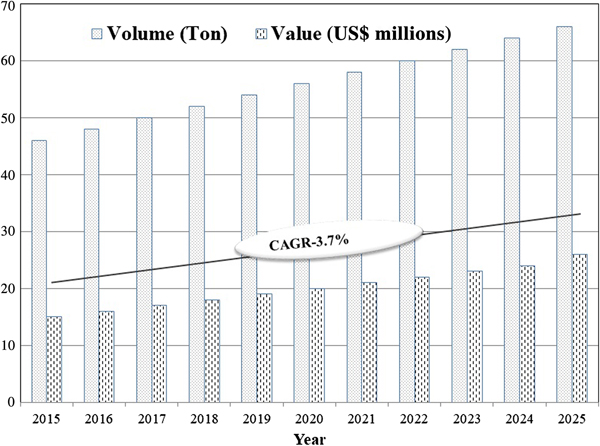
Zeolites are a naturally occurring group of minerals consisting of over 50 different minerals with varying physical and chemical properties. The structure of zeolite aluminosilicates is composed of TO4 tetrahedral units (T = Al and Si) joined covalently by O atoms, which are linked to produce >150 different zeolite framework types. Because of this special, porous, crystalline structure which remains rigid in the presence of water, zeolites may be adapted for a variety of uses. Iranian zeolites vary in terms of purity, composition, crystal size, porosity, pore size and other characteristics (Mazloomi & Jalali, Reference Mazloomi and Jalali2016). Natural zeolites have many important properties and are available in many regions of the world. Natural and synthetic zeolites with large specific surface areas are safe, environmentally friendly and inexpensive, and they find widespread applications as molecular sieves, cation/anion exchangers, adsorbents, catalysts, detergent builders, gas purifiers, soil remediators and effective materials for the removal of heavy metals from wastewaters in industrial processes (Nezamzadeh-Ejhieh & Kabiri-Samani, Reference Nezamzadeh-Ejhieh and Kabiri-Samani2013). They are used in the agricultural sector and in oil/petrochemical industries such as water and wastewater treatment, as well as in energy-related applications such as gas separation and storage processes (Beitollah & Jafari Sadr, Reference Beitollah and Jafari Sadr2009). The global zeolites market is expected to have a compound annual growth rate of 3.7% from 2017 to 2025 (Fig. 1).
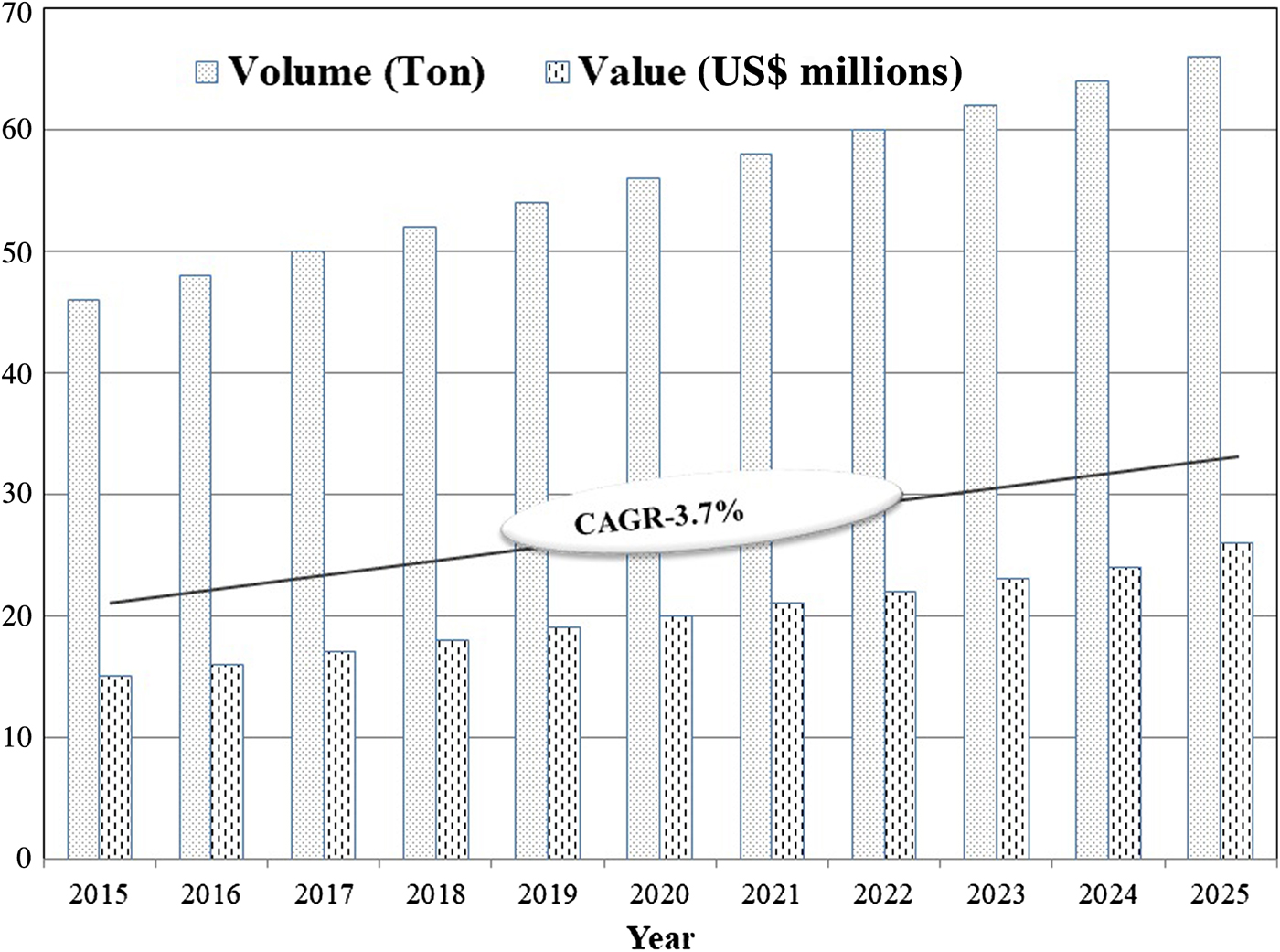
Fig. 1. Global zeolite market (retrieved on 10 December 2018 from www.credenceresearch.com/report/zeolites-market). CAGR = compound annual growth rate.
Commercially available deposits of natural zeolites are limited to a few particular zeolite species, with clinoptilolite (CLP) being the most abundant natural zeolite. Most natural zeolites contain impurities that interfere with many industrial applications; therefore, they do not meet the criteria required for some industrial applications, including catalytic and gas-separation processes. Thus, natural zeolites are used primarily in animal husbandry, agriculture and water and wastewater-treatment applications. To fulfil the market demands for highly efficient molecular sieves and catalysts, high-purity artificial zeolites are manufactured at large scale using different starting materials as sources of Al and Si. Synthetic zeolites are preferred over their natural counterparts due to the possibility of tuning their properties by means of different synthesis and post-synthesis modification techniques.
Iran has a large mine of natural high-purity (75–95 wt.%) CLP zeolite. In spite of the abundant occurrence of high-purity (75–95%), natural zeolite deposits in Iran (Kazemian, Reference Kazemian2002), systematic studies are scarce (Khalili et al., Reference Khalili, Makizadeh and Taghipour2005). This review outlines the major research progress and achievements in the field of natural zeolite science and technology and their applications in Iran.
Natural zeolite deposits in Iran
Iran, with an area of 1,648,000 km2, is among the 15 major mineral-rich countries in the world, hosting some 68 types of minerals, 37 billion tons of proven reserves and more than 57 billion tons of potential reserves worth US$770 billion in 2014. Nevertheless, the mining sector is still underdeveloped in the country due to a lack of proper investment in infrastructure and bureaucratic barriers to exploration projects, among other factors (McCrae, Reference McCrae2016).
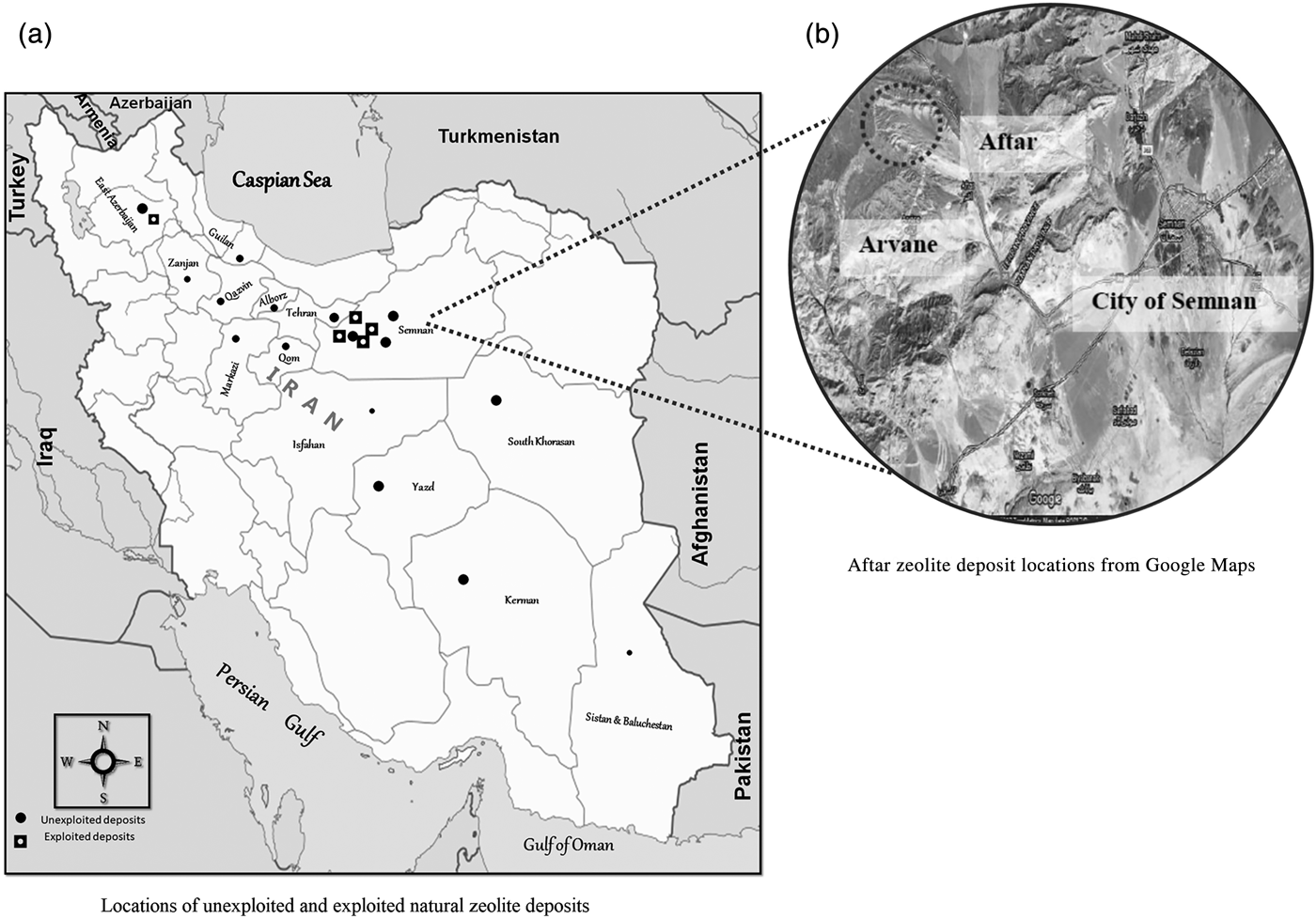
Despite substantial background geological work by government and private companies on the exploration and exploitation of zeolitic deposits, official data and statistics on natural zeolite reserves in the country are very limited. It has been suggested that Iran hosts tens of million tons of natural zeolite reserves – mostly sedimentary – which, according to the National Geoscience Database of Iran (NGDIR), are distributed in various regions around the country. According to reports published by the Iranian Geological Survey Organization (IGSO), natural zeolite is probably the second most common mineral deposit in Iran (after iron) (Fig. 2).
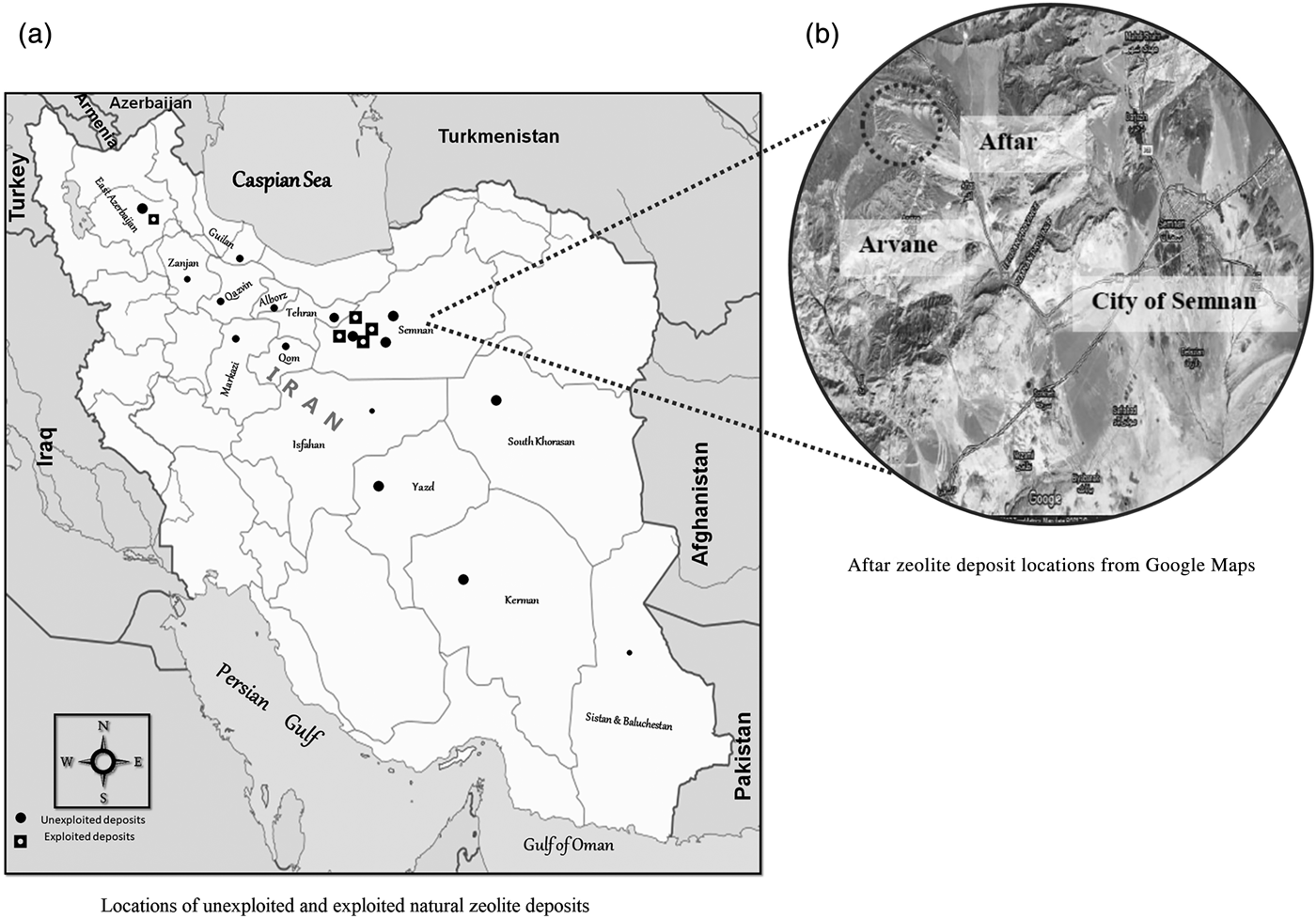
Fig. 2. (a,b) Location of some natural zeolite deposits in Iran (retrieved on 5 December 2018 from www.iza-online.org/natural/Catalog/Iran.pdf).
Although there are several registered Iranian companies that sell natural zeolite products to domestic markets and a few which export natural zeolites to the international market, most Iranian zeolite reserves – some with economic potential – have not been well documented. According to the scientific literature and accessible technical reports (mostly informal/unpublished and in the Persian language), while most of the commercially available natural zeolite deposits are being mined from Semnan Province, there are many more deposits in other provinces with huge potential for commercialization (Fig. 2).
Semnan Province
Semnan Province, located in central-north Iran, stretches along the Alborz mountain range and borders the Dasht-e-Kavir desert in the south. It is the main hub of natural zeolite deposits in Iran. Sedimentary zeolites have been observed south of central Alborz, between the Tehran and Semnan regions. Amygdaloidal zeolites occur in Cenozoic andesitic volcanic sequences in north-central Iran. These zeolites, which might be associated with bentonites in places, contain evaporites and differ from their sedimentary counterparts of Alborz. According to unpublished informal technical reports, Semnan Province contains >16 million tons of proven sedimentary zeolite deposits that are either in active mines of have potential for economic exploitation. Semnan Province supplies >95% of the country's demand for natural zeolites, mainly CLP for the animal husbandry, aquaculture, agriculture, cement and construction industries. Natural CLP layers in Eocene tuffs emerged and formed more than 600,000 tons of high-quality (85–95% CLP content) zeolite deposit. Natural CLP forms an east–west extending bed with outcrop thicknesses of 15–110 m.
Most of the zeolite deposits in the area contain CLP and heulandite associated with calcite, orthoclase, plagioclase, quartz, clay minerals, biotite and volcanic glass. Based on geological and mineralogical evidence, the zeolites formed from alteration of acidic volcanic tuffs in a shallow-sea environment at high pH. The increased pH during sedimentation of the tuffs provided suitable conditions for the conversion of the volcanic glass into zeolites. The zeolite deposits are classified as high-purity or grade 1, with a white to cream colour, or grade 2, with a light-green to cream colour.
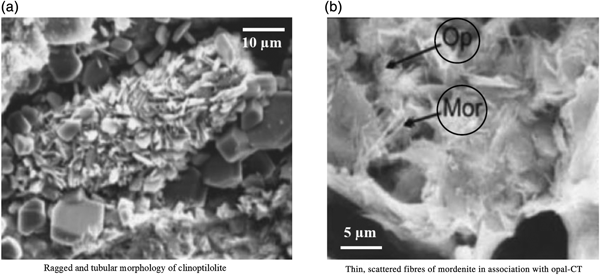
Another zeolite deposit of lower quality located in the Sartakht area (southeast of Semnan Province) is composed of authigenic zeolite (CLP and minor mordenite), smectite and opal-CT (Bazargani-Guilani & Rabbani, Reference Bazargani-Guilani and Rabbani2008). The mordenite occurs as thin, scattered fibres (Fig. 3). Scanning electron microscopy (SEM) examination suggests that CLP is the main constituent of zeolitic tuffs and mordenite crystals formed from the relict glass.

Fig. 3. (a,b) SEM images of zeolite samples of Sartakht/southeast of Semnan Province (retrieved on 5 December 2018 from www.iza-online.org/natural/Catalog/Iran.pdf). Op = opal-CT; Mor = mordenite.
A high-quality, sedimentary, natural zeolite deposit of Eocene age is located in the southeast Semnan area. The deposit forms an anticline, in which the zeolite horizons are >20 m thick. Sodium CLP is the main mineral phase of these zeolite-rich tuffs which also contain smectite, quartz, halite, opal-CT, calcite and dolomite. This deposit is part of the Karaj Formation of Kuh-e-Astaneh in western Semnan, which consists of shales, carbonates (micrite, Mg-micrite, pelagic micrite, limestone and dolostone), radiolarite, tuffite, zeolite and bentonite. Geological studies indicated that the glasses have been altered to bentonite and zeolite. The Karaj Formation contains analcime associated with abundant quartz, minor feldspar and regular mixed-layered illite/smectite. The absence of Si-rich zeolites, CLP or mordenite as analcime precursors in the samples with high K/Na ratios indicates that analcime in the Karaj Formation formed directly from vitric tuffs in a highly saline basin prior to burial. The absence of Si-rich zeolite precursors in the samples with high K/Na ratios is attributed to the very high pH conditions. It is probable that the zeolite precursors were necessary for formation of analcime in alkaline conditions of lower pH values (Bazargani-Guilani & Rabbani, Reference Bazargani-Guilani and Rabbani2008).
Tetranatrolite and natrolite are generally fibrous and acicular and more abundant than analcime and stilbite in Meyamey area in Shahrood. Analcime was found with quartz in the lower layers, with clinoptilolite and halite in the middle layers and with caicite and dolomite in the celestine-bearing samples of low-K tuffs. Amygdaloidal zeolites of Meyamey area are spheroidal, ellipsoidal and unnaturally shaped. The host rocks of the zeolites include trachyandesite, andesite, chloritized andesite and porphyritic andesitic basalt.
Natural zeolite occurrences, mostly natrolite, associated with copper minerals such as malachite in vesicles, amygdales, veins and veinlets at brown andesites in association with nummulitic limestones have been reported east of Shahrood City. Veins of hematite and quartz occur in association with copper minerals. The chemical composition (wt.%) of a typical natural zeolite from Semnan Province deposits is as follows: SiO2 (64.4), Al2O3 (12.8), Fe2O3 (1.31), TiO2 (0.31), CaO (2.37), MgO (1.15), Na2O (1.13), K2O (2.64), P2O5 (0.21) and loss on ignition (LOI; 13.19) (Kazemian et al., Reference Kazemian, Modarress, Kazemi and Farhadi2009).
East Azerbaijan Province
East Azerbaijan Province, with a couple of active zeolite mines, provides ~5–10% of Iran's market demands for CLP. Most of the commercial zeolite deposits are located in the vicinity of Mianeh City in Mianeh County. The deposits are of Eocene age and formed from the alteration of andesite tuffs. They contain mainly CLP and minor quartz, cristobalite, feldspar and montmorillonite and are overlain by Pliocene conglomerate. The zeolite rocks occupy an area of 800 m × 800 m and are 30 m thick (data retrieved from www.iza-online.org/natural/Catalog/Iran.pdf). The typical chemical composition (wt.%) of the zeolite deposits from Mianeh is as follows: SiO2 (65.9), Al2O3 (12.1), Fe2O3 (2.49), TiO2 (0.31), CaO (2.62), MgO, (1.23), Na2O (1.73), K2O (3.43), P2O5 (0.07) and LOI (12.1).
Tehran Province
The Alborz mountain range is divided into the Kopeh-dagh zone in the north and the Central Iranian zone in the south and is a region of active deformation within the broad Arabian–Eurasian collision zone. The 40 km long zeolitized green tuff belt of Eocene age in the Karaj Formation, Central Alborz, consists of volcanoclastic rocks. The area affected by zeolitization is 3–300 m thick. Gypsum lenses are interbedded with the green tuff succession. The zeolitic rocks consist of major CLP and montmorillonite and minor cristobalite (Kazemian, Reference Kazemian2004; Taghipour, Reference Taghipour2010).
Volcanic rocks in the southwest of Tehran contain a variety of amygdale, vein, veinlet and patchy zeolites, including analcime, tetranatrolite, natrolite, stilbite, scolecite, mesolite, laumontite, mordenite, heulandite/CLP and phillipsite. The zeolites are associated with smectite, chlorite and calcite. Laumontite, phillipsite and mordenite have also been reported in amygdales in central Iran. In addition, analcime and quartz, along with diagenetic dolomite and celestite, occur within the lower part of the Karaj Formation, and white opal-CT and smectite occur in the upper part. The analcime in this region is diagenetic (Bazargani-Guilani et al., Reference Bazargani-Guilani, Rabbani, Irajian and Kazemian2008).
Kerman Province
The occurrence of natrolite has been reported northwest of Bardsir City in Kerman Province. In addition, deposits of CLP-rich tuffs with high economic potential occur in the Baft region. Furthermore, zeolite occurrences have been reported in pores and fractures locally accompanied by small amounts of copper south of Jiroft City. The zeolite reserves in this area are very large and are of potential economic significance (Kazemian, Reference Kazemian2002).
Other provinces
High-purity natural CLP deposits with natrolite, tetranatrolite, mesolite, analcime, stilbite, levyne and offretite associated with kaolinite and calcite and traces of chalcopyrite and malachite are exploited in Qom Province. To the best of our knowledge, there are no accessible reports on these deposits. In the vicinity of the Badamcheh region, zeolites are associated with baryte. In addition, natrolite, tetranatrolite, epistilbite and analcime associated with calcite, pyrolusite, hematite, opal-CT, chalcedony and quartz have been reported in amygdales of altered andesite and andesitic–basaltic rocks north of the Parandak City (Markazi Province). The sequence of mineral formation in the amygdales and veins is primary calcite, analcime, natrolite and secondary calcite. A zeolite deposit with substantial economic potential located in the Erjenan region near Ardakan City in Yazd Province contains CLP. A thin layer of zeolite (CLP) has been reported northeast of Zanjan City (Zanjan Province). Because of the small thickness of the zeolite bed, its exploitation may not be economically viable.
Zeolite occurrences have been reported in the Eocene tuffs southeast of Arvan Goosh village near Avaj (Qazvin Province) and in the Taleghan region (Alborz Province). The latter contains analcime, but the reserves are not adequate for commercial mining. In addition, natrolite, tetranatrolite, andesite, analcime and stilbite in amygdales and veins probably of hydrothermal origin occur in the Gilan region. Moreover, a mesolite deposit has been reported southeast of Tabas along the Deyhook-Ravar road (South Khorasan Province) (Faghihian & Kazemian, Reference Faghihian and Kazemian1998), and minable reserves of CLP-rich tuffs are exploited in this province. Natrolite zeolite has been reported in Northern Zahedan (Sistan and Baluchestan Province). The utilization of natural zeolites, with respect to their synthetic counterparts, reduces operation costs and eliminates the need for time-consuming synthesis procedures and expensive reagents and eco-friendly materials (Naderpour et al., Reference Naderpour, Noroozifar and Khorasani-Motlagh2013).
Research, production and applications of natural Iranian zeolites
There are several registered Iranian companies, such as Afrand Tooska, Fath-e-Alborz, Afrazand and Negin-Powder companies, producing and distributing natural zeolite products to the domestic market. Most of the production plants are located in Semnan Province (Fig. 4).

Fig. 4. Typical open-pit zeolite mine (left) and deposit of powdered zeolite (right) in Semnan Province.
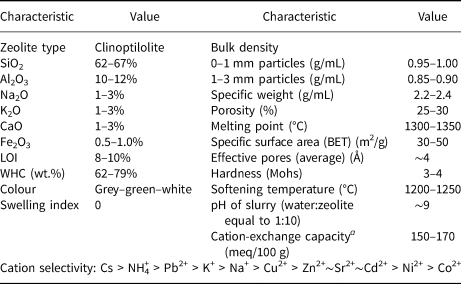
Recently, a few Iranian companies have been exporting their natural zeolite products (i.e. Zeodigest® brand) to other countries in Asia and Africa in an effort to introduce high-quality natural Iranian CLP to the international market (Table 1).
Table 1. Typical composition and properties of the products of Semnan zeolite deposits – Zeodigest® (Zeodigest, 2015).
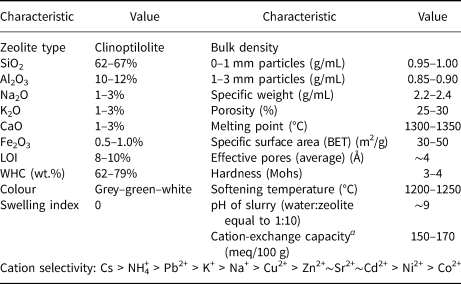
a Measured by the standard ammonium acetate method.
BET = Brunauer-Emmett-Teller; WHC = water-holding capacity.
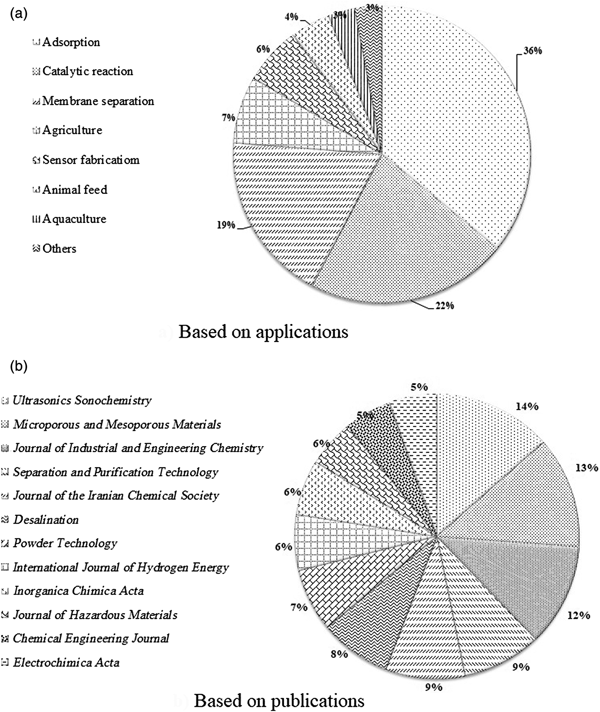
Figure 5a shows the numbers of papers published by Iranian scholars on different applications of zeolite (both natural and synthetic) since 2005. The three main application fields studied are adsorption, heterogeneous catalytic reactions and composite–membrane separation. Moreover, the top 12 Clarivate journals published at least 10 contributions per year per journal by Iranian scholars since 2005 (Fig. 5b). Note that most published papers by Iranian researchers are focused on environmental applications of natural zeolites.
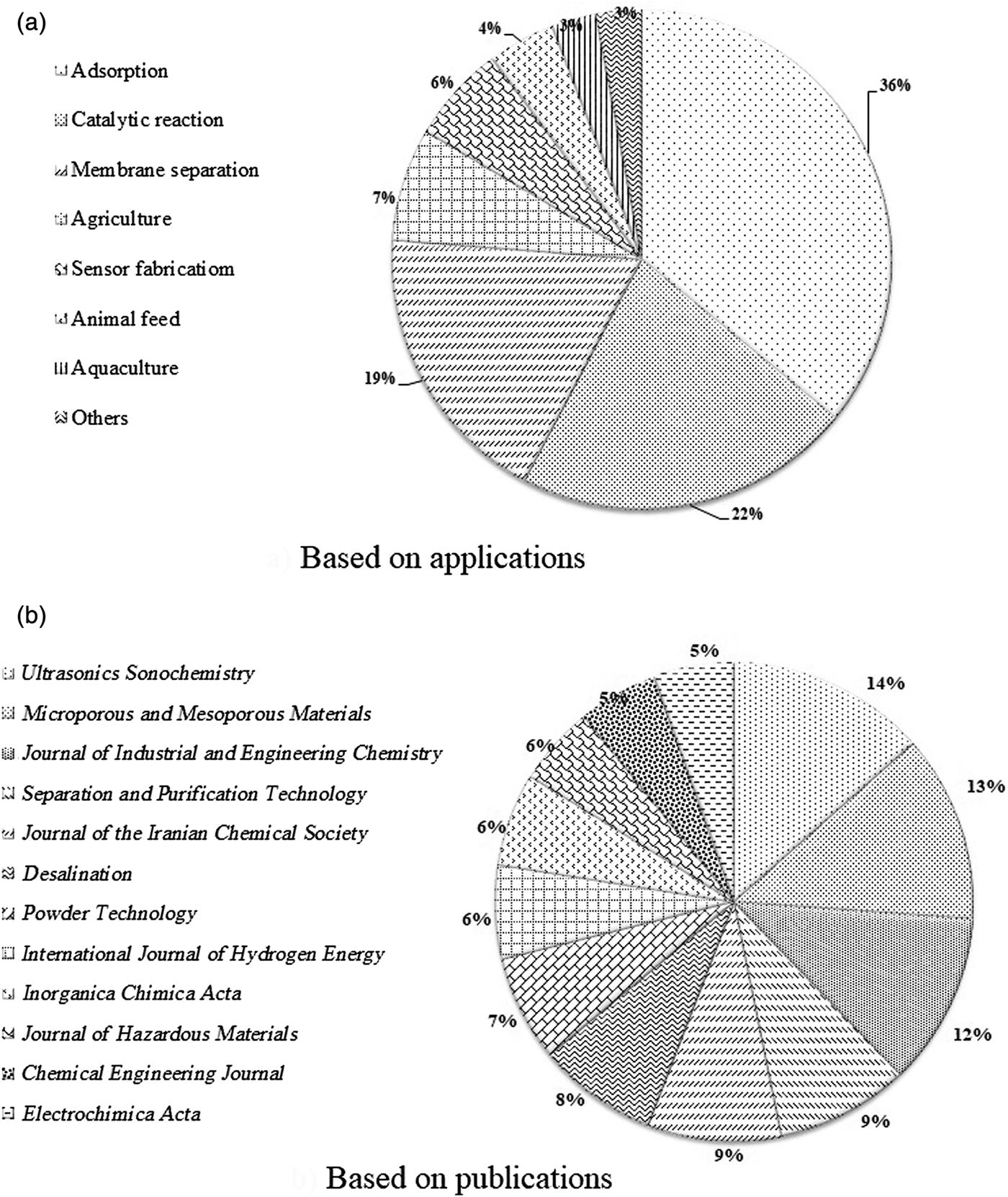
Fig. 5. (a,b) Research papers published on natural zeolite by Iranian researchers since 2005.
So far, Iranian zeolite products (mainly CLP) with various brands have been marketed for, but not limited to, the applications detailed in the sections below.
Environmental applications of natural zeolites
The adsorptive properties of natural CLP from various regions of the country are used in the removal of radiopharmaceutical 131I from nuclear waste effluents (Faghihian et al., Reference Faghihian, Ghannadi-Maragheh and Malekpour2002). Potassium nickel hexacyanoferrate (KNiFC) was incorporated into the porous matrix of natural CLP and zeolite P synthesized from CLP after impregnation with Ni(NO3)2 and K4Fe(CN)6 (Kazemian et al., Reference Kazemian, Zakeri and Rabbani2006a). The KNiFC-CLP was a suitable sorbent for removal of 137Cs, whereas KNiFC-P was more suitable for 90Sr radionuclides. The adverse effect of sodium cations when present at high concentrations in radioactive waste streams was less significant for the modified adsorbents compared to the parent zeolites. A natural CLP zeolite from the Ardakan region (Yazd Province) was used as a starting material to synthesize zeolite P (Kazemian et al., Reference Kazemian, Darybi-Kasmaei, Mallah and Khani2006b). Both zeolites were tested for their removal efficiency of Cs and Sr ions from radioactive waste. The Cs- and Sr-saturated zeolites were vitrified in order to immobilize these radionuclides in borosilicate glass matrices. The glasses produced from CLP were more suitable for immobilization of the simulated wastes than those made of zeolite P, and overall, the glasses were more efficient at stabilizing 90Sr than 137Cs radioisotopes.
Clinoptilolite and its Na derivatives were used for the treatment of low-level radioactive liquid waste including radionuclides and heavy metals (Moattar & Hayeripour, Reference Moattar and Hayeripour2004). The results were compared with two types of shrimp chitin derivatives as natural organic adsorbents. Clinoptilolite showed the weakest performance among the adsorbents; however, the Na-CLP showed the best adsorption for Cs, but less so for Sr, Co and Mn ions. Adsorption of 103Ru, Co2+ and Ni2+ on natural and modified CLP (NH4+ form) was reported from nuclear wastewater (Faghihian, Reference Faghihian, Kabiri-Tadi and Ahmadi2011; Malekpour et al., Reference Malekpour, Edrisi, Hajialigol and Shirzadi2011). The selectivity of zeolite was greater for Co2+ than Ni2+. Based on desorption studies, 97%, 74% and 85% recovery rates of Ru, Co2+ and Ni2+, respectively, were achieved using HCl solution. The adsorption capacity for both cations increased with increasing temperature, and microwave irradiation facilitated the adsorption of the cations onto zeolite channels (Malekpour et al., Reference Malekpour, Edrisi, Hajialigol and Shirzadi2011). A CLP-rich tuff was more effective for radioactive 212Pb removal from aqueous solutions than natrolite (Kazemian et al., Reference Kazemian, Rajec, Macasek and Orechovska Kufcakova2001). In addition, the Na-CLP exhibited good adsorption capacity for Th4+ removal from aqueous solutions (Khazaei et al., Reference Khazaei, Faghihian and Kamali2011).
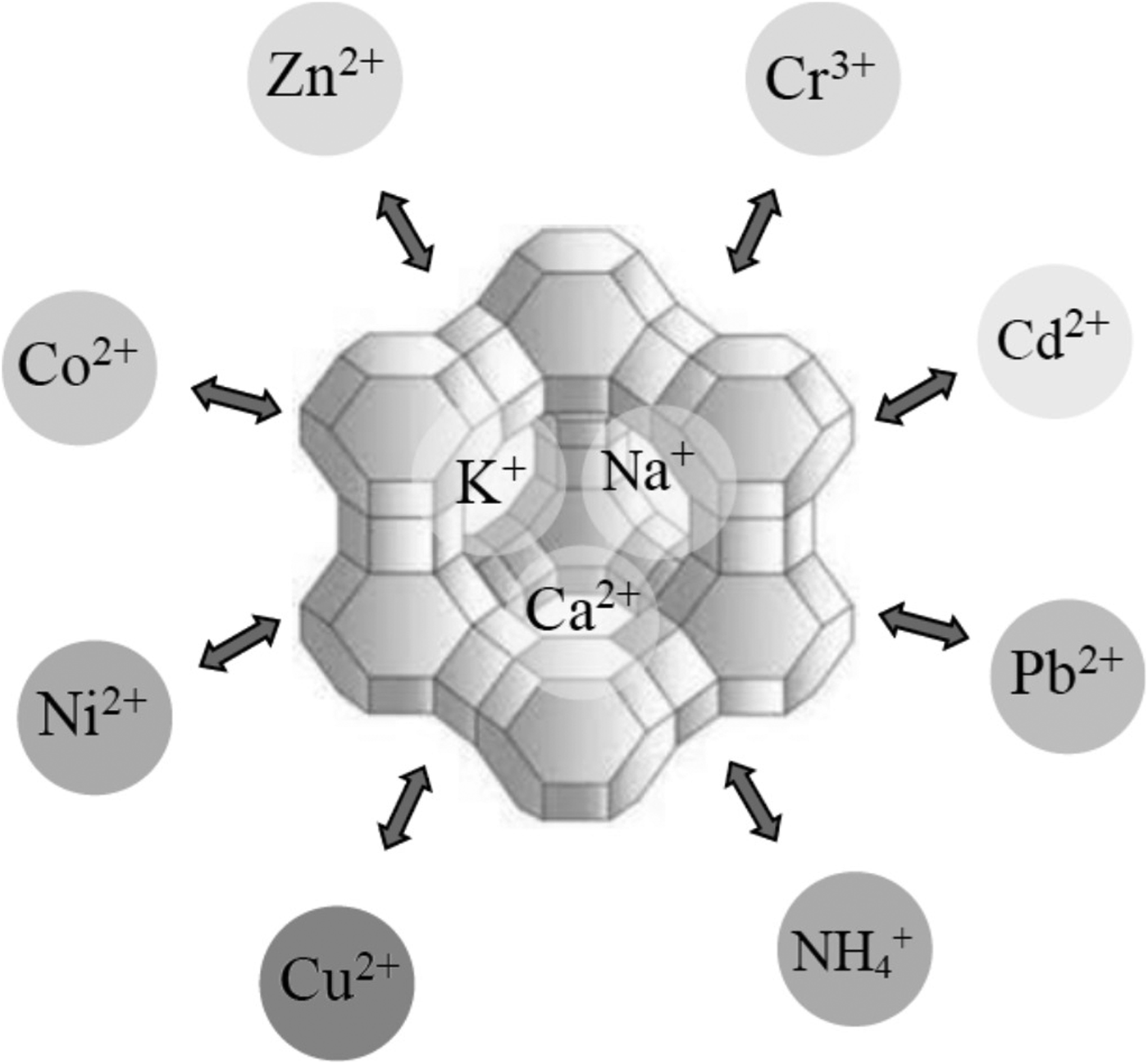
The uptake of Hg by CLP was compared with granular activated carbon and anthracite. The effects of Hg concentration, contact time and pH level on removal efficiencies were studied. The order of removal efficiency was: granular activated carbon > CLP > anthracite (Samadi et al., Reference Samadi, Salimi and Saghi2009). The selectivity of CLP for Cd2+, Cu2+, Ni2+ and Pb2+ was examined in single- and multi-component solutions (Merrikhpour & Jalali, Reference Merrikhpour and Jalali2013; Yousefi et al., Reference Yousefi, Torab-Mostaedi, Charkhi and Aghaei2016). The selectivity sequence obtained from Freundlich distribution coefficients for the single- and multi-component systems was: Pb2+ > Cu2+ > Cd2+ > Ni2+. In the multi-component solutions, the metals exhibited competitive adsorption on the zeolite. The removal of ions followed ion exchange (Fig. 6) for Cd2+ and Ni2+ and ion exchange and precipitation for Cu2+ and Pb2+. Moreover, the efficiency of CLP for the removal of Cr6+, Cd2+ and Zn2+ was heavily dependent on the pH (Irannajad et al., Reference Irannajad, Haghighi and Safarzadeh2016a; Jorfi et al., Reference Jorfi, Ahmadi, Pourfadakari, Jaafarzadeh, Darvishi Cheshmeh Soltani and Akbari2017). In addition, the adsorption of Cu2+ from aqueous solution was greater on Na-CLP than on its natural and Na-exchanged counterparts (Irannajad et al., Reference Irannajad, Haghighi and Safarzadeh2016b).
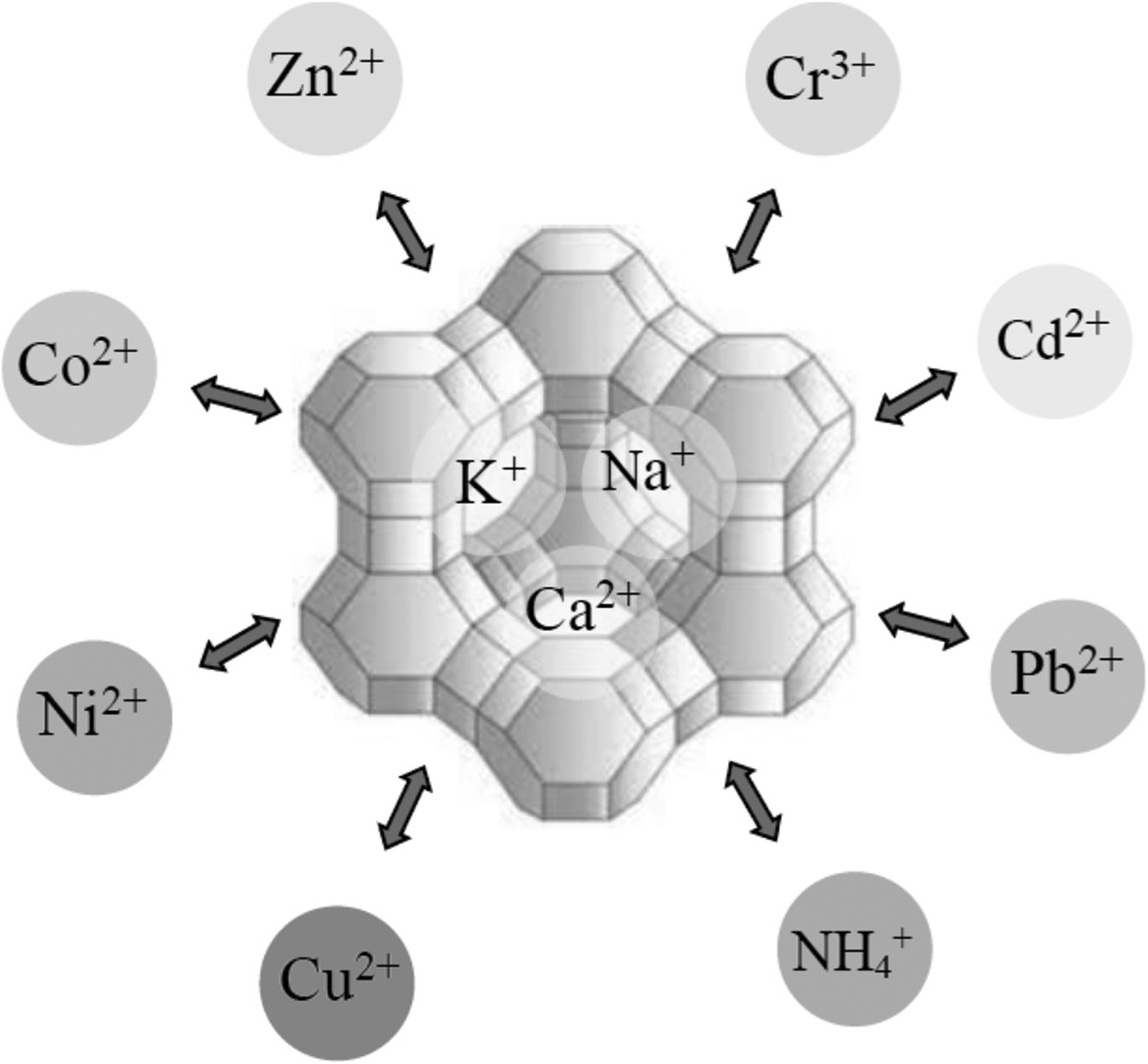
Fig. 6. Schematic representation of the cation-exchange reaction between mobile cations located in the pores of a zeolite with heavy metals and other positively charged ions (e.g. ammonium).
The removal of Ni2+ was optimized by micro- and nano-sized CLP and its modified form by dimethylglyoxime (Nezamzadeh-Ejhieh & Kabiri-Samani, Reference Nezamzadeh-Ejhieh and Kabiri-Samani2013). The modified zeolite displayed good selectivity for Ni2+ in the presence of different multivalent cations compared with the original micro- and nano-sized CLP. The CLP was effective at decreasing the Na, K, Mg, Ca, Co, HCO3, Ni, Cd, Pb, Cr, chemical oxygen demand (COD) and total coliform contents of a composting leachate when land treatment was investigated (Tabatabaei et al., Reference Tabatabaei, Najafi, Mirzaei, Nazem, Heidarpour, Hajrasoliha, Afyuni, Beigi Harchegani, Landi, Akasheh, Zamanian, Barani and Amini2012). Adsorption depended on the zeolite particle size.
A Na+-CLP exchanged with Fe3+ and Al3+ ions displayed high capacity for F– adsorption at acidic pH (Rahmani et al., Reference Rahmani, Nouri, Kamal Ghadiri, Mahvi and Zare2010). Removal of hardness, cations and anions, from drinking water was investigated using CLP (Badalians Gholikandi et al., Reference Badalians Gholikandi, Baneshi, Dehghanifard, Salehi and Yari2010). In addition, a MnO2-supported CLP displayed greater adsorption capacity for Cu2+, Cd2+ and Zn2+ ions than its FeO-supported counterpart (Aghazadeh et al., Reference Aghazadeh, Safarzadeh, Gharabaghi and Irannajad2016; Irannajad et al., Reference Irannajad, Haghighi and Soleimanipour2016c). The removal of Fe2+ from water by MnO2-modified CLP was controlled by liquid-phase molecular diffusion through the mesopores of the zeolite aggregate for particles >150 µm (Pashmineh Azar & Falamaki, Reference Pashmineh Azar and Falamaki2012). Three filtering media – a conventional slow sand filter (SSF), a slag-modified filter (SMF) and a CLP-modified filter (ZMF) – were examined for their water softening capacity. The As3+ removal, coliform bacteria removal, mean turbidity removal, electrical conductivity reduction efficiencies and cation-exchange capacity of the adsorbents tested decreased in the following order: SMF > ZMF > SSF. However, the mean total hardness removal efficiency showed the following order: ZMF > SMF > SSF (Abdolahnejad et al., Reference Abdolahnejad, Ebrahimi and Jafari2014, Reference Abdolahnejad, Jafari, Ebrahimi, Mohammadi and Farrokhzadeh2017). In another study, zeolite-based Raschig ring adsorbents with optimal physical strength and sorption capacity for removal of Pb2+ from aqueous solution were prepared using 47.5 wt.% cement kiln dust, 32.5 wt.% zeolite and 20.0 wt.% bentonite (Salem et al., Reference Salem, Afshin and Behsaz2012).
A CLP was dealuminated with oxalic acid and was subsequently treated with Ni(NO3)2 for deep desulfurization of liquid fuels. The adsorption capacity of the Ni2+-modified CLP for various sulfur compounds followed the order: iso-propyl mercaptan > thiophene > benzothiophene > dibenzothiophene (Mahmoudi & Falamaki, Reference Mahmoudi and Falamaki2016a, Reference Mahmoudi and Falamaki2016b). Moradi et al. (Reference Moradi, Karimzadeh and Moosavi2018) prepared Cu2+- and Ni2+-exchanged CLP for desulfurization of a model fuel (dibenzothiophene and dimethyl dibenzothiophene). Due to the presence of d-electrons in the ion-exchanged adsorbents, sulfur compounds interacted with the cations through π-complexation, which resulted in a significant increase in the adsorption capacity of the samples compared to their original counterparts. A CLP modified with TiO2 nanoparticles via co-precipitation displayed greater removal efficiency of dissolved natural organic matter from water compared to its natural counterpart (Tilaki & Fard, Reference Tilaki and Fard2017).
Acid activation of CLP with HCl and H2SO4 increased the specific surface area of the zeolite and increased its Si/Al ratio. HCl was more effective for activation than H2SO4 (Tehrani & Salari, Reference Tehrani and Salari2005). In addition, CLP activated with HCl showed significant adsorption capacity for acetone CLP (Aghababaei, Reference Aghababaei2016). Optimization of ammonia adsorption from aqueous solution was examined using CLP modified (MZ) with H3PO4, HNO3, H2SO4 and HCl. The adsorption efficiency for ammonia followed the order: HCl-MZ > H3PO4-MZ > HNO3-MZ > H2SO4-MZ > unmodified zeolite (Mokhtari-Hosseini et al., Reference Mokhtari-Hosseini, Ehsan, Reza and Toktam2016). In contrast, the treatment of CLP with HCl to remove carbonate and impurities and to increase porosity for NO3– removal from aqueous solution was not very efficient (Azari et al., Reference Azari, Mahvi, Naseri, Rezaei Kalantary and Saberi2014).
A CLP was used as a starting material to synthesize zeolites A and P for removal of arsenate and arsenite from drinking water (Menhaje-Bena et al., Reference Menhaje-Bena, Kazemian, Ghazi-Khonsari, Hosseini and Shahtaheri2004). The original CLP and the synthetic zeolites were modified with iron (II). The Fe-modified synthetic zeolite A was the most selective sorbent for the removal of arsenic ions.
The capacity of CLP to adsorb NH4+ from synthetic and real wastewater samples of a fertilizer-producing plant was studied in both batch and continuous (fixed bed column) experiments (Ashrafizadeh et al., Reference Ashrafizadeh, Khorasani and Gorjiara2008; Jafarpour et al., Reference Jafarpour, Foolad, Mansouri, Nikbakhsh and Saeedizade2010). The NH4+ removal was controlled by the zeolite particle size and the presence of competitive cations and anions in the solution. The selectivity order for cations was: K+ > Na+ > Ca2+ > Mg2+. For anions, the order was: Cl– > PO43– > SO42–. The presence of K+, Ca2+ and Mg2+ cations in solution decreased NH4+ adsorption, whereas the organic acids increased NH4+ adsorption on CLP. A high level of regeneration (90%) was achieved with a relatively small volume of 1 N NaCl solution. The brine of the regeneration column was transferred to the air-stripping column for conversion of NH4+ ions to gaseous NH3 (Rahmani et al., Reference Rahmani, Samadi and Ehsani2009). The ion-exchange capacity increased with decreasing zeolite particle size. The natural CLP could potentially be used as a controlled-release NH4+ fertilizer (Malekian et al., Reference Malekian, Abedi-Koupai, Eslamian, Mousavi, Abbaspour and Afyuni2011). Moreover, CLP removed NH4+ and humic acid from surface waters. Removal of both components was greater in binary systems compared to experiments with single components (Moussavi et al., Reference Moussavi, Talebi, Farrokhi and Mojtabaee Sabouti2011). In addition, a CLP modified with permaganate adsorbed up to 6.7 mg/g of NO3– from synthetic wastewater under optimal conditions (Mohsenibandpei et al., Reference Mohsenibandpei, Alinejad, Bahrami and Ghaderpoori2016).
Natural Iranian CLP modified with cationic hexadecyltrimethyl ammonium chloride (HDTMA-Cl) and N-cetylpyridinium bromide (CPB) surfactants exhibited significant adsorption capacity for monoaromatic hydrocarbons such as benzene, toluene, ethylbenzene and xylene isomers (BTEX) (Torabian et al., Reference Torabian, Kazemian, Seifi, Bidhendi and Ghadiri2010). By increasing the surfactant loading, increases in adsorption capacity of 60–70% for HDTMA-CLP and of 47–99% for CPB-CLP were observed. Moreover, the granulated nanoparticles of a CLP modified with CPB and HDTMA were evaluated as potential adsorbents for the removal of BTEX (Seifi et al., Reference Seifi, Torabian, Kazemian, Nabi Bidhendi, Azimi and Charkhi2011). The adsorption capacity of the granulated zeolite nanoparticles was significantly higher than those of micron-sized natural zeolite. Finally, surfactant-modified zeolite was used for the removal of methyl tert-butyl ether (MTBE) from aqueous solutions. The HDTMA-modified CLP exhibited higher MTBE adsorption compared to its CPB-CLP counterpart (Ghadiri et al., Reference Ghadiri, Nabizade, Mahvi, Nasseri, Kazemian, Mesdaghinia and Nazmara2010).
Natural zeolites in aquaculture
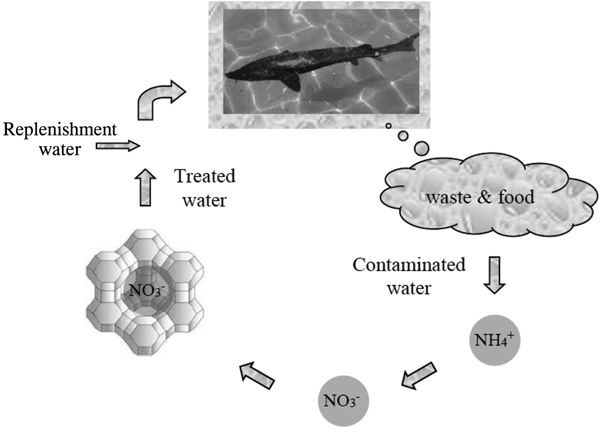
Water quality management in recirculation aquaculture systems requires low-cost treatment methods with high capacity (Fig. 7). Granulated CLP has been applied to help prevent acute toxicity of total ammonia (10–35 mg/L) to Huso huso (Farhangi et al., Reference Farhangi, Gholipour Kanani and Kashani2014), Acipenser persicus (Farhangi & Rostami-Charati, Reference Farhangi and Rostami-Charati2012), beluga (Asgharimoghadam et al., Reference Asgharimoghadam, Gharedaashi, Montajami, Nekoubin, Salamroudi and Jafariyan2012), rainbow trout (Ghiasi & Jasour, Reference Ghiasi and Jasour2012), angelfish (Pterophyllum scalare) (Ghiasi & Jasour, Reference Ghiasi and Jasour2012) and Mazandaran common carp (Cyprinus carpio) (Ghiasi & Jasour, Reference Ghiasi and Jasour2012). Most ammonia adsorption was recorded during the first 12 h of the experiment. Addition of 10–15 g/L of zeolite was the optimal dose for improvement of water quality and fish-growth performance. Total hardness was the same in the treatment groups and the control, while the NH4+ concentration was higher in the control. By increasing the amount of zeolite in each treatment, the survival rate of fish increased significantly. Prior to zeolite application, major lesions included haemorrhage, hyperaemia, hyperplasia, epithelial cell necrosis, degenerated tubules of the kidneys, expansion of Bowman's capsules in the kidneys and hepatocyte necrosis in liver. The use of zeolite improved fish physiological and immunological characteristics and increased immune responses. Some positive effects on physiological functions were observed for juvenile rainbow trout fed with nanostructured zeolite (Alinezhada et al., Reference Alinezhada, Faridi, Falahatkar, Nabizadeh and Davoodi2017). A nanochitosan/CLP composite showed greater potential to increase growth performance, digestive amylase activity and some biochemical parameters in rainbow trout compared to chitosan/CLP (Sheikhzadeh et al., Reference Sheikhzadeh, Kouchaki, Mehregan, Tayefi-Nasrabadi, Divband, Khataminan and Shabanzadeh2017).
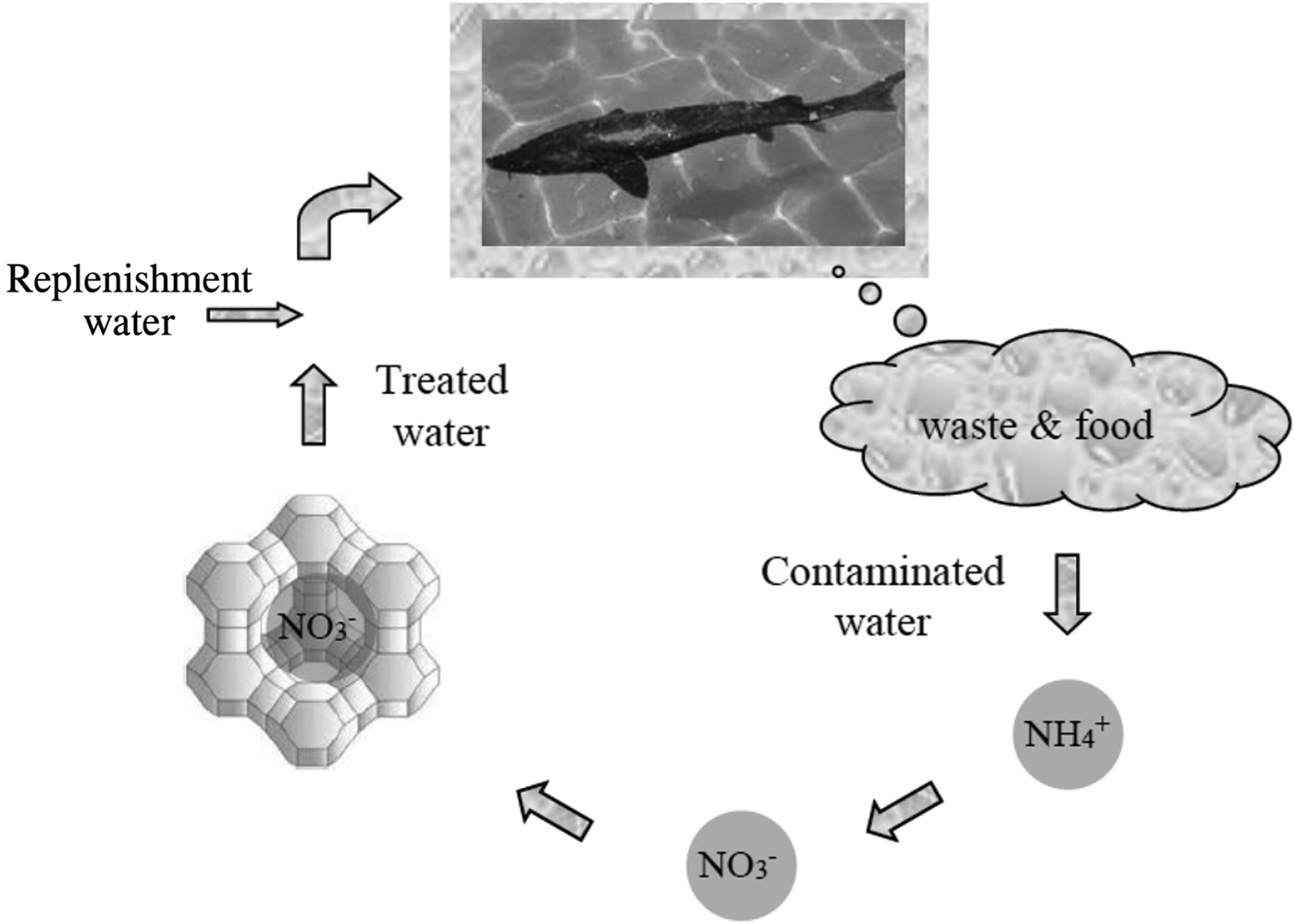
Fig. 7. Ammonia removal by zeolite for the purification of contaminated water in aquaculture.
Natural Iranian zeolites in soil remediation and agriculture
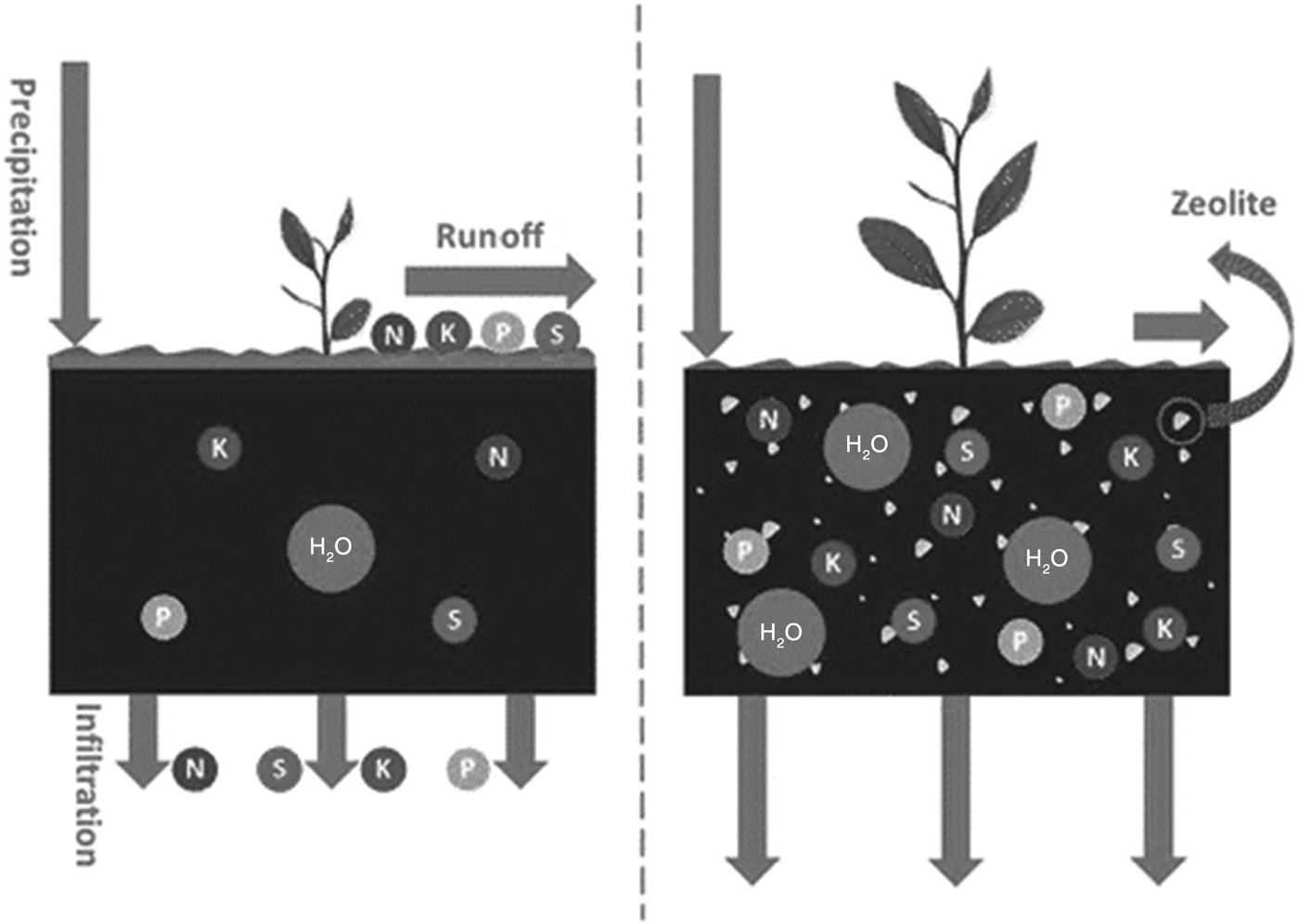
Innovative strategies are needed to improve water and nutrient-use efficiencies for sustainable agricultural production, particularly in sandy soils. For instance, application of slow-release fertilizers in soils would reduce the environmental impact of using soil nutrients such as urea. For better management of fertilizer application and to control NO3– leaching, especially under the flooded irrigation of paddy fields, the application of natural zeolites might be helpful. The existence of large channels allowing a fluid to pass through, the ability to lose and gain water reversibly, the high cation-exchange capacity and the surface properties of zeolites make them suitable for agricultural uses. The application of zeolite at 2 g/kg soil is sufficient to adsorb the NH4+ applied and inhibit its leaching due to water flow (Sepaskhah & Yousefi, Reference Sepaskhah and Yousefi2007). Ammonium-saturated zeolite acts as a slow-release fertilizer, and this may be considered an environmentally friendly strategy to enhance uptake of soil micro- and macro-nutrients by plants. NH4+/K+-saturated CLP and chabazite were prepared and applied in column leaching tests using a sandy soil amended with chemical fertilizers and N/K-saturated zeolites. The NH4+ and K+ losses from soils amended with zeolite were less than those with chemical fertilizers. Chabazite was more effective than CLP at preventing NH4+ loss during leaching, whereas reduction of K+ loss was similar for both zeolites (Eslami et al., Reference Eslami, Khorassani, Coltorti, Malferrari, Faccini, Ferretti, Di Giuseppe, Fotovat and Halajnia2018). The influence of zeolite application was investigated under rainfall simulation to control for the effects of freeze–thaw on basic hydrological variables such as runoff production and soil loss. The application of zeolite had significant effects on the hydrological behaviour of soil induced by freeze–thaw cycles. Employing zeolite may be an effective amendment that could reduce soil erosion in steep and degraded rangelands where the surface is exposed to rainfall and runoff (Fig. 8) (Behzadfar et al., Reference Behzadfar, Sadeghi, Khanjani and Hazbavi2017).
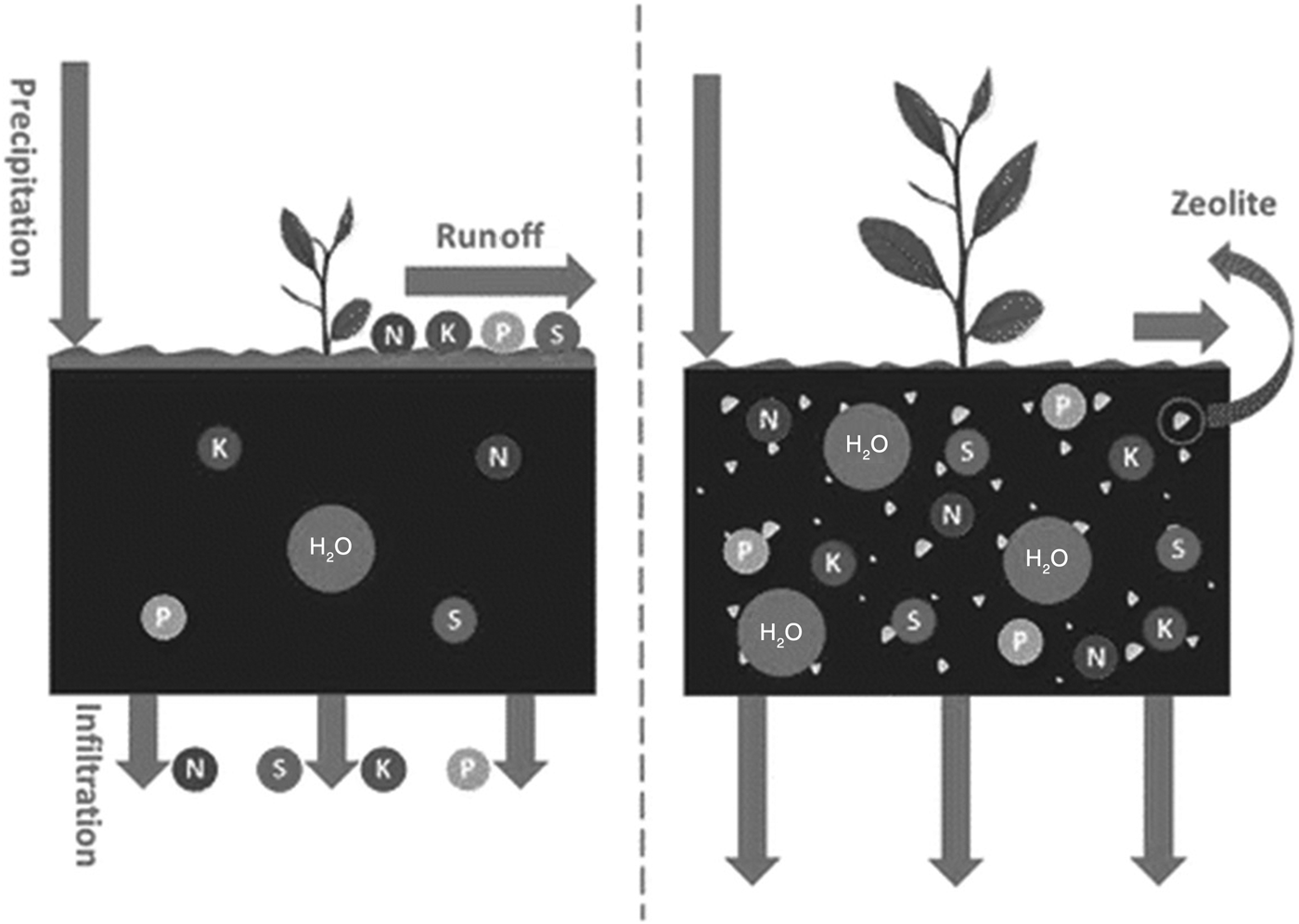
Fig. 8. Schematic illustration of the application of zeolites in soil remediation and nutrient retention.
The production yields of lettuce and red tilapia seedlings were greater with treatment using a small cotton bag containing CLP compared to the control treatment in an aquaponic system without zeolite (Rafiee & Saad, Reference Rafiee and Saad2010). Moreover, the concentration of total ammonia-N in the residual water was significantly lower in the zeolite treatment than in the control sample. The effluent produced from the carp breeding in the experiment unit was entered into an alfalfa culturing medium; therefore, a closed cycle was established. The application of zeolite and nitrifying bacteria to soil and the conversion of ammonia to nitrate occurs during nitrification, which improves the water quality of aquaculture and increases the uptake of nitrate by plants, thereby reducing water and soil pollution (Motesharezadeh et al., Reference Motesharezadeh, Arasteh, Pourbabaee and Rafiee2015). K-CLP application under field conditions in a coarse-textured rice field increased significantly the soil-available K+ and its uptake by rice straw (Kavoosi, Reference Kavoosi2007). The highest K+ uptake was observed in a treatment with zeolite and urea. Application of CLP increased the available N+, K+, P3+, Ca2+ and Mg2+ of the medium, the net photosynthetic rate and the water use efficiency in the growth and flowering of strawberries (Abdi et al., Reference Abdi, Khosh-khui and Eshghi2006). The application of zeolite and foliar selenium (Na2SeO4) to the growth and yields of three canola cultivars in a field trial had significant positive effects on traits related to yield. Zeolite decreased respiration, malondialdehyde and proline in salt-stressed plants. Soluble sugars and potassium contents increased in response to zeolite application, while sodium contents decreased significantly. Selenium led to an increase in plant height, silique number, seed number in silique, biological yield, harvest index and oil percentage, while respiration, malondialdehyde, proline and sodium decreased with selenium application. Similarly, silicon had a significant effect on growth and agronomic traits. Silicon promoted chlorophyll synthesis while preventing malondialdehyde, proline and sodium accumulation in plant tissues (Zahedi et al., Reference Zahedi, Noormohamadi, Shirani Rad, Habibi and Akbar Boojar2009; Bybordi, Reference Bybordi2016).
The K+, Ca2+- and combined K+,Ca2+-zeolites had significant effects on saffron (Corocus sativus), the most valuable medicinal and spice plant, in terms of emergence time and percentage; however, there were no significant differences when using different zeolite levels (Ahmadee et al., Reference Ahmadee, Khashei Suiki and Sayyari2014). The number of surviving plants decreased during the plant growth period under drought stress for two rangeland species (Ziziphose spina-christi and Acacia salicina), but a significant difference was observed between control pots without CLP and pots treated with zeolite surviving (Ghazavi et al., Reference Ghazavi, Vali and Mohammadesmaili2013). Well-watered plants using zeolite produced the greatest seed yield of mung bean (Vigna radiata L.), and irrigation disruption at flowering initiation without CLP application produced the lowest yield (Pirzad et al., Reference Pirzad, Jalilian and Akbari Bavandi2015). The maximum seed protein content in sunflower (Helianthus annuus L.) was achieved with a combination of urea, composted cattle manure and CLP treatment, while the minimum seed protein content was observed in urea treatment (Gholamhoseini et al., Reference Gholamhoseini, Ghalavand, Khodaei-Joghan, Dolatabadian, Zakikhani and Farmanbar2013). The vermin-compost treatment yielded maximum growth characteristics of Zinnia flowers, whereas minimum growth parameters were observed with zeolite treatment (Amjazi & Hamidpour, Reference Amjazi and Hamidpour2012). The effect of drought stress and application of CLP was evaluated in terms of morphological and physiological traits of mallows in greenhouse conditions (Ahmadi Azar et al., Reference Ahmadi Azar, Hasanloo, Imani and Feiziasl2015). The greatest shoot fresh weight, root and shoot length, stomata conductance, electrolytic leakage and chlorophyll contents were observed upon zeolite treatment and 100% moisture field capacity. The application of zeolite in combination with soil prevented water loss and facilitated water availability to the plant. Application of both organic residues (compost) and light-expanded clay aggregates (LECAs)/zeolite conserved soil moisture and reduced evaporation, temperature fluctuation and mechanical resistance (Judy & Movahedi Naeini, Reference Judy and Movahedi Naeini2007). Application of CLP increased essential oil yield of the medicinal plant Dracocephalum moldavica L. and increased N+ and K+ concentration, essential oil content and yield in organic cultivation of German chamomile (Matricaria chamomilla) (Salehi et al., Reference Salehi, Ghalavand, Sefidkon and Asgharzade2011; Karimzadeh et al., Reference Karimzadeh, Sefidkon, Majnoon Hosseini and Peighambari2014). The effects of CLP on salinity and the presence of harmful salts (NaCl and Na2SO4) in soil were studied on Raphanus sativus L. cultivation. Using CLP improved soil quality and increased the quantity and quality of the crop (number of leaves, total leaf area, total fresh weight, total dry weight, root fresh weight, air fresh weight, root dry weight and air dry weight) (Noori et al., Reference Noori, Zendehdel and Ahmadi2006).
The release of Cd2+, Ni2+ and Pb2+ from the metallurgical industry, ceramic manufacturing, electroplating and textile printing in soil causes toxicity in plants and their products. Using 15% CLP in different soil textures (clay, loam and sand) decreased the Cd2+, Ni2+ and Pb2+ contents in the leachate solution by >90% after soil treatment with CaCl2 (Ansari Mahabadi et al., Reference Ansari Mahabadi, Hajabbasi, Khademi and Kazemian2007). After application of zeolite, the soil with a clayey, loamy texture trapped Cr3+, Pb2+, Ni2+ and Cd2+ from influent leachate more strongly than the control soil with a loamy, sandy texture (Mirzaei et al., Reference Mirzaei, Heidarpour, Tabatabaei, Najafi and Hashemi2013). The application of CLP in sewage sludge decreased the Pb2+ and Cd2+ contents in shoots and roots of maize (Zea mays L.). Using superadsorbents is one of the solutions to water shortages in arid and semiarid regions. Application of CLP as a superadsorbent had a positive effect on the establishment of plants and on other indices such as plant height, large and small canopy diameter and collar diameter of Nitraria schoberi L. (Zareian et al., Reference Zareian, Jafari, Javadi and Tavili2018).
Natural Iranian zeolites in animal feed
The recent increase in the use of natural zeolites in animal feed as an anti-caking/flowing agent has improved feed production and efficiency and animal health and, in some cases, has provided protection against mycotoxin intoxication. Applications of natural zeolites in animal husbandry are based on their ability to adsorb ammonia/ammonium, to lose/gain water reversibly and selectively to trap cations such as heavy metals by exchange with alkaline and alkaline earth cations in their porous structure. However, studies on the effects of zeolites on food intake, weight gain, growth rate, egg production, egg weight, shell thickness and internal egg characteristics are contradictory. While some studies report beneficial effects due to the inclusion of zeolites in the diet of birds, other studies were inconclusive or suggested negative effects. The type and dosage of natural zeolite and the impurity contents are the main factors controlling their utilization in animal feeding. Zeolites have beneficial effects on the feed efficiency ratio, water consumption, nutrient utilization, manure and litter condition and, more importantly, on aflatoxicosis (Shariatmadari, Reference Shariatmadari2008). Iranian CLP has already found widespread domestic use in the catfish-rearing industry and as an animal feed supplement (Ashrafizadeh et al., Reference Ashrafizadeh, Khorasani and Gorjiara2008).
A study on the short-term effects of CLP in colostrum and milk on the serum mineral concentrations of neonatal dairy calves based on blood samples taken from calves after birth and after a few weeks of life showed significant effects on the Ca2+, P+, Na+ and Fe2+ concentrations and no effects on K+ and Mg2+ concentrations (Mohri et al., Reference Mohri, Seifi and Maleki2008). A similar study evaluated the effects of CLP on the adsorption of immunoglobulins from colostrum and the incidence of enteric diseases of Holstein calves. Addition of 1 g of CLP per kilogram of body weight per day to colostrum and milk could reduce diarrhoea, but its effect on passive immunity was negligible (Sadeghi & Shawrang, Reference Sadeghi and Shawrang2008). The use of CLP exhibited the best effects on serum immunoglobulins (IgG and IgM) and vitamin A adsorption, average daily gain and reduction of faecal scores in new-born Holstein calves. The crude protein and metabolizable energy conversion ratios of male Varamin lambs were improved by CLP. In addition, Arabic lambs receiving CLP in feed had less plasma glucose and blood urea nitrogen, whereas crude protein was increased (Ghaemnia et al., Reference Ghaemnia, Bojarpour, Mirzadeh, Chaji and Eslami2010).
Diets of broiler chickens containing kaolin and zeolite increased blood total protein, glucose content and growth hormone and decreased triglyceride compared to the control, whereas no significant differences were observed for the levels of globulin, urea, creatinine and cholesterol (Safaeikatouli et al., Reference Safaeikatouli, Jafariahangari and Baharlouei2011). Nanozeolite (~1%) decreased the adverse effects of aflatoxins on the colour and oxidative stability of broiler chicken thigh meat (Shabani et al., Reference Shabani, Dastar, Hassani, Khomeiri and Shabanpour2016). Addition of kaolin, bentonite and zeolite to the broiler chicken diet caused weight increases; faecal moisture in the treatments with the silicate minerals varied between trial group and control samples, but this did not cause any difference in internal organ or faecal pH values (Safaei Katouli et al., Reference Safaeikatouli, Boldaji, Dastar and Hassani2010). Finally, the nutritional responses in terms of weight gain, specific growth rate, food conversion ratio and survival rate were improved considerably after application of CLP as antibacterial, antifungus and antitoxin agents in the digestive system of kutum fish (Rutilus frisii kutum) (Navirian et al., Reference Navirian, Sotohian and Mostafazadeh2011).
Modified natural Iranian zeolites as catalysts and photocatalysts
Iron loading on CLP produced a heterogeneous Fenton-like system that resulted in nearly total elimination of phenol and 70% COD removal (Bayat et al., Reference Bayat, Sohrabi and Royaee2012). The CLP catalyst retained activity and stability for a period of 30 h on continuous stream in a packed bed reactor. A CLP loaded with MnO, Ag2O and MnO–Ag2O displayed photodegradation of methylene blue in the following order: MnO-Ag2O2-CLP > MnO/CLP and Ag2O/CLP (Karimi-Shamsabadi & Nezamzadeh-Ejhieh, Reference Karimi-Shamsabadi and Nezamzadeh-Ejhieh2016). A CLP was loaded with CuFe2O4 and TiO2 nanoparticles using solid-state dispersion and was applied successfully for photocatalytic degradation of acid red in water (Nikazar et al., Reference Nikazar, Gholivand and Mahanpoor2008; Bagheri Ghomi & Ashayeri, Reference Bagheri Ghomi and Ashayeri2012). The CLP composite had superior degradation efficiency in comparison to the CuFe2O4 and TiO2 nanoparticles and the original CLP. A TiO2/CLP nanocomposite prepared by calcination followed by ion exchange with ammonium titanyl oxalate monohydrate was used for photodegradation of tetracycline (pharmaceutical antibiotic) under visible light (Saadati et al., Reference Saadati, Keramati and Mehdipour Ghazi2016) and a mixture of aniline and dinitroaniline aqueous solution (Zabihi-Mobarakeh & Nezamzadeh-Ejhieh, Reference Zabihi-Mobarakeh and Nezamzadeh-Ejhieh2015). The original and the micronized CLP did not display significant photocatalytic activity and the pure TiO2 also displayed lower photocatalytic activity compared to the TiO2-CLP and the TiO2-micronized CLP. The Fe-CLP composite was also used successfully as a catalyst for the photodegradation of furfural in a wastewater sample under Hg lamp irradiation (Mousavi-Mortazavi & Nezamzadeh-Ejhieh, Reference Mousavi-Mortazavi and Nezamzadeh-Ejhieh2016).
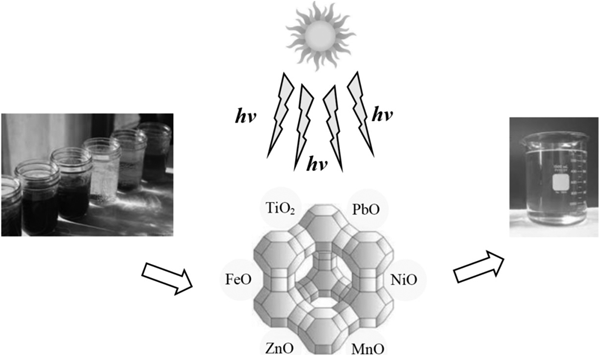
TiO2/Fe2O3 and ZnO/Fe2O3 nanoparticles loaded on CLP were used for photodegradation of diphenhydramine from contaminated water, with the former showing better performance (Davari et al., Reference Davari, Farhadian, Nazar and Homayoonfal2017). A CLP impregnated with Co was used for direct decomposition of N2O, a greenhouse gas (Ghahri et al., Reference Ghahri, Golbabaei, Vafajoo, Mireskandari, Yaseri and Shahtaheri2017). A protonated CLP showed a promising performance for NOx abatement via selective catalytic reduction by propane (Ghasemian et al., Reference Ghasemian, Falamaki and Kalbasi2014). The performance of zero-valent Fe0-coated CLP in the chemical reduction of NO3– in unbuffered conditions was enhanced by coating small amounts of Cu0 onto the freshly prepared Fe0/zeolite composite (Fateminia & Falamaki, Reference Fateminia and Falamaki2013). Nanoparticles ground by ball-milling were ion exchanged in a ferrous solution and calcined at 450°C to obtain FeO-nano-CLP, which was used for photodegradation of tetracycline pharmaceutical capsules in an aqueous solution under Hg lamp irradiation. The order of degradation reactivity of the samples was as follows due to the larger effective surface area of the nanoparticles: FeO-nano-CLP > Fe-nano-CLP > FeO-CLP (Nezamzadeh-Ejhieh & Shirzadi, Reference Nezamzadeh-Ejhieh and Shirzadi2014). Composite NiO/nano-CLP was prepared by a similar procedure from NiCl2 aqueous solution. The catalysts obtained were used in the photodegradation of cefuroxime using Hg lamp irradiation, and they displayed greater photocatalytic activity than pure NiO nanopowders (Pourtaheri & Nezamzadeh-Ejhieh, Reference Pourtaheri and Nezamzadeh-Ejhieh2015). The photocatalytic degradation efficiency of nitrophenol aqueous solution was enhanced by 22% using ZnO/nano-CLP, with respect to ZnO/micro-CLP, under UV irradiation. Proper addition of H2O2 and potassium bromate as electron acceptors might improve the photodegradation rate of nitrophenol (Nezamzadeh-Ejhieh & Khorsandi, Reference Nezamzadeh-Ejhieh and Khorsandi2014). FeO, ZnO and the hybridized FeO–ZnO were supported on nano-CLP particles and used in the photocatalytic degradation of the pollutants present in a fish pond (Bahrami & Nezamzadeh-Ejhieh, Reference Bahrami and Nezamzadeh-Ejhieh2014). In addition, CuO was incorporated onto CLP via wet impregnation with CuSO4 aqueous solution followed by calcination and was used efficiently as a photocatalyst to degrade a p-aminophenol aqueous solution (Nezamzadeh-Ejhieh & Amiri, Reference Nezamzadeh-Ejhieh and Amiri2013). Photodegradation of organic dyes by metal oxide-loaded zeolite is illustrated schematically in Fig. 9.
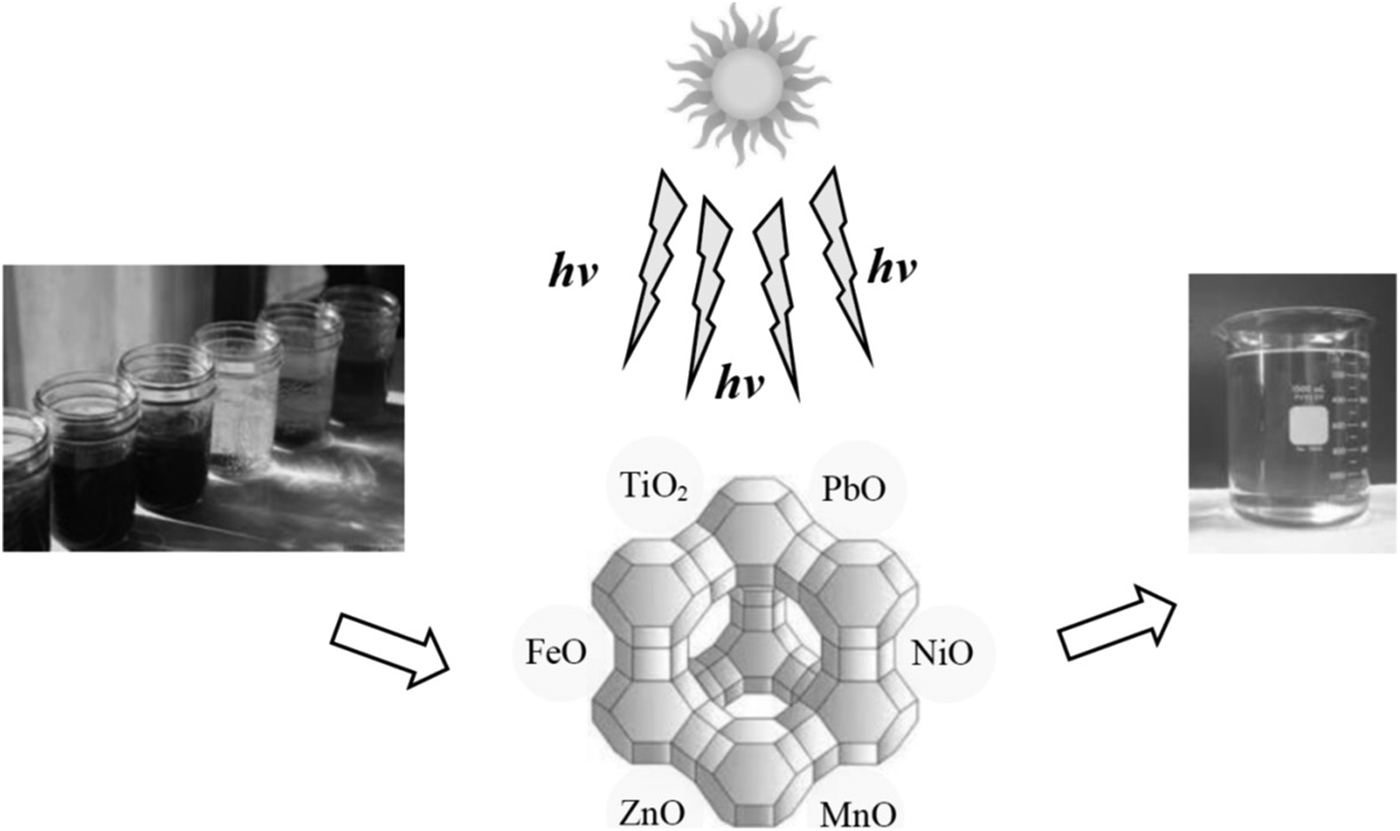
Fig. 9. Schematic representation of photodegradation of organic dyes by metal oxide-loaded zeolite.
NiO/PbO and NiS/PbS were supported on CLP nanoparticles and were used in photodegradation of nitrophenol. The NiO/PbO-NCP demonstrated greater photocatalytic activity than its NiS/PbS counterpart (Babaahamdi-Milani & Nezamzadeh-Ejhieh, Reference Babaahamdi-Milani and Nezamzadeh-Ejhieh2016). CdS loaded onto CLP by ion exchange and precipitation procedures was used for the photodegradation of p-aminophenol in aqueous solution under UV irradiation (Nezamzadeh-Ejhieh & Shirvani, Reference Nezamzadeh-Ejhieh and Shirvani2013). In a similar procedure, a NiS-CLP composite was prepared and used for photodegradation of furfural in aqueous solution under UV irradiation and showed greater efficiency than direct photolysis (Nezamzadeh-Ejhieh & Moeinirad, Reference Nezamzadeh-Ejhieh and Moeinirad2011). Photodegradation of a mixture of methyl orange (MO) and bromocresol was investigated using NiO, CdO, ZnO, FeO, CuS, NiS, CdS, ZnS and FeS doped in CLP. The catalysts were prepared by calcination (oxide forms) and sulfurization (sulfide forms) methods followed by ion exchange. The order of decolourization efficiency of the various photocatalysts was: CuS > ZnO > FeO > CdS > NiS > ZnS > CdO > NiO > FeS (Nezamzadeh-Ejhieh & Moazzeni, Reference Nezamzadeh-Ejhieh and Moazzeni2013). The photo-decolourizing ability of MO from aqueous solution was reported using Fe0 nanoparticles supported on natrolite zeolite nanoparticles (NANPs-FeNPs). The order of reactivity of the catalysts was: photo-NANPs-FeNPs-H2O2 > photo-NANPs-H2O2 > photo-NANPs-FeNPs > photo-H2O2 > NANPs-FeNPs-H2O2 (Naderpour et al., Reference Naderpour, Noroozifar and Khorasani-Motlagh2013).
Nano-sized CLP was employed as a catalyst for the synthesis of pharmaceutically active 2-amino-4H-chromene derivatives and demonstrated its greater preference compared to other tested catalysts (Baghbanian et al., Reference Baghbanian, Rezaei and Tashakkorian2013). A heterogeneous catalyst for the liquid-phase epoxidation of alkenes has been synthesized by introducing nanocluster polyoxomolybdate into a CLP (Bagherzadeh & Hosseini, Reference Bagherzadeh and Hosseini2017). In addition, a polyaniline/CLP nanocomposite with enhanced corrosion protection (anticorrosive) properties in acidic environments in comparison to pure polyaniline coating was prepared by chemical oxidative polymerization of anilinium cations (Olad & Naseri, Reference Olad and Naseri2010). Moreover, plasma-modified CLP nanorods were prepared from CLP using environmentally friendly corona discharge plasma. The catalytic performance of a plasma-modified clinoptilolite in the heterogeneous sono-Fenton-like process was greater than that of CLP for the treatment of phenazopyridine (Khataee et al., Reference Khataee, Sadeghi Rad, Vahid and Khorram2016).
Some Iranian natural CLP zeolites have displayed catalytic properties in different systems such as methanol and ethanol/bioethanol dehydration (Karimi et al., Reference Karimi, Ghobadian, Omidkhah, Towfighi and Yaraki2016) and as catalyst supports for liquid-phase cation reduction and para-nitrophenol hydrogenation (Ghasemian et al., Reference Ghasemian, Falamaki and Kalbasi2014). CLP nanopowders (<100 nm) of desirable crystal order were produced by application of appropriate wet and dry ball-milling. Nevertheless, this method led to a wide size distribution of ground powders in the range of <100 nm–30 µm and loss of crystal order of ~55–100% (Charkhi et al., Reference Charkhi, Kazemian and Kazemeini2010). The potential of nano-sized CLP (53.9 nm) for methanol dehydration has been investigated in the direct conversion of syngas to dimethyl ether after modification by HNO3 treatment. The catalytic performance of synthesized samples showed that the optimal CuO–ZnO–Al2O3 to CLP ratio was 4:1 (Khoshbin et al., Reference Khoshbin, Haghighi and Asgari2013). Dehydration of methanol to dimethyl ether using CLP was also studied in a continuous fluidized bed reactor. Among the operating parameters, partial pressure of methanol had the greatest impact on the process yield (Kasaie & Sohrabi, Reference Kasaie and Sohrabi2009).
Antibacterial and health-related applications of natural zeolites
Clinoptilolite is an adsorbent with a significant adsorption efficiency, and it is capable of the filtration and purification of air contaminated with Pseudomonas aeruginosa bacteria. The indirect use of Ag nanoparticles for reduction of fungal infections during the incubation period of fertilized rainbow trout eggs was investigated. Different concentrations of nanosilver-coated zeolite (AgNPs) were compared with unmodified CLP used as water filter media in semi-recirculation systems. The filters containing 0.5% AgNPs increased the survival rate by 4.5% from fertilization to the swim-up stage compared to the control. Moreover, the additional application of activated carbon (as adsorbent media) along with AgNP-coated media in filters led to an increase of nearly 11.2% in the survival rate for the larval stage (Johari et al., Reference Johari, Kalbassi, Soltani and Yu2015). The antibacterial properties of Cu2+ and Cu2+/Ni2+ nanoparticles loaded on NaP zeolite (obtained by hydrothermal conversion of CLP) against Bacillus subtilis and Escherichia coli have been investigated. The ultrasonic-assisted loaded nanoparticle had greater activity against bacteria compared to the conventionally loaded CLP (Behin et al., Reference Behin, Shahryarifar and Kazemian2016a, Reference Behin, Kazemian and Rohani2016b). A Zn2+-loaded zeolite/graphene oxide nanocomposite as a new drug carrier was synthesized. The prepared nanocomposite was cytocompatible and had a high loading capacity and slow release performance for doxorubicin, which is a cancer drug (Khatamian et al., Reference Khatamian, Divband and Farahmand-zahed2016).
Addition of 5% and 12.5% of CLP to rat feed improved significantly their short-term, medium-term and long-term memory performance (Nikpey et al., Reference Nikpey, Kazemian, Safari-Varyani, Rezaie and Sirati-Sabet2013). The improvement in memory function may be attributed in part to the indirect effects of the cation-exchange property of zeolites that reduced the bioavailability of lead.
Concrete additive and other applications
Nano-silica and micron-sized CLP may replace Portland cement constituents. The effects of these additives on the compressive strength, tensile strength and durability of cylindrical specimens were investigated. The mechanical properties displayed moderate improvement and the penetration of chloride ions decreased and electrical resistivity increased significantly, thus increasing the corrosion control of reinforced concrete structures (Eskandari et al., Reference Eskandari, Vaghefi and Kowsari2015). The study of LECAs and CLP as internal curing agents showed that LECAs have significant adsorption capacities and are able to release water at high relative humidity, whereas CLP adsorbs most of the water in nanometre-sized pores and retains the water down to low relative humidity levels (Ghourchian et al., Reference Ghourchian, Wyrzykowski, Lura, Shekarchi and Ahmadi2013).
A modified PVC-membrane electrode with tetra-butylammonium bromide-CLP nanoparticles was fabricated and used successfully as an indicator electrode in the titration of oxalate ions with CaCl2 solution. The proposed electrode was also used in direct potentiometric determination of oxalate in many real samples, such as mushrooms, black and green tea, spinach and beets (Hoseini & Nezamzadeh-Ejhieh, Reference Hoseini and Nezamzadeh-Ejhieh2016).
Finally, a new carbon-paste electrode was developed using CLP modified by the Fe2+ ion for the measurement of monohydrogen arsenate (Mazloum Ardakani et al., Reference Mazloum Ardakani, Karimi, Mashhadizadeh, Pesteh, Azimi and Kazemian2007). The electrode exhibited a linear response to monohydrogen arsenate over a wide concentration range. The ion-selective electrode was accurate in the pH range of 7–11 and was tested successfully for the determination of arsenic in water and wastewater samples.
Conclusion
While finding official reports and consistent statistical data on Iran's natural zeolite deposits may be difficult, in this review, a comprehensive report was compiled based on reliable and trusted sources and some technical reports in the Persian language in order to present a clear picture of Iran's natural zeolite reserves. High-quality zeolite deposits containing mainly CLP are widespread in various regions throughout the country. Tens of millions of tons of natural zeolites were proven, which are still underdeveloped. Currently, Semnan Province is the main hub of zeolite deposits, with numerous registered and active zeolite mines and zeolite suppliers. East Azerbaijan and Tehran Provinces also have good potential for the extraction of high-quality natural zeolites.
Extensive studies have been conducted on natural zeolites by numerous research teams in Iran. These include the development of new value-added products and processes for various applications, from water/wastewater treatment to soil remediation, agriculture and horticulture, animal husbandry and aquaculture. Based on the scientific reports published on natural zeolites by Iranian researchers since 2005, ion-exchange (adsorption), separation and catalytic applications of natural and modified zeolites are the main areas of active research in the country. Conducting more applied research towards developing value-added products based on natural zeolites is an obvious necessity not only for zeolite researchers in Iran, but also worldwide.
Author ORCIDs
H. Kazemian, 0000-0002-8021-4548.