INTRODUCTION
Leishmania is a digenetic parasite with extracellular motile promastigotes present in the alimentary tract of their insect vector, the phleblotomine sandfly, and intracellular non-motile amastigotes that live in the mononuclear phagocytes of mammalian hosts. Parasites of this genus are responsible for the broad spectrum of cutaneous, mucocutaneous, and visceral leishmaniasis. These diseases occur throughout the world and present a major public health issue in tropical areas. In the New World, members of the L. mexicana complex and Viannia subgenus are associated with human cutaneous leishmaniasis, and mucocutaneous leishmaniasis, respectively (Lainson and Shaw, 1987; Herwaldt, 1999).
Surface glycoconjugates of parasites play an important role in the process of infection. Binding of Leishmania sp. promastigotes and amastigotes to the host cell depends on the presence of membrane proteins, glycoproteins, glycolipids (GLs), and lipophosphoglycan (LPG) (Straus et al. 1993; Handman, 1999; Ritting and Bogdan, 2000; Guha-Niyogi, Sullivan and Turco, 2001; Suzuki, Takahashi and Straus, 2002). Leishmania GLs are also involved in macrophage invasion, and in macrophage/monocyte signalling modulation events such as the NO synthesis and oxidative burst (McNeely et al. 1989; Straus et al. 1993, 1997; Tachado, Mazhaari-Tabiri and Schofield, 1999; Suzuki et al. 2002; Giorgio et al. 2003). Characteristic carbohydrate composition of glycoinositolphospholipids (GIPLs) has been found in Old and New World species of the L. (Leishmania) subgenus e.g. L. (L.) major; L. (L.) donovani, and L. (L.) mexicana. GIPLs from these species usually belong to 1 of 3 types: type 1, having the core sequence Manα1-6Manα1-4GlcNα1-6myo-inositol; type 2, having the core sequence Manα1-3Manα1-4GlcN1-6myo-inositol; or hybrid type, having both 3- and 6-linked mannose branches on the first mannose. All these cores are usually extended with 1–3 galactose residues (McConville and Ferguson, 1993).
Two species in the Leishmania Viannia subgenus, L. (V.) braziliensis and L. (V.) panamensis, cause facial disfigurement with high morbidity, and present an increasing public health problem in South America. Little is known about their surface glycoconjugates or other surface molecules involved in interaction with host cells. Muskus et al. (1997) showed that LPG levels in these species are only about 10% as high as in L. (Leishmania) species. Soares et al. (2005) recently demonstrated that L. (V.) braziliensis synthesizes only ~5% the amount of LPG expressed in other Leishmania species, and has unique sugar substitutions of metacyclic LPG. Regarding low molecular weight GLs, Zawadzki et al. (1998) showed that GIPLs are the major cell surface components of L. (V.) panamensis promastigotes, which present type 2 or hybrid-type glycan cores, with unusual glycan moieties attached to the 3-position of α1-3 linked mannose, such as Galα1-2Galβ- and Galα1-2/3Galα1-2Galβ1. Here, we describe the isolation and characterization of specific GLs from L. (V.) braziliensis promastigotes.
MATERIALS AND METHODS
Parasites
L. (V.) braziliensis promastigotes were cultured at 26 °C by several passages of log-phase parasites in Medium 199 supplemented with 10% heat-inactivated fetal calf serum (Cultilab, Campinas, SP, Brasil). Table 1 shows the International Codes of all Leishmania species and serodemes used.

Immunization procedure
Membranes were obtained by disrupting 1×109 promastigotes of L. (V.) braziliensis (M11272) (third passage) by nitrogen cavitation using bomb Parr (1500 psi, 25 min). The material was centrifuged at 3000 g for 10 min, and the supernatant was ultracentrifuged at 100000 g for 60 min. The two sediments were pooled and lyophilized. The material (8·4 mg) was dissolved in 2·5 ml of phosphate-buffered saline (PBS) and mixed with 34 mg acid-treated, heat-inactivated Salmonella minesotae (Silveira et al. 2001). The mixture was stirred for 1 h at 56 °C, lyophilized, and resuspended in 2·5 ml of distilled water. Aliquots (100 μl) of this suspension were used to immunize male BALB/c mice intravenously, once per week, over 3 weeks. After a 2-week resting period, the immune response of the mice was boosted with 200 μl of the immunogenic complex. Three days later, mice were sacrificed, and spleen cells were fused with NS-1 cells. Hybridomas secreting immunoglobulin reactive with L. (V.) braziliensis GLs were detected by solid-phase radioimmunoassay (RIA). Clones showing positive reactivity with GLs were re-cloned by limiting dilution.
Extraction and purification of GLs
Promastigotes (1×1011) were extracted 3 times with 30 ml of chloroform/methanol (CM) (2[ratio ]1; v/v) and 3 times with 30 ml isopropanol/hexane/water (IHW) (55[ratio ]20[ratio ]25; v/v/v) (Toledo et al. 1995). The extracts were rotary evaporated, dissolved in sodium acetate buffer (0·1 M, pH 6·5) containing 5% 1-propanol, and applied to an octyl-Sepharose column (20×150 mm) (McConville and Blackwell, 1991). The column was eluted with sodium acetate buffer containing an increasing concentration of 1-propanol (5%, 10%, 35%, 50%). The eluted fractions were rotary evaporated, dissolved in water, dialysed against distilled water, and dried. GLs were eluted with 35% 1-propanol, separated from phospholipids by Silica Gel 60 chromatography (10×150 mm column), equilibrated with CM (90[ratio ]10; v/v) and eluted by increasing methanol concentration (CM ratios 90[ratio ]10, 70[ratio ]30, 50[ratio ]50, 30[ratio ]70, 10[ratio ]90; v/v) and in IHW (55[ratio ]20[ratio ]25; v/v/v). Fractions were dried under a nitrogen stream at 37 °C, and resuspended in CM (2[ratio ]1; v/v) containing 5% water. GLs were identified by HPTLC on Silica Gel 60 plates using CM/ CaCl2 0·02% (60[ratio ]40[ratio ]9; v/v/v). GLs were visualized under UV as purple spots after spraying with 0·01% primulin in 80% aqueous acetone, spraying with 0·5% orcinol in 3 M H2SO4, and heating for 5 min at 120 °C (Takahashi, Metoki and Hakomori, 1988). GLs were further purified by HPLC (Varian model 9010) on Iatrobeads column (4·6×300 mm, 6R-8010; Iatron, Tokyo) (Straus et al. 1993), and eluted on a gradient of IHW from 55[ratio ]43[ratio ]2 to 55[ratio ]30[ratio ]15 (v/v/v, 175 min, flow rate 2·0 ml/min). Phosphodiester linkages were detected by staining with Dittmer-Lester reagent (Dittmer and Lester, 1964). GLs were quantified by phenol-sulfuric acid reaction, a colorimetric method for carbohydrate determination (Dubois et al. 1956).
High molecular weight GLs such as LPG were purified from delipidated residue (i.e. precipitate of parasites extracted with CM and IHW) by incubation of this residue with 40 ml of water saturated with 1-butanol (McConville and Blackwell, 1991). The resulting suspension was kept overnight at 4 °C under agitation, the extract was rotary evaporated, dissolved in sodium acetate buffer (0·1M, pH 6·5) containing 5% 1-propanol, and applied to an octyl-Sepharose column (20×150 mm). The column was eluted with sodium acetate buffer containing increasing concentration of 1-propanol (5%, 10%, 35%, 50%). The LPG was eluted with 35% 1-propanol.
HPTLC immunostaining
GLs were separated on HPTLC plates using CM/CaCl2 0·02% (60[ratio ]40[ratio ]9; v/v/v). After development, plates were dried, soaked in 0·5% polymethacrylate in petroleum ether, dried, and blocked for 2 h with 1% bovine serum albumin (BSA) in PBS. Plates were incubated overnight with monoclonal antibody or serum from a patient infected with L. (V.) braziliensis, followed by sequential incubation with rabbit anti-mouse IgG or rabbit anti-human Ig and 125I-labelled protein A (2×106 cpm per 50 ml BSA/PBS) for 45 min, as described by Straus et al. (1993).
Binding assay
For solid-phase RIA binding assay, GLs were adsorbed on 96-well plates (Falcon Microtest III, Oxnard, CA) (Suzuki et al. 1997). GLs dissolved in ethanol were added (100 ng, first well), serially diluted, dried at 37 °C, and blocked with 1% BSA in PBS (200 μl/well) for 2 h. Plates were incubated overnight with mAb SST-1 (100 μl) at 4 °C. The amount of mAb bound to GL was determined by reaction with 50 μl of rabbit anti-mouse IgG. Plates were washed 3 times with PBS, and incubated with 50 μl 125I-labelled protein A in 1% BSA in PBS (about 105 cpm/well) for 1 h. Radioactivity in each well was measured by use of a gamma counter.
Cellular radioimmunoassay
The 96-well plates were pre-coated with 0·1% poly-L-lysine (molecular weight 500 kDa; Sigma) for 30 min. Promastigotes (2×106 per well) were added, plates were centrifuged for 10 min at 850 g, and parasites were fixed for 15 min with 0·5% glutaraldehyde in cold PBS. Plates were washed with PBS, unbound aldehyde groups were blocked by addition of 0·1 M glycine, pH 8·0, for 30 min, and plates were washed again with PBS. For solid-phase RIA the plates were blocked with 1% BSA in PBS (200 μl) for 2 h, and incubated with mAb for 2 h. The amount of bound mAb was determined as described in the preceding section.
Periodate oxidation
The 96-well plates were coated with purified GLs of L. (V.) braziliensis and treated with 50 mM sodium m-periodate in sodium acetate buffer (0·1 M, pH 4·5) at room temperature for 1 h (Straus et al. 1993). The plates were washed with PBS, incubated with 50 mM sodium borohydride in PBS (50 μl/well) at 25 °C for 30 min in the dark, rinsed in PBS, incubated with blocking solution, and tested by solid phase RIA.
Purification of mAbs by Sepharose-protein A chromatography
Ascites of mAbs SST-1 and MEST-1 (an IgG3 mAb that does not react with L. (V.) braziliensis; Suzuki et al. 1997) were diluted 10-fold in Tris-HCl 0·05 M, pH 8·2, containing 0·15 M NaCl, and applied on a 5-ml Sepharose-protein A column. The column was extensively washed with the same buffer, and bound mAb was eluted with glycine-HCl 0·05 M, pH 3·0, containing NaCl 0·15 M. Purity of mAb preparations was tested by SDS-PAGE, and activity was checked by solid-phase RIA. Protein was quantified by the Bradford method (Bradford, 1976).
Preparation of Fab fragments
Purified mAbs were dialysed against 20 mM phosphate buffer (pH 7·0) containing 10 mM EDTA. Approximately 10 mg pure mAb was incubated with 0·5 ml of agarose-immobilized papain (Pierce) in 20 mM phosphate buffer containing 10 mM EDTA and 20 mM cysteine-HCl. The mixture was kept at 37 °C for 5 h with constant stirring, centrifuged, and the supernatant was dialysed against PBS for 24 h. Fab fragments were separated from intact mAbs and Fc fragments on a Sepharose-protein A column. Purity of Fab fragments was analysed by SDS-PAGE, and protein concentration was determined by the Bradford method.
Indirect immunofluorescence
Parasites (1×108) were fixed with 1% formaldehyde in PBS for 10 min. Cells were washed, resuspended in 1 ml of PBS, and 20 μl of the suspension was added to cover-slips. Air-dried preparations were delipidated with 150 μl of IHW (55[ratio ]20[ratio ]25; v/v/v) for 15 min and with 150 μl CM (2[ratio ]1; v/v) for 15 min (Silveira, Takahashi and Straus, 2003). Cells on the cover-slips were blocked with 5% BSA in PBS, and incubated sequentially with primary antibody (SST-1) or serum from a patient infected with L. (V.) braziliensis (1 h), and FITC-conjugated goat anti-mouse IgG or anti-human Ig (1 h). Cover-slips were washed 5 times with PBS after each incubation, and examined by epifluorescence microscopy. Alternatively, live parasites were incubated with mAb SST-1 for 30 min, washed with PBS, fixed with formaldehyde, and placed on cover-slips. Air-dried cover-slips were blocked with 5% BSA, and incubated with FITC-conjugated goat anti-mouse IgG for 1 h. In control experiments, an irrelevant IgG3 mAb (CU-1; Takahashi et al. 1988) was used, and no fluorescence was observed. Negative reactivity was defined as complete lack of fluorescence on the parasites.
Preparation of peritoneal macrophages
Peritoneal macrophages were harvested by washing the peritoneal cavity of BALB/c mice with PBS. Macrophages were washed 3 times with cold PBS, centrifuged at 400 g, and the final pellet was resuspended in RPMI 1640 supplemented with 10% fetal calf serum, 10 mM HEPES, and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively). Macrophages (~5×105) were placed on 13mm sterile glass cover-slips in 24-well plates. Non-adherent cells were removed by several washes with RPMI 1640, and the plates were kept at 37 °C in a CO2 incubator (Straus et al. 1993).
Inhibition of Leishmania infectivity in macrophages by mAb
Promastigotes (5×106 per 150 μl) were pre-incubated with various concentrations of Fab fragment (0·25 μg) for 1 h, washed with RPMI 1640, and incubated with peritoneal macrophages (10 parasites/macrophage, 5×106 parasites/well) for 2 h in RPMI 1640 without serum at 37 °C. Non-adherent parasites were removed by washing the monolayers with medium. Infected macrophages were maintained in RPMI 1640 with 5% fetal calf serum in a CO2 incubator for 24 h. The macrophages were fixed with methanol and stained with Giemsa solution. The phagocytic index was determined by multiplying the percentage of macrophages that had phagocytosed at least 1 parasite by the average number of parasites per infected macrophage (300 cells were examined) as described by Straus et al. (1993). Inhibition was expressed as percentage phagocytosis in the presence of mAb, relative to control (no mAb).
RESULTS
HPTLC of GL fraction in Leishmania (Viannia) braziliensis promastigotes
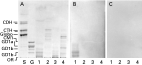
GL fraction purified from promastigotes of L. (V.) braziliensis was analysed by HPTLC. Promastigotes express GLs with chromatographic migration slower than GLs having 4 carbohydrate chains (tetraosylceramide standard) (Fig. 1A). The chromatographic pattern of L. (V.) braziliensis promastigote GLs was different from those observed for L. (V.) braziliensis amastigotes, which presented more hydrophobic components, and for L. (L.) amazonensis promastigotes and amastigotes. Total carbohydrate content of the GL fraction was estimated as 1·5 mg per 1010 cells by the phenol/sulfuric acid method.

Fig. 1. HPTLC immunostaining of GL fractions from Leishmania (V.) braziliensis and L. (L.) amazonensis. GLs were extracted and purified as described in the Materials and Methods section, and subjected to HPTLC developed in CM/ aqueous CaCl2 0·02% (60[ratio ]40[ratio ]9; v/v/v). (A) Orcinol/H2SO4 staining. (B) Immunostaining with mAb SST-1. (C) Immunostaining with a pool of sera (dil. 1: 100) from patients infected with L. (V.) braziliensis. Lane S, neutral GL standard mixture containing ceramide dihexoside (CDH), ceramide trihexoside (CTH), and globoside (globo). Lane G, ganglioside standard mixture containing GM1, GD1a, GD1b, and GT1b. Lane 1, GL fraction from L. (V.) braziliensis promastigotes. Lane 2, GL fraction from L. (V.) braziliensis amastigotes. Lane 3, GL fraction from L. (L.) amazonensis promastigotes. Lane 4, GL fraction from L. (L.) amazonensis amastigotes. OR, origin.
Production of mAb directed to L. (V.) braziliensis promastigote GLs
After the immunization procedure described above, several positive hybridomas directed to promastigotes were produced, as detected by solid-phase RIA using 96-well plates adsorbed with L. (V.) braziliensis promastigotes. Only 1 of these also recognized purified promastigote GL fraction. This hybridoma was selected, cloned, and characterized. The mAb (IgG3 isotype) was termed SST-1, and its specificity to L. (V.) braziliensis GLs was analysed by HPTLC immunostaining (Fig. 1B). The mAb SST-1 reacted only with the GL fraction of L. (V.) braziliensis promastigotes, not with GLs from L. (V.) braziliensis amastigotes, or from L. (L.) amazonensis promastigotes or amastigotes. These antigens were also not reactive with sera of patients infected with L. (V.) braziliensis.
Reactivity of mAb SST-1 with GLs from various Leishmania species
SST-1 reactivity with GL fractions from various Leishmania species was determined by solid-phase RIA. The mAb was specific to L. (V.) braziliensis, reacting with as little as 10 ng of total GL. It was not reactive with GLs from promastigotes of the Leishmania subgenus (i.e. L. (L.) amazonensis, L. (L.) major, or L. (L.) chagasi) (Fig. 2A), nor with L. (V.) braziliensis LPG. To ascertain the importance of the carbohydrate moiety for SST-1 reactivity, we performed sodium m-periodate oxidation of L. (V.) braziliensis GLs followed by reduction with NaBH4. Under this condition the vicinal hydroxyl groups of carbohydrate were oxidized, and SST-1 reactivity with the GLs was abolished (Fig. 2B).

Fig. 2. Reactivity of mAb SST-1 with Leishmania GLs. Promastigote GL fraction and LPG preparation were serially diluted in ethanol and adsorbed on 96-well plates (200 ng carbohydrate in the first well). SST-1 binding was determined by RIA as described in the Materials and Methods section. (A) GL fraction of various species of Leishmania. (B) GLs of L. (V.) braziliensis adsorbed on 96-well plates oxidized with 50 mM sodium m-periodate in sodium acetate buffer (0·1 M, pH 4·5) shown as open circles, versus control (GL not oxidized) shown as solid circles.
GLs were also purified from 5 species of the Viannia subgenus, and from 6 L. (V.) braziliensis serodemes (Shaw et al. 1986). GL fractions were resuspended to normalize the number of parasites, and SST-1 reactivity was analysed by RIA. SST-1 reacted with GLs from all 7 L. (V.) braziliensis serodemes (Fig. 3A), and to a lesser extent with GLs from L. (V.) guyanensis and L. (V.) naiffi (Fig. 3B).

Fig. 3. Reactivity of mAb SST-1 with GLs of various Leishmania species. GL fractions from 1·5×106 promastigotes (corresponding to 200 ng carbohydrate in L. (V.) braziliensis) were adsorbed on 96-well plates. SST-1 binding was determined by solid-phase RIA as described in the Materials and Methods section. (A) GLs of various L. (V.) braziliensis serodemes were tested. (B) GLs from various species of the Viannia subgenus were serially diluted and tested.
Isolation of GL antigens from L. (V.) braziliensis
GLs were extracted from L. (V.) braziliensis promastigotes, purified, and fractionated by HPLC as described in the Materials and Methods section. A typical HPLC elution pattern of L. (V.) braziliensis promastigote GLs is shown in Fig. 4A. Fractions containing antigenic bands were visualized by immunostaining with SST-1 (Fig. 4B). Fractions 58–62 and 69–73, corresponding respectively to bands 3 and 5, were reactive with SST-1. These fractions were eluted in a gradient of 11–15% water. Bands 1 (fraction 51–55), and 4 (fractions 63–65) did not react with SST-1. Purified GL fractions were stained by Dittmer-Lester's reagent to indicate the presence of phosphodiester linkages.

Fig. 4. HPLC elution profile of GL fractions from Leishmania (V.) braziliensis promastigotes, and their reactivity with mAb SST-1. HPLC fractions, eluted with a gradient of IHW from 55[ratio ]43[ratio ]2 to 55[ratio ]30[ratio ]15 (v/v/v), were applied on an HPTLC plate and developed in CM/ aqueous CaCl2 0·02% (60[ratio ]40[ratio ]9; v/v/v). (A) Orcinol/H2SO4 staining. (B) SST-1 immunostaining. Lane S, neutral GL standard mixture containing CDH, CTH, and globoside. Lane G, ganglioside standard mixture containing GM1, GD1a, GD1b, and GT1b. Lane NF, GLs not fractionated. OR, origin. Fractions 58–62 and 69–73, corresponding to Bands 3 and 5 respectively, were reactive with mAb SST-1. These fractions were eluted in a gradient containing 11–15% water.
Indirect immunofluorescence of promastigotes with mAb SST-1
The indirect immunofluorescence technique revealed a strong fluorescence with SST-1 on entire L. (V.) braziliensis promastigotes, using either formaldehyde-fixed (Fig. 5C) or non-fixed parasites (Fig. 5A). When fixed parasites were delipidated by a mixture of IHW, no SST-1 reactivity was observed (Fig. 5E). Under this condition, other antigens such as glycoproteins and proteins are not removed, as shown by reactivity of sera from patients infected with L. (V.) braziliensis (Fig. 5G). These results indicate that SST-1 recognizes GL antigens present on the surface of L. (V.) braziliensis promastigotes.

Fig. 5. Indirect immunofluorescence of Leishmania (V.) braziliensis promastigotes using mAb SST-1. (A, C, E and G) Fluorescence. (B, D, F and H) Phase contrast. (A and B) Live parasites incubated with SST-1. (C and D) Fixed parasites incubated with SST-1. (E and F) Fixed promastigotes delipidated with IHW (55[ratio ]20[ratio ]25; v/v/v) and CM (2[ratio ]1; v/v) incubated with SST-1. (G and H) Fixed promastigotes delipidated with IHW (55[ratio ]20[ratio ]25; v/v/v) and CM (2[ratio ]1; v/v), incubated with sera of cutaneous leishmaniasis patients.
Reactivity of mAb SST-1, by indirect immunofluorescence, with different species of Leishmania is summarized in Table 2. Positive fluorescence was observed for promastigotes of all L. (V.) braziliensis serodemes tested, as well as L. (V.) braziliensis reference strains such as M2903 and M2904. SST-1 recognized L. (V.) guyanensis and L. (V.) naiffi promastigotes, but did not react with L. (V.) braziliensis amastigotes, or promastigotes from the Leishmania subgenus.

Effect of SST-1 Fab fragments on Leishmania infectivity
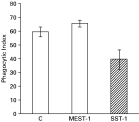
To test whether antigens recognized by mAb SST-1 are involved in Leishmania-macrophage interaction, L. (V.) braziliensis promastigotes were pre-incubated with Fab fragments of SST-1 or of an irrelevant antibody, and infectivity on macrophage monolayers was analysed. SST-1 Fab fragments reduced the phagocytic index ~34% (Fig. 6), but this reduction was not significant (P>0·05). MEST-1 Fab fragments had no effect, as expected, since this mAb does not recognize L. (V.) braziliensis.

Fig. 6. Macrophage phagocytic index of promastigotes incubated with Fab fragments of SST-1 and MEST-1. Fab fragments of both mAbs (0·25 μg) were pre-incubated with 5×106 parasites, then incubated with mouse macrophages for 2 h at 37 °C. Non-adherent parasites were removed and infected macrophages were maintained in a CO2 incubator for 24 h, as described in the Materials and Methods section. Data represent mean values (±S.E.M.) from triplicate experiments. The phagocytic index is the percentage of infected macrophages multiplied by the average number of parasites per infected macrophage. For control (C), parasite pre-incubation was carried out in the absence of antibody.
DISCUSSION
Previous studies on GLs of the L. Viannia subgenus were focused on L. (V.) panamensis (Muskus et al. 1997; Zawadzki et al. 1998). The present study showed that GLs of L. (V.) braziliensis promastigotes, termed bands 1, 2, 3, and 4, have low chromatographic migration, and a concentration of ~1·5 mg per 1010 promastigotes. In contrast, amastigotes virtually do not express these GLs but express a set of more hydrophobic glycolipids, indicating that a change in cell membrane glycoconjugate composition is associated with cell phase differentiation in L. (V.) braziliensis, and may be associate with their survival and proliferation in host macrophages.
The presence of epitope Galα1-3Gal in L. (V.) panamensis and L. (L.) major was demonstrated by Avila and Rojas (1990) using sera of cutaneous leishmaniasis patients. In contrast, in the present study, GLs from L. (V.) braziliensis did not react with sera of leishmaniasis patients. This strongly suggests that L. (V.) braziliensis GLs differ from L. (V.) panamensis or L. (L.) major GLs, which present Galα1-3Gal epitope recognized by anti-Galα1-3 antibodies (Avila, Rojas and Galili, 1989).
Characterization of L. (V.) braziliensis GLs was carried out using mAb SST-1. This mAb, produced against L. (V.) braziliensis promastigote membranes, is able to detect by RIA a low amount of GL antigen – from ~50 ng promastigote GL fraction. SST-1 recognizes a carbohydrate epitope present in lipidic molecules, as demonstrated by the great decrease in SST-1 reactivity following oxidation of GL with sodium m-periodate. The epitope recognized by SST-1 is present exclusively in the low-molecular weight GL fraction, as demonstrated by observations that only bands 3 and 5 were recognized by SST-1 in HPTLC immunostaining, and SST-1 did not cross-react with LPG fraction purified from promastigotes. Likewise, in an indirect immunofluorescence study, SST-1 reactivity was abolished when parasites underwent delipidation with IHW. In SST-1 immunoprecipitation of parasites surface labelled with 125I, no radioactivity was detected in the immunoprecipitate (data not shown). No difference in SST-1 reactivity was observed between promastigotes isolated from logarithmic versus stationary phase.
The GLs extracted from L. (V.) braziliensis are glycoglycerolipids, since they were susceptible to alkaline hydrolysis. Preliminary GC-MS analysis of band 2, the major SST-1-reactive antigen, indicated the presence of palmitic acid, inositol, glucosamine, mannose, and glucose (Yoneyama et al., unpublished data).
GLs recognized by SST-1 were detected only in promastigotes of the L. braziliensis complex, specifically in L. (V.) braziliensis, L. (V.) guyanensis, and L. (V.) naiffi. SST-1-reactive GLs were expressed in all 7 L. (V.) braziliensis serodemes tested. Although SST-1-reactive GL antigens are present in the parasite surface, pre-incubation of parasites with SST-1 Fab fragments resulted in insignificant (~34%) inhibition of macrophage infectivity. Since other GLs, such as bands 1, 2 and 4 are also expressed in these parasites, we cannot rule out the possibility that GLs not reactive with SST-1 may also be involved in L. (V.) braziliensis promastigote-macrophage interaction.
Studies are in progress to determine whether these GLs specific to the L. Viannia subgenus are associated with the preferential development of these promastigotes in the hindgut of the sandfly vector, or with high metastatic potential in the mammalian host.
The authors thank Dr Stephen Anderson for editing of the manuscript and Maria Vieira Seles for technical assistance. This work was supported by FAPESP, CNPq and CAPES.