial scaffolds should aim to resemble the microstructures and regulatory mechanisms of natural ECMs for the purpose of stimulating the best regenerative ability of damaged tissues or organs. It is very important but difficult to modulate cell behaviours (e.g. proliferation, differentiation and migration) within peptide nanofibre scaffolds because these regulatory mechanisms in natural ECMs are very complicated and hard to manipulate. The design of biomaterial scaffolds mimicking natural ECMs may significantly increase the possibility of controlling cell functions.
Acknowledgements
D.M. Jiang was supported in part by China National ‘‘973 Project’' and by the grant from the Natural Science Foundation Project of CQ CSTC (2009AB5080), and National Natural Science Foundation of China (NSFC, 81171685/H0604). We apologize to all the scientists whose work we could not cite due to space restrictions.