INTRODUCTION
Pelagic sharks are important components of the oceanic pelagic ecosystem, functioning as apex predators and scavengers, exerting significant impact on other species of the marine food web (Cortés, Reference Cortés1999; Kitchell et al., Reference Kitchell, Essington, Boggs, Schindler and Walters2002). The population dynamics of most of these sharks are characterized by slow growth, late maturity and low fecundity and therefore they can withstand only modest level of fishing without depletion and stock collapse (Camhi et al., Reference Camhi, Fowler, Musick, Bräutigam and Fordham1998). Due to increasing demands for shark flesh for human consumption, fins for shark fin soup, liver oil for vitamin extraction and hides for leather (Compagno, Reference Compagno2002), sharks are increasingly exploited both by targeted fisheries and as by-catch of fisheries targeting tunas and swordfish (Stevens et al., Reference Stevens, Bonfil, Dulvy and Walker2000). Scientists have documented growing concern over the widespread decline of shark populations due to this increased fishing mortality and had advocated for immediate adoption of management measures for arresting the rapid depletion of sharks (Stevens et al., Reference Stevens, Bonfil, Dulvy and Walker2000). Detailed information on the life history traits is essential for identifying suitable management measures for a given species. Studies on the maturity on sharks are of high importance in their management, since the attainment of sexual maturity has substantial impact on their distribution, behaviour and biology (Francis & Duffy, Reference Francis and Duffy2005).
The pelagic thresher (Alopias pelagicus Nakamura, Reference Nakamura1935), bigeye thresher (A. superciliosus Lowe, 1841), oceanic whitetip shark (Carcharhinus longimanus (Poey, 1861)), tiger shark (Galeocerdo cuvier (Péron & Lesueur, 1822)), shortfin mako (Isurus oxyrinchus Rafinesque, 1810), longfin mako (I. paucus Guitart, 1966) and blue shark (Prionace glauca (Linnaeus, 1758)) constitute a significant proportion of oceanic sharks harvested in India (Varghese et al., Reference Varghese, Vijayakumaran, Tiburtius and Mhatrein press). Sarangdhar (Reference Sarangdhar1943, Reference Sarangdhar1949) described the pregnant tiger sharks and their embryos collected from the shark landings at Bombay, India. However, there is a dearth of detailed information on the reproductive aspects of these oceanic pelagic sharks in Indian seas. In this perspective, the present study aimed to describe the size, sex and maturity of seven pelagic sharks caught in the oceanic fishery of the eastern Arabian Sea.
MATERIALS AND METHODS
Pelagic shark specimens for this study were collected from the sharks landed at the Cochin fisheries harbour (south-west India, 9°56′N 76°15′E) during January 2013 to December 2014. Monthly samplings were made on the shark landings by mechanized drift gillnet-cum-longline fleet based at Cochin. This fishery deploys about 210 mechanized boats of 10–20 m overall length (L OA), targeting large pelagics. The drift gillnets operated by this fishery are of maximum 2000 m length and 11 m hung depth with mesh size 100–350 mm, whereas monofilament longlines with steel wire leaders and circle, ‘J’ or tuna hooks, usually with live baits are used in longline operations, deploying 500–1000 hooks in a day's operation. The fishing area spans the entire western Indian Exclusive Economic Zone (EEZ, Figure 1). Fishing operations were suspended in June and July every year due to the closed fishing season.

Fig. 1. Map of the study area, the western Indian Exclusive Economic Zone (eastern Arabian Sea).
The total lengths (L T) of individual sharks were measured using a measuring tape and sexes were discriminated by examining the presence of claspers. In males, the outer clasper length (C LO) was measured from the clasper tip to the edge of pelvic fin and the condition of the claspers were noted before the fish was eviscerated at the harbour. Males were classified as immature (claspers are non-calcified or semi-calcified, testes are soft, elongated and not lobated or starting to lobate) and mature (claspers are fully calcified and can be rotated 180 degrees, rhipidion can be splayed easily and testes lobated). Similarly, female specimens were classified as immature (ovary small, oocytes white or translucent, thin oviducts, oviducal glands and uteri poorly developed) and mature (well developed ovary, uteri, oviducal glands and egg capsules, fertile eggs or embryos present in the uteri). Testes from males and ovary, eggs, oviducal glands and uteri from females were measured and weighed. In pregnant females, the embryos were extracted from the uteri, enumerated, sexed, measured and weighed. Following the method from Harry et al. (Reference Harry, Tobin and Simpfendorfer2013), a logistic regression was fitted to binomial maturity data (0 = immature; 1 = mature) vs total length using GLM procedure in R with the argument family ‘logit’ (R Core Team, 2013) to predict the lengths at which 50% (length at maturity, L T50) and 95% (L T95) of the individuals are mature. The length at birth (L b) was estimated from the maximum size of embryos recovered from the uterus and the minimum size of free-swimming specimens captured (Liu et al., Reference Liu, Chen, Liao and Joung1999).
RESULTS
Overall, 1449 specimens of pelagic sharks in the L T range of 65–361 cm were subjected for biological studies during the study period (Table 1). Sex ratio was biased to males in blue, threshers, longfin mako and tiger sharks, while females dominated in the specimens of oceanic whitetip shark. The length frequency distributions indicated variations in the total lengths of male and female specimens in all the species. However, a Kolmogorov–Smirnov test did not reveal any statistically significant differences in the length frequencies. The length at maturity, litter size and size at birth varied widely among different species studied. However, reproductive strategy in both the thresher shark species was similar, including litter size (always two), mode of nutrition to embryos (oophagy) and seasonality and periodicity in reproduction (non-seasonal reproduction with annual reproductive cycle).
Table 1. Length, sex ratio, maturity and litter size of pelagic sharks in the eastern Arabian Sea (L T, Total length; SD, standard deviation).

a Pregnant females were absent in the specimens collected.
Alopias pelagicus
A total number of 656 pelagic threshers, in the L T range of 142–319 (248.16 ± 31.92; mean ± standard deviation) cm were studied. The overall sex ratio (F:M) was 1:1.61, which significantly varied from the expected ratio of 1:1 (χ2 = 36.15, P < 0.05). Male specimens collected were in the L T range of 142–312 (249.98 ± 29.95) cm, whereas the L T of females ranged between 144 and 319 (245.02 ± 34.37) cm (Figure 2). However, the length frequencies distributions of males and females did not show significant differences (Kolmogorov–Smirnov test: D = 0.22; P > 0.05).

Fig. 2. Total length frequency of pelagic threshers sampled from the eastern Arabian Sea.
The C LO of smallest male was 2.3 cm while that of largest specimen was 18.7 cm and noticeable increase in the C LO starts with the size class 230–235 cm (Figure 3). Smallest mature male specimen was 245 cm while the largest immature male was L T 270 cm. The L T50 estimated was 254.96 cm (Figure 4) and the L T95 estimated was 278.04 cm (Table 2). The females start maturing at 268 cm L T and all the females larger than 286 cm were mature. The immature specimens had ovaries in the weight range of 10.7–323 g, whereas those of mature specimens were in the range of 185.3–1215.7 g, and the ovary weights of pregnant females were in the range of 480.3–2834 g. In females, the L T50 estimated was 271.39 cm and the L T95 was 287.18 cm. In pelagic threshers, only two eggs are fertilized; these are then enclosed in egg capsules, reach one uterus each and develop there. Overall, 21 pregnant females, in the L T range of 282–319 cm were recorded, almost throughout the year. All pregnant females carried two embryos, one in each uterus. The sex ratio of embryos did not vary significantly from parity (χ2 = 0.381, P > 0.05). The embryos were in the L T range of 16.8–137.8 cm and the temporal analysis of mean length of embryos could not reveal seasonal reproduction of pelagic threshers in the eastern Arabian Sea. The largest embryo of L T 137.8 cm was recorded during May 2014, and had the typical colouration and features of free-swimming specimens. The smallest free-swimming specimen was a 142 cm (L T) male recorded in September 2013. It is therefore inferred that the size at birth of pelagic threshers in the eastern Arabian Sea will be 137.8–142 cm. Only the right ovary was functional and pregnant females had huge ovaries, containing thousands of vitelline eggs which were supplied to the embryos in the form of nutritive egg capsules. Nutritive egg capsules contained varying numbers of eggs (12–62) and a steady increase in the size and number of eggs in the nutritive egg capsules were noticed with increasing embryo size (linear regression, nutritive egg capsule length = 0.058 embryo length + 2.628; R 2 = 0.947 and number of eggs in egg capsule = 0.385 embryo length + 5.293; R 2 = 0.931).

Fig. 3. Total length and clasper length of male pelagic threshers sampled from the eastern Arabian Sea.

Fig. 4. Logistic curves fitted to maturity in relation to total lengths of pelagic threshers of the eastern Arabian Sea. Dashed lines indicate length at maturity.
Table 2. Length at 50% maturity (LT50) and the length at 95% maturity (LT95) of pelagic sharks in the eastern Arabian Sea (SE, standard error).

a Length at maturity could not be estimated due to inadequate sample size.
Alopias superciliosus
A total of 217 specimens of bigeye threshers, in the L T range of 135–361 (254.35 ± 38.92) cm were sampled. The overall sex ratio (1F:1.44M) significantly varied from the expected ratio of 1:1 (χ2 = 7.01, P < 0.05). Male specimens collected were in the L T range of 135–327 (250.89 ± 32.68) cm, whereas the L T of females ranged between 145 and 361 (259.33 ± 46.20) cm (Figure 5). However, the total lengths of males and females did not show significant differences (Kolmogorov–Smirnov test: D = 0.15; P > 0.05).

Fig. 5. Total length frequency of big eye threshers sampled from the eastern Arabian Sea.
The outer clasper lengths of males were in the range of 3.2–22.4 cm and noticeable increase in the C LO starts when L T reaches 224 cm (Figure 6). Smallest mature male specimen was 247 cm while the largest immature male was of L T 280 cm. The L T50 estimated was 263.50 cm and the L T95 was estimated to 282.98 cm. The females start maturing at 282 cm L T and all the females larger than 322 cm were mature. Total number of immature females recorded was 76, number of mature females excluding pregnant was 5 and the number of pregnant females was 8. The immature specimens had ovaries in the weight range of 1.9–325.5 g, whereas those of mature specimens were in the range of 870.3–1112.9 g, while the ovary weights of pregnant females were in the range of 1823.4–2615 g. In females, the L T50 estimated was 310.69 cm (Figure 7) and the L T95 was estimated to 345.13 cm. Similar to pelagic threshers, only two eggs become fertilized in bigeye threshers, which are then enclosed in egg capsules, reach one uterus each and develop there. Eight pregnant females, in the L T range of 283–361 cm were recorded, almost throughout the year. All pregnant females carried two embryos, one in each uterus. The sex ratio of embryos (1F:0.78M) did not vary significantly from parity (χ2 = 0.25, P > 0.05). The embryos collected were in the L T range of 19.0–118.0 cm and the temporal analysis of mean length of embryos could not reveal seasonal reproduction in this species either. The largest embryo of L T 118.0 cm weighing 3.5 kg was recorded during March 2014, which had the typical colouration and features of free-swimming specimens. The smallest neonate was a male specimen of L T 135 cm recorded during April 2013. The size at birth of bigeye threshers in the eastern Arabian Sea will be 118–135 cm. Similar to the pelagic threshers, in bigeye threshers the functional right ovary of pregnant females was huge, containing thousands of vitelline eggs which were supplied to the embryos in the form of nutritive egg capsules. Nutritive egg capsules contained varying number of eggs and, similar to pelagic threshers, the size and number of eggs in the egg capsules increased with increasing embryo length in this species (Figure 8).

Fig. 6. Total length and clasper length of male bigeye threshers collected from the eastern Arabian Sea.

Fig. 7. Logistic curves fitted to maturity in relation to total lengths of bigeye threshers of the eastern Arabian Sea. Dashed lines indicate length at maturity.

Fig. 8. Nutritive egg capsules supplied to bigeye thresher embryos of 34 cm L T (A) and 118 cm L T (B).
Carcharhinus longimanus
The total lengths of 212 oceanic whitetip shark specimens sampled during the study were in the range of 65–265 (155.43 ± 38.32) cm. The overall sex ratio was near to parity (1:0.93; F:M) (χ2 = 0.30, P > 0.05). Male specimens collected were in the L T range of 65–265 (164.30 ± 44.03) cm, whereas the L T of females ranged between 69 and 246 (147.20 ± 30.08) cm (Figure 9). Statistically significant differences in the length frequencies of males and females were also absent in the species (Kolmogorov–Smirnov test: D = 0.17; P > 0.05).

Fig. 9. Total length frequency of oceanic whitetip sharks sampled from the eastern Arabian Sea.
The C LO of the smallest male was 1.5 cm while that of the largest specimen was 17.2 cm and a noticeable increase in the C LO starts when L T reaches 187 cm (Figure 10). The smallest mature male specimen was 202 cm while the largest immature male was of L T 225 cm and the length at maturity (L T50) estimated was 207.19 cm while the L T95 was estimated to 226.74 cm. The females start maturing at 185 cm L T and all the females longer than 203 cm were mature. The oviducal gland widths of immature specimens were in the range of 0.5–2.2 cm, those of mature specimens were in the range of 4.0–5.5 cm, and in pregnant females the oviducal gland widths were in the range of 5.6–6.4 cm. The uterus widths in immature specimens were in the range of 0.8–2.3 cm, mature specimens in the range of 4.9–5.7 cm, and in pregnant females the uterus widths were in the range of 12.2–16.2 cm. In females, the L T50 estimated was 187.74 cm (Figure 11) and the L T95 estimated was 203.27 cm. Only five pregnant females, in the L T range of 183–246 cm were recorded, during the months February, May and September. A total number of 29 embryos, in the L T range of 7.2–64.2 cm were recovered from the uteri. Numbers of embryos in females were in the range of 3–9 (5.8 ± 2.39) and the relationship between the L T of mothers and number of embryos was given by y = 0.0697x − 8.524 (R 2 = 0.55), indicating a moderate relationship between the mother's length and litter size. No significant differences in the number of embryos in left and right uteri (Student's t-test, P > 0.05) were observed. The sex ratio of embryos (1F:0.81M) did not vary significantly from parity (χ2 = 0.31, P > 0.05). The near term embryo of L T 64.2 cm was recorded during February 2014, and considering the smallest neonate was 65 cm, the size at birth will be 64.2–65.0 cm. Since the near term embryo was recorded during February and the smallest neonate was recorded during March, it is concluded that the parturition will be taking place during March–May. Considering the record of smallest embryo during May and the largest in February, it is postulated that the gestation period may be little less than one year.

Fig. 10. Total length and clasper length of male oceanic whitetip sharks collected from the eastern Arabian Sea.

Fig. 11. Logistic curves fitted to maturity in relation to total lengths of oceanic whitetip sharks of the eastern Arabian Sea. Dashed lines indicate length at maturity.
Galeocerdo cuvier
A total of 217 specimens of tiger sharks, in the L T range of 85–398 (198.23 ± 59.54) cm were sampled. The overall sex ratio was 1F:1.09M, which did not significantly vary from the expected ratio of 1:1 (χ2 = 0.37, P > 0.05). Male specimens collected were in the L T range of 89–355 (199.39 ± 54.06) cm, whereas the L T of females ranged between 85 and 398 (196.97 ± 65.21) cm (Figure 12). However, the total lengths of males and females did not show significant differences (Kolmogorov–Smirnov test: D = 0.063; P > 0.05) in this species also.

Fig. 12. Total length frequency of tiger sharks sampled from the eastern Arabian Sea.
The outer clasper lengths of male specimens were in the range of 1.9–24.0 cm and noticeable increase in the C LO starts with the L T 247 cm (Figure 13). Smallest mature male specimen was 272 cm while the largest immature male was L T 287 cm. The L T50 estimated was 286.56 cm (Figure 14) and the L T95 was 306.87 cm. The females start maturing at 274 cm L T and all the females longer than 310 cm were mature. In females, the L T50 estimated was 300.31 cm while the L T95 was 347.26 cm.

Fig. 13. Total length and clasper length of male tiger sharks collected from the eastern Arabian Sea.

Fig. 14. Logistic curves fitted to maturity in relation to total lengths of tiger sharks of the eastern Arabian Sea. Dashed lines indicate length at maturity.
Total number of immature females recorded was 94, the number of mature females excluding pregnant was 2 and the number of pregnant females was 8. Average number of yolked eggs in mature females was 23.4. Ovarian eggs measured 0.2–7.1 cm diameter and the diameters of oviducal glands were in the range of 1.3–11.2 cm. During the study, eight pregnant females were sampled, almost throughout the year. Smallest pregnant female was 285 cm (L T) and the mean L T of all pregnant females sampled was 325.13 (±38.82) cm. The oviducal glands of pregnant females were in the range of 8.8–11.2 (10.1 ± 0.89) cm. A total of 282 embryos, in the L T range of 6.8–79.6 (37.06 ± 22.24) cm, were recovered from the uteri. Numbers of embryos in females were in the range of 22–51 (35.25 ± 8.94). The uteri were obliquely compartmentalized and the embryos were individually placed in compartments of uterus. The embryos as well as the fertilized eggs in the uterus were covered with a thin fluid-filled sac, which in turn was covered by thin, soft, transparent, membranous sheath of nearly 100 × 30 cm. Both ends of these sheaths formed an iridescent wrinkled and highly folded structure. Smaller embryos had external yolk sacs, largest of which was 10.1 cm in diameter recorded for an embryo of 9.6 cm total length. This external yolk sac disappears in the embryos of total length 63.3 cm. Seasonal analysis of mean lengths of embryos showed pronounced seasonal change in mean embryo size. Smallest embryos were recovered from a mother of L T 285 cm collected during January 2014. Fertilized eggs, which were also collected from the uterus of this mother, indicate that gestation in tiger sharks of eastern Arabian Sea starts in January. Largest embryo of L T 79.6 was recovered from a 347 cm (L T) mother caught during May 2014. The smallest free-swimming specimen of L T 85.2 cm was caught in May 2013 (Figure 15). This shows that the parturition takes place in May and the gestation period will be about 16 months and the length at birth will be in the range of 79.6–85.2 cm. The sex ratio of embryos (1F:0.97M) did not significantly vary from the expected ratio of 1:1.

Fig. 15. Fertilized egg (A) and embryo (B) collected from a 285 cm L T female collected during January 2014 and the smallest neonate (85.2 cm L T) collected during May 2013 (C).
Isurus oxyrinchus
The total lengths of 96 specimens of shortfin makos sampled were in the range of 97–269 (168.73 ± 37.56) cm. The overall sex ratio (1F:0.96M) did not significantly vary from parity (χ2 = 0.04, P > 0.05). Male specimens collected were in the L T range of 128–221 (161.68 ± 23.88) cm, whereas the L T of females ranged between 97 and 269 (175.24 ± 46.06) cm (Figure 16). However, the total lengths of males and females did not show significant differences (Kolmogorov–Smirnov test: D = 0.26; P > 0.05).

Fig. 16. Total length frequency of shortfin makos sampled from the eastern Arabian Sea.
The C LO of the smallest male was 2.8 cm while that of the largest specimen was 18.9 cm and a noticeable increase in the C LO starts at the L T 149 cm (Figure 17). Smallest mature male specimen was 166 cm while the largest immature male was of L T 205 cm. Only eight mature males were recorded in the samples studied. The L T50 estimated was 189.05 cm and the L T95 was estimated at 222.88 cm. Pregnant females could not be encountered in the specimens landed at the harbour. However, three mature specimens having uterus widths greater than 4 cm were recorded during February and September 2014. These limited data indicate that females start maturing at 257 cm L T and the largest immature was 267 cm. In females, the L T50 estimated was 266.42 cm and the L T95 was estimated at 289.14 cm (Figure 18).

Fig. 17. Total length and clasper length of male shortfin makos collected from the eastern Arabian Sea.

Fig. 18. Logistic curves fitted to maturity in relation to total lengths of shortfin makos of the eastern Arabian Sea. Dashed lines indicate length at maturity.
Isurus paucus
The longfin mako samples for the study were collected during May–August. A total of 25 specimens, in the L T range of 140–258 (169.72 ± 30.07) cm were sampled. The overall sex ratio was 1F:1.27M, which did not significantly vary from the expected ratio of 1:1 (χ2 = 0.36, P > 0.05). Male specimens collected were in the L T range of 140–258 (167.57 ± 35.05) cm, whereas the L T of females ranged between 149 and 227 (172.45 ± 23.61) cm (Figure 19). However, the total lengths of males and females did not show significant differences (Kolmogorov–Smirnov test: D = 0.083; P > 0.05) in this species either.

Fig. 19. Total length frequency of longfin makos sampled from the eastern Arabian Sea.
The C LO of smallest male was 3.2 cm while that of largest specimen was 19 cm and noticeable increase in the C LO starts with L T 189 cm (Figure 20). Only two mature specimens, of L T 225 and 258 cm could be sampled and due to the lack of enough mature specimens, the L T50 (206.77 cm) and L T95 (209.07 cm) estimated (Figure 21) was not reliable, since the standard errors in the estimations were too high (Table 2). However, the length distribution of largest immature (L T 189 cm) and smallest mature specimens indicate that the length at maturity of males will be in the range of 189–225 cm. Since all the females sampled were immature, size at maturity in females also could not be estimated.

Fig. 20. Total length and clasper length of male longfin makos collected from the eastern Arabian Sea.

Fig. 21. Logistic curves fitted to maturity in relation to total lengths of longfin makos of the eastern Arabian Sea. Dashed lines indicate length at maturity.
Prionace glauca
Only 26 specimens of blue sharks, in the L T range of 186–280 (218.93 ± 20.30) cm could be sampled during the entire sampling period. The specimens were collected during the months May and August. The overall sex ratio was 1F:5.5M, which significantly varied from the expected ratio of 1:1 (χ2 = 12.46, P < 0.05). Male specimens collected were in the L T range of 186–280 (216.96 ± 21.42) cm, whereas the L T of females ranged between 222 and 236 (229.75 ± 6.13) cm (Figure 22). However, the total lengths of males and females did not show significant differences (Kolmogorov–Smirnov test: D = 0.4; P > 0.05) in this species.

Fig. 22. Total length frequency of blue sharks sampled from the eastern Arabian Sea.
The C LO of males were in the range of 7.9–14.6 cm (Figure 23). Since smaller specimens were absent in the samples, point of inflection in the length of claspers could not be identified. Smallest mature male specimen was 195 cm while the largest immature male was of L T 212 cm. More than 63% of the male specimens sampled were mature with calcified claspers. The L T50 estimated was 207.11 cm while the L T95 estimated was 228.56 cm (Figure 24). Pregnant females could not be encountered in the specimens landed at the harbour. However, all the four females sampled were mature specimens. Small sample size and absence of immature specimens in the samples precluded the estimation of size at maturity in females.

Fig. 23. Total length and clasper length of male blue sharks collected from the eastern Arabian Sea.
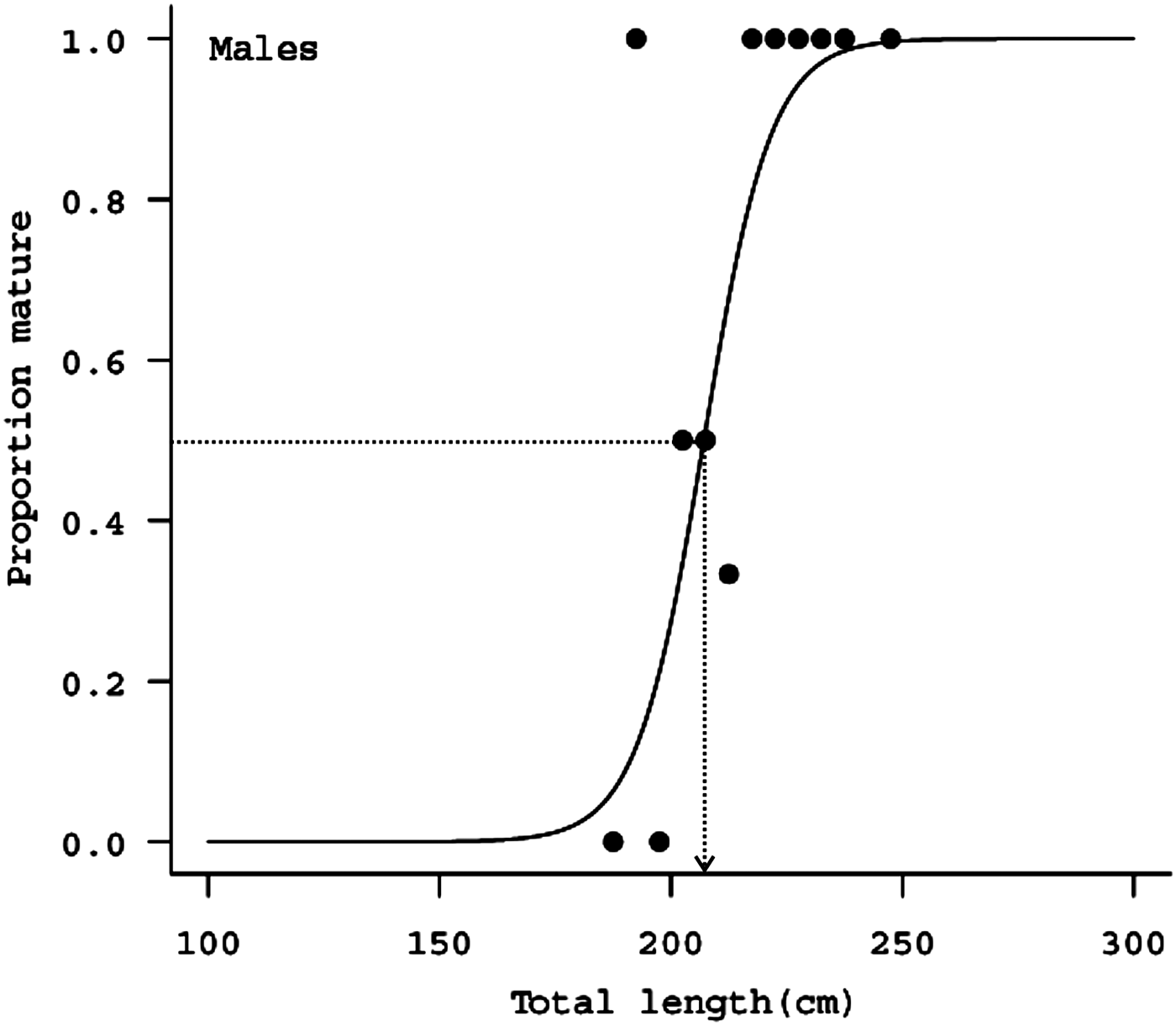
Fig. 24. Logistic curves fitted to maturity in relation to total lengths of male blue sharks of the eastern Arabian Sea. Dashed lines indicate length at maturity.
DISCUSSION
Alopias pelagicus
Total lengths of pelagic threshers caught by gillnet-cum-longline fishery based at Cochin during the years 2013 and 2014 were in the range of 144–319 cm (females) and 142–312 cm (males), which was substantially shorter than maximum sizes recorded in Taiwanese and Indonesian waters (Liu et al., Reference Liu, Chen, Liao and Joung1999; White, Reference White2007a). The length of the smallest free-living specimen in the present study was similar to the length of the smallest specimen caught from the western Indian Ocean (137 cm, Compagno, Reference Compagno2002). Sex ratio in the present study was significantly biased to males, whereas the specimens in earlier studies conducted off Taiwan, Indonesia and Ecuador had sex ratios biased to females (Liu et al., Reference Liu, Chen, Liao and Joung1999; White, Reference White2007a; Romero-Caicedo et al., Reference Romero-Caicedo, Galván-Magaña and Martinez-Ortiz2014). However, the sex ratios of embryos in all the above studies including the present study were close to parity indicating sexual segregation in free-swimming specimens.
Male and female pelagic threshers of eastern Arabian Sea become sexually mature at smaller L T (254.96 cm and 271.39 respectively), than those in Taiwanese (267–276 cm and 282–292 cm, Liu et al., Reference Liu, Chen, Liao and Joung1999), Indonesian (264.8 and 285.3 cm, Drew et al., Reference Drew, White, Harry and Huveneers2015) and Ecuadorian (268.6 and 282.6 cm, Romero-Caicedo et al., Reference Romero-Caicedo, Galván-Magaña and Martinez-Ortiz2014) waters. Bass et al. (Reference Bass, D'Aubrey and Kistnasamy1975a) reported that a 277 cm female caught off South Africa was an immature specimen. Liu et al. (Reference Liu, Chen, Liao and Joung1999) estimated the ratio of length at maturity to maximum observed length (L T50/L max) of pelagic threshers off Taiwan as 0.74–0.77, whereas in the present study the estimation was in the range of 0.82–0.85. The size at birth estimated in this study (139.9 cm) was similar to the estimations from Indonesian waters (130–144 cm, White, Reference White2007a), which was smaller than estimations for population off Taiwan (159–190 cm, Liu et al., Reference Liu, Chen, Liao and Joung1999).
The present study failed to establish seasonal reproduction of pelagic threshers in the eastern Arabian Sea. Similarly, seasonal reproduction in pelagic threshers was less evident in studies conducted off Taiwan and Indonesia (Liu et al., Reference Liu, Chen, Liao and Joung1999; White, Reference White2007a), whereas Romero-Caicedo et al. (Reference Romero-Caicedo, Galván-Magaña and Martinez-Ortiz2014) reported an annual pattern for reproduction with a gestation period of 9 months for pelagic threshers off Ecuador. Pregnant females in the present study had larger ovaries than non-pregnant specimens and continuous vitellogenesis was observed in mature and pregnant females indicating an annual reproductive cycle with concurrent vitellogenesis and gestation without any resting period. Similar observations were made by Castro (Reference Castro2009) and Romero-Caicedo et al. (Reference Romero-Caicedo, Galván-Magaña and Martinez-Ortiz2014) for pelagic threshers of the eastern Pacific. Similar to the observations in earlier studies (Otake & Mizue, Reference Otake and Mizue1981; Liu et al., Reference Liu, Chen, Liao and Joung1999; White, Reference White2007a; Castro, Reference Castro2009; Romero-Caicedo et al., Reference Romero-Caicedo, Galván-Magaña and Martinez-Ortiz2014), this study also established oophagy in pelagic thresher embryos. The present study could establish significant linear relationship between the size and number of nutritive eggs in the nutritive egg capsules and embryo size.
Alopias superciliosus
Maximum lengths (L T) of bigeye threshers caught by gillnet-cum-longline fishery based at Cochin during 2013–2014 were 361 cm (females) and 327 cm (males), which were substantially shorter than maximum sizes recorded in previous studies (females – 460.7 cm (Nakamura, Reference Nakamura1935) males – 410 cm (Moreno & Moron, Reference Moreno and Moron1992)). Sex ratio in the present study was significantly biased to males, whereas the specimens in earlier studies conducted off Taiwan and Indonesia had sex ratios biased to females (Chen et al., Reference Chen, Liu and Chang1997; White, Reference White2007a). However, the sex ratios of embryos in all the above studies including the present study were close to parity indicating sexual segregation in free-swimming specimens.
Male and female bigeye threshers of the eastern Arabian Sea become sexually mature at smaller L T (263.5 and 310.69 cm respectively), than those in the north-eastern Atlantic (276 and 341 cm, Moreno & Moron, Reference Moreno and Moron1992), Taiwanese (270.1–287.9 cm and 332–341.1 cm, Chen et al., Reference Chen, Liu and Chang1997) and Indonesian (279–283 cm and 350.8 cm, White, Reference White2007a) waters. Chen et al. (Reference Chen, Liu and Chang1997) estimated the ratio of length at maturity to maximum observed length (L T50/L max) as 0.79, whereas in the present study the estimation was in the range of 0.81–0.86. The size at birth estimated in this study (118–135 cm) was smaller than estimations for population off Taiwan (135–140 cm, Chen et al., Reference Chen, Liu and Chang1997).
The reproduction in bigeye threshers of the eastern Arabian Sea was non-seasonal. Similarly, seasonal reproduction in pelagic threshers was less evident in studies conducted off Taiwan (Chen et al., Reference Chen, Liu and Chang1997). The present study revealed an annual reproductive cycle in bigeye threshers of eastern Arabian Sea with concurrent vitellogenesis and gestation without any resting period. Similar to the observations of earlier studies (Chen et al., Reference Chen, Liu and Chang1997), this study also established that bigeye thresher embryos are oophagous.
Carcharhinus longimanus
Oceanic whitetip sharks caught by gillnet-cum-longline fishery based at Cochin were in the total length range of 65–265 cm. The 65 cm specimen recorded during this study was smaller than the smallest free-swimming specimen reported in the literature (69 cm, White, Reference White2007b). The length at birth reported for this species is in the range of 60–65 cm L T off South Africa (Bass et al., Reference Bass, D'Aubrey and Kistnasamy1973) and 55–75 cm in the north-west Pacific (Seki et al., Reference Seki, Taniuchi, Nakano and Shimizu1998). The size at birth estimated in the present study was 64.2–65 cm. The sex ratio of free-swimming specimens as well as embryos of whitetip sharks in the present study was near to parity, similar to the reports in earlier studies conducted in the north-west Pacific and north-eastern Brazil (Seki et al., Reference Seki, Taniuchi, Nakano and Shimizu1998; Lessa et al., Reference Lessa, Paglerani and Santana1999).
Earlier studies conducted in the Atlantic and Pacific Oceans reported lower length at maturity for males than females (Seki et al., Reference Seki, Taniuchi, Nakano and Shimizu1998; Coelho et al., Reference Coelho, Hazin, Rego, Tambourgi, Oliveira, Travassos, Carvalho and Burgess2009), whereas, similar to the observations in the present study, the size at maturity was higher in males than females in the Indian Ocean stocks (Bass et al., Reference Bass, D'Aubrey and Kistnasamy1973; White, Reference White2007b). Since the pregnant females could be collected during February, May and September only, gestation period could not be identified in the present study. However, the specimens with L T less than 70 cm in this study were recorded during the month of March which indicates that in the Arabian Sea, the parturition may be taking place during summer months (March–May). Further, considering the record of pregnant female with smallest embryos (7.2 cm) during May, largest (64.2 cm) during February and record of smallest neonates in March, it can be concluded that the gestation period may be a little less than or equal to 1 year. However, detailed studies are needed to reach a definitive conclusion. Seki et al. (Reference Seki, Taniuchi, Nakano and Shimizu1998) reported that the parturition period in this species is extended over a longer duration, with no distinct seasonal reproductive cycle, whereas seasonal parturition and mating occurring in spring and early summer were reported by Backus et al. (Reference Backus, Springer and Arnold1956) and Gohar & Mazhar (Reference Gohar and Mazhar1964). In the south-west Indian Ocean, near term foetuses were recorded during September–October, indicating early summer parturition in the whitetip sharks of the south-west Indian Ocean. Brood size of whitetip sharks in the present study was 3–9, averaging 5.8 embryos, which is in agreement with 1–14 embryos per litter reported by Seki et al. (Reference Seki, Taniuchi, Nakano and Shimizu1998). Bass et al. (Reference Bass, D'Aubrey and Kistnasamy1973) reported that in the south-west Indian Ocean, the brood size was 6–8, averaging 7 embryos, whereas Gohar & Mazhar (Reference Gohar and Mazhar1964) reported a litter size of 10–15 for the Red Sea specimens.
Galeocerdo cuvier
During the study period, the gillnet-cum-longline fishery at Cochin fisheries harbour landed tiger sharks in the L T range of 85–398 cm. The majority of the specimens caught were sub-adults, in the length range of 150–230 cm. The length at birth reported for this species is in the range of 51–90 cm L T (Compagno, Reference Compagno1984; Randall, Reference Randall1992; Simpfendorfer, Reference Simpfendorfer1992; Whitney & Crow, Reference Whitney and Crow2007) and the length at birth estimated in the present study (79.6–85.2 cm) falls in this range. The sex ratio of tiger sharks in the present study was near to parity. Similarly, the specimens caught off Indonesia and off Hawaii had sex ratios near to parity (White, Reference White2007b; Whitney & Crow, Reference Whitney and Crow2007).
Size at maturity of male tiger sharks in the eastern Arabian Sea estimated in this study was 286.56 cm, which was similar to the estimations of Whitney & Crow (Reference Whitney and Crow2007) (292 cm), Clark & von Schmidt (Reference Clark and von Schmidt1965) and Bass et al. (Reference Bass, D'Aubrey and Kistnasamy1975b) (290 cm each). The smallest mature female in the samples of the present study was 274 cm L T, whereas the total length of smallest pregnant specimen was 285 cm. Pregnant females with total lengths as small as 210 cm have been reported in Brazil (Alves, Reference Alves1977). The size at maturity of females estimated in the present study is 300.31 cm, which falls within the range of 297–320 cm reported from the Atlantic Ocean (Clark & von Schmidt, Reference Clark and von Schmidt1965; Rivera-Lopez, Reference Rivera-Lopez1970; Branstetter et al. Reference Branstetter, Musick and Colvocoresses1987). However, this estimation was lower than the size at maturity reported for tiger sharks off Madagascar (340 cm, Fourmanoir, Reference Fourmanoir1961), South-east Australia (330 cm, Stevens, Reference Stevens1984) and Hawaii (330–345 cm, Whitney & Crow, Reference Whitney and Crow2007), while it was higher than the value estimated for female tiger sharks off Australia (287 cm, Simpfendorfer, Reference Simpfendorfer1992).
In the present study, presence of pregnant females with smallest embryos (6.8 cm) along with fertilized eggs in the uterus during January indicates that the gestation starts during December–January months. Similarly, pregnant mothers with largest embryos resembling neonates were recorded during the month of May. Smallest free-swimming specimens also were appearing in the landings during May. Further, embryos of intermediate sizes were recorded during the months February, May, September, October and January. Following Whitney & Crow (Reference Whitney and Crow2007), it is concluded that reproduction in tiger sharks in the eastern Arabian Sea is seasonal, with gestation starting during January followed by parturition during May of the following year, and the gestation period estimated was 16 months. Considering the postulation of Whitney & Crow (Reference Whitney and Crow2007) on the 4–5 months storage of sperm in the oviducal gland before fertilization, it is concluded that tiger shark breeding in the eastern Arabian Sea may be taking place during August–September. Sarangdhar (Reference Sarangdhar1943, Reference Sarangdhar1949) has given detailed accounts of pregnant tiger sharks and their embryos collected from the shark landings at Bombay, India. Female with smallest embryos (6.8–7.5 cm L T) was collected on 15 December 1941, females with embryos in the length range of 52.07–48.26 cm on 17 January 1942, whereas females with near-term embryos of 68.58–74.93 cm L T was collected on 4 May 1943. The above findings of Sarangdhar (Reference Sarangdhar1943, Reference Sarangdhar1949) corroborate the conclusions in the present study on a 16 months gestation period for the tiger sharks of Arabian Sea. In Hawaii, mating takes place in January–February and gestation lasts 15–16 months until parturition in September–October (Whitney & Crow, Reference Whitney and Crow2007). However, Castro (Reference Castro2009) proposed a 12 months gestation period for tiger shark. The percentage of mature females that are pregnant in the present study (80%) was substantially higher than reports in previous studies conducted off Hawaii (Whitney & Crow, Reference Whitney and Crow2007), the Caribbean (Rivera-Lopez, Reference Rivera-Lopez1970), Brazil (Alves, Reference Alves1977) and Australia (Simpfendorfer, Reference Simpfendorfer1992), which may indicate a shorter resting period for the tiger sharks than proposed by Whitney & Crow (Reference Whitney and Crow2007) and that the tiger sharks of eastern Arabian Sea give birth once in 2 years. Castro (Reference Castro2009) postulated that tiger sharks may have a biennial reproductive cycle, whereas, Whitney & Crow (Reference Whitney and Crow2007) suggested that tiger sharks in Hawaii give birth once every 3 years. However, the small sample size of mature females in the present study was a major limiting factor for drawing definitive conclusions on the reproductive cycle.
Numbers of embryos in pregnant females in the present study (range: 22–51; mean – 35.25) was in the range of litter size of tiger sharks (10–80) reported in earlier studies (Bigelow & Schroeder, Reference Bigelow, Schroeder, Tee-Van, Breder, Hildebrand, Parr and Schroeder1948; Bass et al., Reference Bass, D'Aubrey and Kistnasamy1975b; Simpfendorfer, Reference Simpfendorfer1992; Whitney & Crow, Reference Whitney and Crow2007). Embryos collected during the present study have not shown any indication of the placenta formation. The tiger shark is the only species of the family Carcharhinidae that is ovoviviparous and aplacental (Compagno, Reference Compagno1988). Embryos <63.3 cm in the present study consume the yolk contained in the external yolk sac, whereas the sources of nutrition in the embryos greater than this length could not be verified, since the external yolk sacs were lost in these embryos. Sarangdhar (Reference Sarangdhar1943) speculated that the ‘watery liquid’ in the sacs covering the developing embryos has a nutritive function. Castro (Reference Castro1983) also postulated that embryos receive additional nourishment in the form of periembryonic fluid.
Isurus oxyrinchus
During the study period, the gillnet-cum-longline fishery at Cochin fisheries harbour landed shortfin makos in the L T range of 97–269 cm. Shortfin makos landed in Indonesia were in the total length range of 116.7–310 cm (White, Reference White2007a). Majority of the shortfin makos (88.54%) recorded in the present study as well as those landed in Indonesia were immature specimens. The sex ratio of shortfin makos in the present study was near to parity. Similarly, the specimens caught off eastern Australia and Indonesia also had sex ratios near to parity (Stevens, Reference Stevens1983; White, Reference White2007a), whereas the sex ratio was biased to females in the north-western Pacific (Joung & Hsu, Reference Joung and Hsu2005).
Female shortfin makos in the eastern Arabian Sea mature at a larger size than males, similar to the earlier reports from other regions of world oceans (Pratt & Casey, Reference Pratt and Casey1983; Stevens, Reference Stevens1983; Joung & Hsu, Reference Joung and Hsu2005). Size at maturity of males in the eastern Arabian Sea estimated in this study (189.05 cm) was similar to the estimations for the Baja California (Conde-Moreno & Galván-Magaña, Reference Conde-Moreno and Galván-Magaña2006), south-east Pacific (Bustamante & Bennett, Reference Bustamante and Bennett2013), Indonesia (185.7 cm, White, Reference White2007a) and California (Cailliet & Bedford, Reference Cailliet and Bedford1983) stocks. However, the length at maturity estimated for male shortfin makos in the western and central North Pacific (156 cm, Semba et al., Reference Semba, Aoki and Yokawa2011) was smaller, while those off eastern Australia (195 cm, Stevens, Reference Stevens1983), southern Africa (194–206 cm, Cliff et al., Reference Cliff, Dudley and Davis1990), north-western Pacific (210 cm, Joung & Hsu, Reference Joung and Hsu2005) and New Zealand (180–185 cm, forklength, Francis & Duffy, Reference Francis and Duffy2005) were greater than the estimations in the present study.
Only 6.12% of the females were mature and pregnant specimens could not be collected in the present study. Similarly, pregnant specimens were absent in the shortfin makos sampled off Mexico (Conde-Moreno & Galván-Magaña, Reference Conde-Moreno and Galván-Magaña2006), Indonesia (White, Reference White2007a), and off the south-west Portuguese coast (Maia et al., Reference Maia, Queiroz, Cabral, Santos and Correia2007), whereas extensive studies by Duffy & Francis (Reference Duffy and Francis2001) and Francis & Duffy (Reference Francis and Duffy2005) recorded a single pregnant female in the New Zealand waters. Gilmore (Reference Gilmore1993) reported that pregnant shortfin makos are usually captured in higher latitudes (between 20° and 30°N or S), which may be due to the migration of the females to these latitudes for parturition. However, none of the 640 female specimens collected from the south-east Pacific (20°S to 27°30′S) off Chile were pregnant (Bustamante & Bennett, Reference Bustamante and Bennett2013) indicating less abundance of pregnant females even in samples caught from higher latitudes. The smallest mature female in the present study was 257 cm (L T) and the size at maturity estimated was 266.42 cm, while the size at maturity estimated in previous studies is in the range of 256–300 cm (Stevens, Reference Stevens1983; Mollet et al., Reference Mollet, Cliff, Pratt and Stevens2000; Francis & Duffy, Reference Francis and Duffy2005; Joung & Hsu, Reference Joung and Hsu2005; Semba et al., Reference Semba, Aoki and Yokawa2011). However, based on a single mature female sampled, White (Reference White2007a) concluded that the female shortfin mako off Indonesia matures at a L T of 240–250 cm, whereas Gohar & Mazhar (Reference Gohar and Mazhar1964) reported a pregnant female of 263 cm with six embryos from the Red Sea and the smallest pregnant female reported by Joung & Hsu (Reference Joung and Hsu2005) from the north-western Pacific was of L T 272 cm. Since the size at maturity of females in the present study was estimated based on three mature specimens, results have to be considered as a preliminary estimation from the area where such reports are rare.
Isurus paucus
Reproduction in longfin mako is aplacental viviparity with oophagy and uterine cannibalism, with a litter size of 2–8 (Reardon et al., Reference Reardon, Gerber and Cavanagh2006). Total length of smallest mature male in the present study (225 cm) was closely similar to the L T of smallest mature male in recorded off Indonesia (228 cm, White, Reference White2007a). In Maldivian waters, Anderson et al. (Reference Anderson, Adam and Saleem2011) reported two male specimens of forklengths 177 and 181 cm assumed to be mature. Female longfin makos of the eastern Arabian Sea mature at greater lengths than males, since the largest female specimen collected in the present study (L T 227 cm) was an immature specimen. Females of the western North Atlantic mature at >245 cm total length (Compagno, Reference Compagno2002).
Prionace glauca
Sex ratio of blue sharks sampled in the present study was strongly biased to males (1F:5.5M). Similarly, presence of significantly more males than females has been reported in most of the earlier studies (Stevens, Reference Stevens1984; Skomal & Natanson, Reference Skomal and Natanson2003; White, Reference White2007b; Anderson et al., Reference Anderson, Adam and Saleem2011). Spatially, blue sharks show high degrees of sexual segregation, since, in lower latitudes, the species is less common and males predominate (Compagno, Reference Compagno1984; Anderson et al., Reference Anderson, Adam and Saleem2011). More than 63% of the male specimens sampled in the present study were mature with calcified claspers and the length at maturity was estimated at 207.11 cm. Males become sexually mature at 184 cm total length off Baja California Sur (Carrera-Fernández et al., Reference Carrera-Fernández, Galván-Magaña and Ceballos-Vázquez2010), at about 210–227 cm off Indonesia (White, Reference White2007a), at 220–227 cm off New South Wales (Stevens, Reference Stevens1984) and at 221 cm off southern New England (Pratt, Reference Pratt1979). All the four females sampled in the present study were mature specimens. Although the length at maturity of females could not be estimated due to small sample size and absence of immature specimens in the samples, our data indicate that the female blue sharks in the eastern Arabian Sea become sexually mature at total lengths lower than 222 cm. Pratt (Reference Pratt1979) reported that female blue sharks are fully mature at 221 cm, whereas Carrera-Fernández et al. (Reference Carrera-Fernández, Galván-Magaña and Ceballos-Vázquez2010) estimated the length at first maturity of females at 196 cm.
Sharks have low resilience to over-exploitation by fisheries because of their K-selected life history strategy (Stevens et al., Reference Stevens, Bonfil, Dulvy and Walker2000). In the light of the increasing fishing pressure on the sharks in the high seas, management measures are urgently needed for ensuring the long-term sustainability of oceanic shark fishery of the Indian Ocean. The biological information collected in the present study will be useful in identifying suitable management measures for these shark species.
Shark finning is prohibited in the Indian Exclusive Economic Zone (EEZ) and the export of fins of all species of shark is prohibited from India. Further, India observes an annual uniform ban on fishing in the Indian EEZ by all mechanized fishing for 61 days for the conservation and sustainable management of its marine resources. However, the annual seasonal fishing ban implemented in the Indian EEZ may not be effective for ensuring the sustainability of highly migratory stocks like sharks, unless similar management measures are adopted in the neighbouring EEZs and high seas. An ocean-wide seasonal fishing ban for the entire Indian Ocean and fleet reduction in the high seas could be useful for reducing the fishing mortality of sharks in the Indian Ocean.
ACKNOWLEDGEMENTS
We are grateful to Shri Premchand, Director General, Fishery Survey of India for all facilities received from the Institute to undertake this study. The authors are indebted to the shark fishermen, auctioneers and fish merchants at the Cochin fisheries harbour and processing plants for allowing biological sampling of sharks. We thank Swapnil Shirke for sharing information on the pelagic thresher embryos collected on board research cruises in the Andaman and Nicobar waters. We are also thankful to the anonymous referees for their valuable comments and suggestions to help us improve this manuscript.




























