In 2009, a new strain of influenza A (H1N1) spread rapidly around the world and caused the first global influenza pandemic of the 21st century. The “early phase” started in April 2009, with outbreaks in Mexico and the United States. The World Health Organization (WHO) declared it a “public health emergency of international concern.” 1 On 27 April 2009, the first cases were confirmed in Europe. 2 The WHO then declared a phase 4 pandemic alert. 3 Two days later, 148 cases were reported in 9 different countries. Furthermore, 7 deaths were reported in Mexico and 1 in the United States. 4 The WHO responded by raising the pandemic alert to level 5. 5 On 10 June 2009, a total of 27,737 cases and 141 deaths were reported in 74 countries. 6 During the early phase, most countries implemented measures according to a containment/delaying strategy, which aimed to limit the spread of the virus. This strategy included the use of antiviral drugs for early treatment of cases or prophylaxis of close contacts, isolation of cases, and quarantining of contacts. The “pandemic peak phase” started on 11 June 2009, when the pandemic alert was raised to phase 6. 7 In the second half of June, the first deaths in Europe and Asia were confirmed and the first case of oseltamivir (Tamiflu; Genentech) resistance was found in Denmark. 8 , 9 On 31 July 2009, 1154 deaths were reported in 5 of the 6 WHO regions. 10 During this peak phase, most countries focused on a mitigation strategy aimed at minimizing the impact of the pandemic by recommending personal protective measures, including frequent hand washing, covering the mouth when coughing, and social distancing (eg, maintaining physical distance from people with flu symptoms and avoiding crowded places). In August 2009, the intensity of most outbreaks was similar to that of seasonal epidemics, and the virus did not mutate to a more pathogenic form. Therefore, on 10 August 2009, the WHO declared a “post-pandemic phase.” 11 Despite the end of the pandemic, the H1N1 vaccine first became available during the post-pandemic phase.
The general public plays an important role in controlling the spread of a virus and in minimizing the impact of a pandemic by adopting government-recommended preventive measures. Theoretical models, like the Protection Motivation Theory, have suggested that behavioral action may be influenced by public perceptions of disease severity, personal susceptibility to the disease, effectiveness of recommended measures, and self-efficacy (confidence in the ability to perform the recommended measures).Reference Norman, Boer and Seydel 12 Public behavior may also be influenced by knowledge and more affective factors, like feelings of anxiety.Reference Rogers 13 , Reference Chapman and Coups 14 Insight into public perceptions and behaviors during a pandemic can provide useful information for risk communication. The influenza A (H1N1) pandemic was a unique situation; it was characterized by changes in risk, publicity, and recommended measures during the different phases. This scenario provides a unique opportunity to gain insight into public perceptions and behaviors, changes over time, and differences between countries. From 2009 to 2012, studies were conducted worldwide on this topic. Systematic literature reviews were performed by Bish et al,Reference Bish and Michie 15 , Reference Bish, Yardley and Nicoll 16 Blasi et al,Reference Blasi, Aliberti and Mantero 17 Brien et al,Reference Brien, Kwong and Buckeridge 18 and Nguyen et al,Reference Nguyen, Henningsen and Brehaut 19 but these examined predictors of behavior. In the present systematic literature review, we aimed to describe public perceptions and behaviors with a special focus on (1) trends over time, and (2) differences between inhabitants of various countries.
Methods
Search Strategy and Search Criteria
A systematic literature search for studies on public perceptions and behaviors during the pandemic was performed on 13 October 2011 and updated on 14 December 2012. We searched the PubMed, Embase, and PsychINFO databases with predefined online search terms. We used terms that represented public perceptions of risk (perceived disease severity and vulnerability), feelings of anxiety, intentions to take preventive measures, and actual behavior. The online search terms are given in detail in the online data supplement to this article.
Inclusion criteria were as follows: studies that focused on the general population and measured actual perceptions or behaviors during the pandemic (publication date of 2009 or later). Data had to be obtained with a quantitative study methodology, and only articles published in the English language were included.
Studies were excluded when they targeted a specific group, like health care workers, parents, pregnant women, students, or patients at risk. Furthermore, we excluded editorials, letters (unless they provided data), posters, and qualitative studies.
We followed the PRISMA guidelines for the literature search and preparation of the article.Reference Moher, Liberati and Tetzlaff 20 Of the 5498 records identified, 5385 records were excluded by the first author (MB) on the basis of title or abstract. The full-text articles (n=113) were independently screened by both the first author (MB) and the second author (HV). Any disagreement between the reviewers was discussed and resolved by consensus. The quality of the included papers was assessed by creating a quality score based on response rate and sample methodology. In social/behavioral science, response rates above 30% are indicated as appropriate. We gave scores for the response rate ranging from 1 to 3 as follows: 1, response rate <10% or not described; 2, response rate of 10% to 30%; and 3, response rate >30%. Sample methodology was scored from 1 to 3 as follows: 1, convenient samples or not described; 2, representative sample methodology for a defined geographic area; and 3, representative sample methodology for the whole country. A quality score for each paper was created by summing the scores for response rate and sample methodology, ranging from 2 (low quality) to 6 (high quality). All studies are described in the tables, regardless of quality score. However, in the Results, we focus mainly on studies with a score of ≥2 for response rate and ≥2 for sample methodology (quality score ≥4). When lower-quality studies are described, the response rate or quality of sample methodology is noted. Data from the eligible studies were extracted (by MB) and categorized according to the pandemic phase and region, as defined by the WHO.Reference About WHO. 21 Trends over time were extracted from follow-up studies or studies with multiple cross-sections, ie, measuring real trends over time. Regional differences were mostly extracted from those studies that included multiple countries or regions. These data are available in the online data supplement.
Results
The total search identified 5498 records, and 5385 were excluded on the basis of title or abstract. A total of 113 full-text articles were assessed, and 70 met the final inclusion criteria (Figure 1). The characteristics of the studies included in this review are described in Table 1. Studies were conducted in Europe (n=23), Asia (n=18), the United States (n=14), Australia (n=8), the Eastern Mediterranean (n=3), and North America (n=1); 3 studies collected data in more than one country or region. Most studies collected data during the post-pandemic phase only (n=38); some collected data over 2 or more phases (n=18) (Table 2). The number of respondents per study ranged from 186 to 22 050, with response rates ranging from 3% to 98%. Most studies were telephone-based surveys (n=39), most used a representative sampling methodology for the whole country (n=35) or for a defined geographic area (n=18), and most used a cross-sectional design (n=60). Ten studies used a time series design with multiple cross-sections (range, 2-36), and 10 studies followed the same respondents over time. Sixteen studies described one or more specific behavioral theories in the study rationale or for development of the questionnaire. On the basis of the quality assessment, most studies reached a quality score of 5 or 6 (n=46).
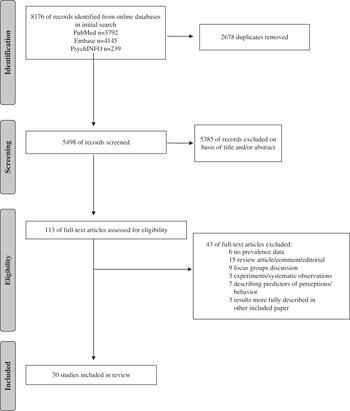
Figure 1 Systematic Review Process.
Table 1 Characteristics of the Studies Included in the Review (n=70)Footnote a

a CS indicates cross-sectional, CSM, Common Sense Model; EPPM, Extended Parallel Process Model; FU, follow-up; HBM, Health Belief Model; PAPM, Precaution Adoption Process Model; PMT, Protection Motivation Theory; RCF, Risk Communication Framework; SEM, Social Ecological Model; SRM, Self-regulation Model; TCM, Trust and Confidence Model; TDM, Trust Determination Model; TPB, Theory of Planned Behavior; TRA, Theory of Reasoned Action.
b Phase 1 = early phase (end of April to 11 June 2009); phase 2 = pandemic peak phase (11 June to 10 August 2009); phase 3 = post-pandemic phase (from 10 August 2009 onward).
c Specific behavioral theory described in the introduction or used for developing the questionnaire.
d Specific data collection period not defined, results included in description pandemic peak phase.
Table 2 Included Studies (Reference Numbers) on Public Perceptions and Behavioral Responses, by Pandemic Phase and World Health Organization RegionFootnote a

a ID indicates infectious diseases. World Health Organization regions: http://www.who.int/about/regions/en/index.html.
Public Knowledge About Influenza A (H1N1)
High knowledge levels about the main modes of transmission of the H1N1 virus (ie, through droplets or close contact with infected people) were observed among the general public in different countries during the various pandemic phases (online data supplement).Reference Aburto, Pevzner and Lopez-Ridaura 22 , Reference Balkhy, Abolfotouh and Al-Hathlool 24 , Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Lau, Yeung and Choi 53 , Reference Lin, Huang and Nie 59 , Reference Marshall, Tooher and Collins 60 , Reference Wong and Sam 88 , Reference Wong and Sam 90 , Reference Yi, Nonaka and Nomoto 91 Nevertheless, several misconceptions or unconfirmed beliefs were apparent, for example, about other modes of transmission of the H1N1 virus, such as transmission through an oral-fecal route, across long distances, via water sources, via insect bites, by eating improperly cooked pork or pork products, or via a sexual route.Reference Balkhy, Abolfotouh and Al-Hathlool 24 , Reference Cowling, Ng and Ip 31 , Reference Lau, Griffiths and Au 52 , Reference Wong and Sam 88 , Reference Wong and Sam 90 Changes in influenza terminology (“swine flu” and “H1N1”) were reported to have caused some confusion, as reported in a study conducted in the United States (Arizona) during the post-pandemic phase.Reference Jehn, Kim and Bradley 44 Furthermore, suboptimal knowledge levels were observed regarding recommended preventive measures. For example, a substantial proportion of Hong Kong respondents during the early phase erroneously believed that the government recommended that the public regularly use face masks in public venues and avoid visiting crowded places.Reference Lau, Griffiths and Choi 51 Although high awareness of personal hygiene measures was observed in studies in the United States (Arizona),Reference Jehn, Kim and Bradley 44 Italy,Reference Ferrante, Baldissera and Moghadam 34 and China (7 urban regions and 2 rural areas)Reference Lin, Huang and Nie 59 during the post-pandemic phase, interpretation of these general recommendations varied widely.Reference Kiviniemi, Ram and Kozlowski 47
In particular, regarding the H1N1 vaccine, part of the public in the Netherlands,Reference Bults, Beaujean and de Zwart 29 Hong Kong,Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 53 and South Australia,Reference Marshall, Tooher and Collins 60 respectively, had the misconception that during the early and pandemic peak phases a vaccine was available, that a seasonal influenza vaccination could effectively prevent H1N1, and that the efficacy of the H1N1 vaccination had been confirmed in clinical trials.
Perceived Severity of Influenza A (H1N1)
Declining trends were observed in the perceived severity of H1N1, as reported in follow-up studies or studies with multiple cross-sections conducted in the United States,Reference Gidengil, Parker and Zikmund-Fisher 37 Netherlands,Reference Bults, Beaujean and de Zwart 29 and Hong KongReference Cowling, Ng and Ip 31 , Reference Lau, Griffiths and Au 52 (online data supplement). For example, the study in the Netherlands described that the percentage of respondents who perceived high severity decreased from 80% during the early phase in May to 39% during the post-pandemic phase in August.Reference Bults, Beaujean and de Zwart 29 A Hong Kong study reported that the perceived severity of H1N1 was high in April 2009 but declined to lower levels by the time the local epidemic began.Reference Cowling, Ng and Ip 31 Also, in studies with a low response rate (United States)Reference Ibuka, Chapman and Meyers 43 and using convenience sampling (Germany),Reference Reuter and Renner 70 declining trends in perceived severity were observed.
Although declining trends were observed in all regions, differences were found in the absolute levels of perceived H1N1 severity. For example, Blank et alReference Blank, Bonnelye and Ducastel 26 conducted a study in 5 countries late in the post-pandemic phase and described that perceived severity of H1N1 was higher in Mexico (3 largest cities), with over one-half of the respondents (51%) considering the severity of H1N1 to be serious, compared to China (3 largest cities; 26%), the United States (19%), France (9%), and Germany (5%).
Perceived Vulnerability to Influenza A (H1N1)
The perceived vulnerability among the general public increased over time during the early and pandemic peak phases, as reported in the United States,Reference Gidengil, Parker and Zikmund-Fisher 37 Netherlands,Reference Bults, Beaujean and de Zwart 29 , Reference van der Weerd, Timmermans and Beaujean 86 and Hong Kong (online data supplement).Reference Cowling, Ng and Ip 31 For example, the US study reported that the mean perceived risk of contracting H1N1 increased over the summer with a peak in September 2009.Reference Gidengil, Parker and Zikmund-Fisher 37 The study in the Netherlands described that in April 2009, 18% of the respondents perceived that they were quite or very susceptible to infection with H1N1, which increased to 30% in August 2009.Reference Bults, Beaujean and de Zwart 29 Also, a study with a low response rate (US) identified increasing trends in perceived vulnerability during the early phase.Reference Ibuka, Chapman and Meyers 43 Although increasing trends were observed during the early and pandemic peak phases, declining trends in perceived vulnerability were observed late within the post-pandemic phase, as reported in studies conducted in the United States,Reference Gidengil, Parker and Zikmund-Fisher 37 Germany,Reference Walter, Bohmer and Reiter 84 and Italy.Reference Ferrante, Baldissera and Moghadam 34
Despite increasing trends in the early and pandemic peak phases, absolute levels of perceived vulnerability remained relatively low in most countries, even during the pandemic peak and post-pandemic phases.Reference Blank, Bonnelye and Ducastel 26 , Reference Bults, Beaujean and de Zwart 29 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 53 , Reference Quinn, Kumar and Freimuth 67 , Reference Schwarzinger, Flicoteaux and Cortarenoda 73 , Reference Wong and Sam 90 , Reference Yi, Nonaka and Nomoto 91 Regional differences in perceived vulnerability were reported in a study by Blank et al,Reference Blank, Bonnelye and Ducastel 26 which was conducted late within the post-pandemic phase. They described that perceived vulnerability was higher in Mexico (3 largest cities), with 35% of the respondents considering the risk of catching H1N1 as serious, compared to the United States (19%), China (3 largest cities; 15%), France (10%), and Germany (4%). Furthermore, studies conducted during the pandemic peak phase showed that respondents perceived themselves as being less likely to get infected with H1N1 than were other individuals.Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Lau, Yeung and Choi 53 , Reference Quinn, Kumar and Freimuth 67
Feelings of Anxiety Regarding Influenza A (H1N1)
Studies that measured perceived anxiety tended to focus on 2 separate topics: the perceived anxiety about the pandemic/H1N1 virus in general and the perceived anxiety about becoming personally infected (online data supplement). The perceived anxiety about the pandemic/H1N1 virus in general showed decreasing trends in studies in the NetherlandsReference Bults, Beaujean and de Zwart 29 and Italy,Reference Ferrante, Baldissera and Moghadam 34 where it was reported that perceived anxiety waned in concert with the waning perception that the virus was an immediate threat. Comparable trends were observed in studies with a low response rate (United States)Reference Jones and Salathe 45 and using convenience sampling (Germany).Reference Reuter and Renner 70 The perceived anxiety about becoming personally infected increased, according to a UK study;Reference Rubin, Potts and Michie 72 the percentage of respondents who were worried about becoming personally infected increased from 10% to 17% during the early phase in May 2009 and to 33% during the peak phase in mid-July 2009.
Perceived anxiety about the pandemic/H1N1 virus in general varied among different countries in the Western Pacific and Southeast Asia. During the pandemic peak phase, high anxiety levels were reported in studies conducted in Australia (Queensland),Reference Leggat, Brown and Aitken 56 with over 50% of respondents concerned about H1N1 while traveling, and in Malaysia (Kuala Lumpur),Reference Wong and Sam 88 , Reference Wong and Sam 89 with 73% of respondents being (slightly) fearful of H1N1 infection. In Hong Kong,Reference Cowling, Ng and Ip 31 however, anxiety remained fairly low, with most respondents reporting no anxiety. In the Netherlands also, anxiety levels were generally low.Reference Bults, Beaujean and de Zwart 29 The perceived anxiety of becoming personally infected varied regionally. Rather low levels were observed in studies conducted in Hong Kong,Reference Cowling, Ng and Ip 31 , Reference Lau, Griffiths and Au 52 Australia,Reference Eastwood, Durrheim and Jones 33 , Reference Taylor, Stevens and Agho 81 the United Kingdom,Reference Rubin, Potts and Michie 72 France,Reference Schwarzinger, Flicoteaux and Cortarenoda 73 and Germany,Reference Walter, Bohmer and Reiter 84 whereas in the United StatesReference Horney, Moore and Davis 41 , Reference Jehn, Kim and Bradley 44 the fear of personal infection remained fairly high.
Perceived Efficacy of Preventive Measures
Studies conducted in the United States (New York state),Reference Kiviniemi, Ram and Kozlowski 47 Netherlands,Reference Bults, Beaujean and de Zwart 29 and Hong KongReference Lau, Griffiths and Au 52 showed that improving hygienic practice (ie, more frequent hand washing, using tissues when coughing or sneezing, cleaning or disinfecting things) was perceived as the most effective preventive measure (online data supplement). Only one study in the Netherlands investigated trends over time in perceived efficacy of measures.Reference Bults, Beaujean and de Zwart 29 That study showed inconsistent patterns: the perceived efficacy of some measures, like antiviral medication, tended to increase at first and then decrease later; other measures, like avoiding crowded places, tended to show the opposite pattern.
Perceived efficacy of vaccination was relatively high, although some variance was observed among countries. During the post-pandemic phase, vaccination against H1N1 was perceived as effective by 82% of respondents participating in a study in Taiwan,Reference Huang, Miao and Kuo 42 81% in the United States (New York state),Reference Kiviniemi, Ram and Kozlowski 47 76% in Malaysia (Kuala Lumpur),Reference Wong and Sam 90 and 53% in the Netherlands.Reference Bults, Beaujean and de Zwart 29
Perceived Self-Efficacy Regarding H1N1 Prevention
Perceived self-efficacy (confidence in the ability to prevent H1N1 infection or perform preventive measures) was measured in only 4 studies: 2 in Hong Kong,Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 54 1 in Malaysia (Kuala Lumpur),Reference Wong and Sam 88 and 1 in the Netherlands (data not shown).Reference Bults, Beaujean and de Zwart 29 The studies conducted in Malaysia (Kuala Lumpur)Reference Wong and Sam 88 and the Netherlands,Reference Bults, Beaujean and de Zwart 29 and one of the Hong Kong studies,Reference Lau, Griffiths and Au 52 measured trends over time in perceived self-efficacy to prevent H1N1 infection. They concluded that, during the early and pandemic peak phases, a decreasing percentage of respondents were confident that they or their family members could prevent an H1N1 infection in the next year. The study in the Netherlands showed that the perceived self-efficacy to perform preventive measures tended to decrease from May to August 2009.Reference Bults, Beaujean and de Zwart 29
Despite the declining trends, all 4 studies found relatively high levels of perceived self-efficacy to perform preventive measures.Reference Bults, Beaujean and de Zwart 29 , Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 54 , Reference Wong and Sam 88 For example, the study in the Netherlands reported that during the different pandemic phases, the majority of respondents felt confident in their ability to improve hygienic practice (88–91%), to seek medical consultation with the onset of flu symptoms (86–91%), and to get vaccinated against H1N1 (70–79%).Reference Bults, Beaujean and de Zwart 29 The Hong Kong study reported that, during the pandemic peak phase, 77% of respondents believed that they or their family members would be able to get an H1N1 vaccination.Reference Lau, Yeung and Choi 54
Intention to Take Measures
Declining trends were observed in the intention to receive H1N1 vaccination, in particular during the post-pandemic phase, as reported in studies conducted in the United States,Reference Gidengil, Parker and Zikmund-Fisher 37 Italy,Reference Ferrante, Baldissera and Moghadam 34 and the NetherlandsReference Bults, Beaujean and de Zwart 29 (online data supplement). For example, the US studyReference Gidengil, Parker and Zikmund-Fisher 37 reported that vaccination intention was highest at the beginning of the pandemic and decreased over time with the lowest point in January 2010. Also, in 2 other studies with an unreported response rate, conducted in GreeceReference Sypsa, Livanios and Psichogiou 80 and Germany,Reference Walter, Bohmer and Heiden 85 decreasing trends in vaccination intention were observed.
During the early phase, the intention to improve hygienic practice, seek medical consultation at the onset of flu symptoms, and take antiviral medication was generally high, as reported in studies conducted in the NetherlandsReference Bults, Beaujean and de Zwart 29 and Hong Kong.Reference Lau, Griffiths and Au 52 Furthermore, in Hong Kong most respondents reported that they would comply with quarantine measures and, if infected, would wear a facemask when going out.Reference Lau, Griffiths and Au 52 During the pandemic peak and post-pandemic phases, the intention to take preventive measures remained relatively high in most countries, including the United States (Arizona and New York state),Reference Jehn, Kim and Bradley 44 , Reference Kiviniemi, Ram and Kozlowski 47 the Netherlands,Reference Bults, Beaujean and de Zwart 29 and Australia (Queensland, New South Wales, and South Australia),Reference Brown, Aitken and Leggat 28 , Reference Marshall, Tooher and Collins 60 , Reference Taylor, Stevens and Agho 81 particularly in improving hygienic practice and social distancing. During the pandemic peak, 40% to 77% of respondents in the studies included in this review reported that they were willing to accept an H1N1 vaccination if offered.Reference Bults, Beaujean and de Zwart 29 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Lau, Yeung and Choi 53 , Reference Marshall, Tooher and Collins 60 , Reference van der Weerd, Timmermans and Beaujean 86 However, the intention to get vaccinated was highly sensitive to the availability of scientific evidence on efficacy and safety, the vaccination provider, and the cost. For example, in a US study,Reference Quinn, Kumar and Freimuth 67 only 9% of respondents were willing to get a new vaccine that had not been approved; in a Hong Kong study,Reference Lau, Yeung and Choi 53 only 5% would accept a vaccination in the absence of data on efficacy and safety. During the post-pandemic phase, the intention to get vaccinated against H1N1 varied widely. As reported in studies conducted in the United States, 30 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Horney, Moore and Davis 41 , Reference Jehn, Kim and Bradley 44 , Reference Kiviniemi, Ram and Kozlowski 47 , Reference Kumar, Quinn and Kim 49 , Reference Maurer, Uscher-Pines and Harris 61 the intention to get vaccinated varied between 9% and 64%. In European studies, the intention to get vaccinated varied, from 17% to 27% in France,Reference Raude, Caille-Brillet and Setbon 68 , Reference Schwarzinger, Flicoteaux and Cortarenoda 73 , Reference Setbon, Le Pape and Letroublon 77 10% to 36% in Italy,Reference Ferrante, Baldissera and Moghadam 34 56% in the United Kingdom,Reference Rubin, Potts and Michie 72 and 43% to 63% in the Netherlands.Reference Bults, Beaujean and de Zwart 29 , Reference van der Weerd, Timmermans and Beaujean 86 Studies conducted in AsiaReference Huang, Miao and Kuo 42 , Reference Wong and Sam 90 , Reference Yi, Nonaka and Nomoto 91 and AustraliaReference Eastwood, Durrheim and Jones 33 , Reference Taylor, Stevens and Agho 81 reported vaccination intention rates between 57% and 78%, and 65% and 67%, respectively.
Actual Behavior
Improved hygienic practice and social distancing were the most often reported preventive behaviors, as reported in studies conducted in Mexico,Reference Aburto, Pevzner and Lopez-Ridaura 22 , Reference SteelFisher, Blendon and Ward 79 the United States,Reference Jehn, Kim and Bradley 44 , Reference SteelFisher, Blendon and Ward 79 Argentina,Reference SteelFisher, Blendon and Ward 79 Saudi Arabia (Riyadh and Jeddah),Reference Balkhy, Abolfotouh and Al-Hathlool 24 Europe,Reference Bults, Beaujean and de Zwart 29 , Reference Prati, Pietrantoni and Zani 66 , Reference Setbon, Le Pape and Letroublon 77 , Reference SteelFisher, Blendon and Ward 79 and AsiaReference Cowling, Ng and Ip 31 , Reference Lau, Griffiths and Au 52 , Reference Lin, Huang and Nie 59 , Reference Miao and Huang 63 , Reference SteelFisher, Blendon and Ward 79 , Reference Wong and Sam 87 , Reference Wong and Sam 89 , Reference Yi, Nonaka and Nomoto 91 (online data supplement). A study in the Netherlands reported increasing trends in improving hygienic practice during the pandemic peak phase.Reference Bults, Beaujean and de Zwart 29 A study conducted in Malaysia reported increasing trends in staying at home, taking preventive medicine, and wearing masks, whereas washing hands regularly declined from August 2009.Reference Wong and Sam 87 Furthermore, decreasing trends were observed regarding social distancing measures (eg, avoiding public transport and crowded places) as reported in studies conducted in Hong KongReference Cowling, Ng and Ip 31 , Reference Lau, Griffiths and Au 52 and Italy.Reference Ferrante, Baldissera and Moghadam 34
Regional differences were observed during the post-pandemic phase. As reported by Steelfisher et al,Reference SteelFisher, Blendon and Ward 79 improved hygiene practice was higher among respondents in Mexico, Argentina, and the United States than in respondents in Japan and the United Kingdom. The use of facemasks was higher among respondents in Mexico and Japan (71% and 63%, respectively) than among those in Argentina (19%), the United Kingdom (11%), and the United States (8%).Reference SteelFisher, Blendon and Ward 79 Furthermore, social distancing behaviors were higher among respondents in Mexico (33–69%) and Argentina (15–61%) than among respondents in the United States (4–56%), Japan (4-43%), and the United Kingdom (2–21%).Reference SteelFisher, Blendon and Ward 79 Vaccination acceptance was rather low and varied from 2% to 19% as reported in studies conducted in Europe,Reference Blank, Bonnelye and Ducastel 26 , Reference Bohmer, Walter and Falkenhorst 27 , Reference Gilles, Bangerter and Clemence 38 , Reference Prati, Pietrantoni and Zani 66 , Reference Raude, Caille-Brillet and Setbon 68 , Reference Schwarzinger, Flicoteaux and Cortarenoda 73 , Reference SteelFisher, Blendon and Ward 79 , Reference Vaux, Van Cauteren and Guthmann 82 in ChinaReference Blank, Bonnelye and Ducastel 26 , Reference Lin, Huang and Nie 59 and JapanReference SteelFisher, Blendon and Ward 79 , Reference Yi, Nonaka and Nomoto 91 (10% to 25%), in Mexico (13% to 33%),Reference Blank, Bonnelye and Ducastel 26 , Reference SteelFisher, Blendon and Ward 79 and in the United States (9% to 41%).Reference Blank, Bonnelye and Ducastel 26 , Reference Galarce, Minsky and Viswanath 35 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Kumar, Quinn and Kim 49 , Reference Li, Chapman and Ibuka 57 , Reference Maurer, Uscher-Pines and Harris 61 , Reference SteelFisher, Blendon and Ward 79
Discussion
The public in the different countries was generally well informed about the main modes of H1N1 virus transmission, and the knowledge level remained relatively stable during the pandemic phases.Reference Aburto, Pevzner and Lopez-Ridaura 22 , Reference Balkhy, Abolfotouh and Al-Hathlool 24 , Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Lau, Yeung and Choi 53 , Reference Lin, Huang and Nie 59 , Reference Marshall, Tooher and Collins 60 , Reference Wong and Sam 88 , Reference Wong and Sam 90 , Reference Yi, Nonaka and Nomoto 91 Nevertheless, there were several misconceptions and unconfirmed beliefs, for example, about recommended preventive measures, especially vaccination, and about other modes of transmission of the H1N1 virus (eg, oral-fecal and sexual routes, water sources, insect bites, and eating pork products).Reference Balkhy, Abolfotouh and Al-Hathlool 24 , Reference Cowling, Ng and Ip 31 , Reference Kiviniemi, Ram and Kozlowski 47 , Reference Lau, Griffiths and Choi 51 , Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 53 , Reference Marshall, Tooher and Collins 60 , Reference Wong and Sam 88 , Reference Wong and Sam 90 This was caused, in part, by changes in influenza terminology (“Mexican flu,” “swine flu,” and “H1N1”). During past outbreaks of infectious diseases (eg, SARS and avian influenza), there were also public misconceptions and unconfirmed beliefs; these were associated with emotional distress of the general public.Reference Leung, Lam and Ho 92 , Reference Lau, Tsui and Kim 93
Declining trends were observed in perceived severity and feelings of anxiety about the pandemic/H1N1 virus.Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Ferrante, Baldissera and Moghadam 34 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Ibuka, Chapman and Meyers 43 , Reference Jones and Salathe 45 , Reference Lau, Griffiths and Au 52 , Reference Reuter and Renner 70 This was probably caused by intense media attention in most countries in the early phase. Representatives of international and national health organizations were predicting worst-case scenarios with large numbers of fatalities on the basis of influenza pandemics in the past. However, most local outbreaks of H1N1 turned out to be similar in intensity to seasonal flu epidemics. This led to declining trends in perceived severity and feelings of anxiety.
Increasing trends were observed in perceived vulnerability during the early and pandemic peak phases.Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Ibuka, Chapman and Meyers 43 , Reference van der Weerd, Timmermans and Beaujean 86 This was consistent with the fact that the number of infected and fatal cases increased rapidly during these phases. Despite this increasing trend, the perception of perceived vulnerability remained relatively low in most studies.Reference Blank, Bonnelye and Ducastel 26 , Reference Bults, Beaujean and de Zwart 29 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 53 , Reference Quinn, Kumar and Freimuth 67 , Reference Schwarzinger, Flicoteaux and Cortarenoda 73 , Reference Wong and Sam 90 , Reference Yi, Nonaka and Nomoto 91 Furthermore, most respondents believed that they were less likely to become infected with H1N1 than were other people during the pandemic peak phase.Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Lau, Yeung and Choi 53 , Reference Quinn, Kumar and Freimuth 67 This suggested that, during the pandemic, the general public in most countries was unrealistically optimistic (or had an “optimistic bias”) regarding the risk of contracting H1N1. This unrealistic optimism may have been influenced by the belief that the illness would be mild and the fact that people could protect themselves by taking preventive measures, which gave the general impression that the pandemic was under control.
Improving hygienic practice (ie, more frequent hand washing, using tissues when coughing or sneezing, cleaning or disinfecting things) was perceived as more effective than other nonpharmaceutical measures, like quarantining or face mask use.Reference Bults, Beaujean and de Zwart 29 , Reference Kiviniemi, Ram and Kozlowski 47 , Reference Lau, Griffiths and Au 52 Pharmaceutical measures, including vaccinations and antiviral medications, are generally very effective in preventing the spread and minimizing the impact of diseases. However, producing a vaccine against a new virus takes time, and resistance against antiviral drugs may occur; both of these factors occurred during the 2009 H1N1 pandemic. In the first phases of the pandemic, nonpharmaceutical measures were available and recognized by the WHO as potentially useful in reducing transmission of influenza.Reference Bell 94 A recent review of the efficacy of measures against influenza found evidence that hygiene measures and respiratory etiquettes reduced the spread of the virus.Reference Balinska and Rizzo 95 Some studies have shown efficacy for other nonpharmaceutical measures, including quarantine or face mask use, but correct implementation of these measures is often difficult, particularly for long periods of time.Reference Brienen, Timen and Wallinga 96 , Reference Aledort, Lurie and Wasserman 97
Of the 48 studies included in this review, only 4 measured perceived self-efficacy regarding preventive measures (ie, confidence in the ability to prevent H1N1 infection or to perform preventive measures).Reference Bults, Beaujean and de Zwart 29 , Reference Lau, Griffiths and Au 52 , Reference Lau, Yeung and Choi 54 , Reference Wong and Sam 88 Although self-efficacy is a construct within the Protection Motivation Theory, and comparable to “perceived behavioral control” in the Theory of Planned Behavior,Reference Connor and Sparks 98 this construct is not included in many other health behavioral theories. This may explain why only a few studies included perceived self-efficacy. Surprisingly, only around 20% (n=16) of the reviewed studies described one or more behavioral theories in the study rationale or for development of the questionnaire.
During the pandemic peak phase, the majority of respondents in most studies reported that they would be willing to accept an H1N1 vaccination if offered.Reference Bults, Beaujean and de Zwart 29 , 30 , Reference Horney, Moore and Davis 41 , Reference Huang, Miao and Kuo 42 , Reference Jehn, Kim and Bradley 44 , Reference Kiviniemi, Ram and Kozlowski 47 , Reference Maurer, Uscher-Pines and Harris 61 , Reference Rubin, Potts and Michie 72 , Reference Wong and Sam 90 , Reference Yi, Nonaka and Nomoto 91 However, the intention to get vaccinated was highly sensitive to the availability of scientific evidence on efficacy and safety, the vaccination provider, and personal cost.Reference Lau, Yeung and Choi 53 , Reference Quinn, Kumar and Freimuth 67 Furthermore, declining trends were observed in intention to receive an H1N1 vaccination, particularly during the post-pandemic phase.Reference Ferrante, Baldissera and Moghadam 34 , Reference Gidengil, Parker and Zikmund-Fisher 37 , Reference Sypsa, Livanios and Psichogiou 80 , Reference Walter, Bohmer and Heiden 85 As reported in several studies, reasons for the low rates of vaccination intention included a belief that the vaccine might be unsafe, a fear of side effects, doubts about vaccine efficacy, a belief that the risk of infection was low, and a belief that, if infected, the illness would be mild.Reference Blank, Bonnelye and Ducastel 26 , Reference Bohmer, Walter and Falkenhorst 27 , 30 , Reference Liao, Cowling and Lam 58 , Reference Seale, McLaws and Heywood 74 , Reference Sypsa, Livanios and Psichogiou 80 Actual vaccination acceptance was much lower than expected, because the vaccine was not available until the post-pandemic phase when the virus had run its course. Furthermore, the vaccination policies varied among (neighboring) countries; for example, there were differences in the target groups, the number of recommended doses, and the content of available vaccines.Reference Mereckiene, D'Ancona and Giambi 99 Some countries may have faced logistical and organizational issues, which caused poor uptake of vaccination. These factors elicited public debate, fueled by the media, about whether the benefits of the H1N1 vaccine outweighed the possible risks.
Regional differences in actual behavior were also observed. For example, Steelfisher et alReference SteelFisher, Blendon and Ward 79 reported that improved hygiene practice, face mask use, and social distancing were higher among respondents in Mexico than among respondents of other countries. The regional differences in the actual behavior may have been due to differences in the number of (fatal) cases and the information people received. Furthermore, in some countries, a specific preventive measure might be more acceptable than others. For example, in Mexico, the government advised citizens to use face masks on public transport and the Mexican army distributed 6 million masks.Reference Condon and Sinha 100 However, in other countries, face mask use was not widely recommended. In those countries, face masks appeared to be associated with negative feelings, like disease victimization and stigmatization.
A clear strength of this review is that it included only articles that measured actual perceptions or behaviors during the pandemic. This in contrast with other studies performed at times when pandemic influenza was not regarded as a high threat and scenarios were based on a hypothetical situation. Another strength was that trends over time were extracted from follow-up studies or studies with multiple cross-sections, ie, measuring real trends over time. Regional differences were mostly extracted from those studies that included multiple countries or regions.
The present literature review also had some limitations. First, a number of studies used a nonrepresentative sampling methodology (eg, convenient sampling); were conducted in a single state, city, or region; or had low response rates. This brought into question whether those results could be generalized to the general public of that country or region. Second, studies varied in the specific formulation of questionnaire items and answer scales.Reference Bults, Beaujean and de Zwart 29 , Reference Cowling, Ng and Ip 31 , Reference Lau, Griffiths and Au 52 , Reference Rubin, Potts and Michie 72 Third, regional differences were mostly extracted from studies including multiple countries. However, for perceived anxiety, (self-)efficacy, and intention, we assessed regional differences by comparing single-country studies because no multi-country studies were available. Furthermore, note that, although the WHO declared each specific alert phase for the entire world, variation existed in the number of cases and deaths among different regions and countries. For example, in Asia the timeline was slightly different and the actual peak occurred later, whereas in some countries there was a second peak of the epidemic in November–December 2009. Therefore, the phase announcements most likely had differential influences on public perceptions and behaviors. Fourth, most studies (n=39) were telephone-based surveys; 15 were internet-based, 7 were face-to-face interviews, 4 were paper-based, and 4 used a combination of these methods. Different data collection methods may have introduced biases, eg, the telephone-based surveys may have elicited more socially desirable answers compared with internet-based surveys. Fifth, most studies had short data collection periods and therefore only provided an indication of the perceptions and behaviors of the public at that specific point during the pandemic. Sixth, we presented the main constructs of the Protection Motivation Theory. However, other health behavior theories describe constructs that may also influence public behavior, like perceived barriers and benefits, social influence (social norms/pressure), and trust in government.Reference Connor and Sparks 98 , Reference Champion 101 Some constructs were included in many studies, in different pandemic phases, and in different WHO regions, but other constructs were measured in only a few studies during a specific phase or in a particular region. Therefore, it was difficult to extract the most important findings or to identify certain general patterns. Finally, a gray literature search was not performed. Therefore, the findings may be subjected to publication bias.
Despite these limitations, the findings of this review provide useful information for risk communication practice, policy, and further research during outbreaks of infectious diseases, which can contribute to achieving successful changes in public behavior that reduce the spread and impact of disease. First, concerning recommendations for risk communication practice, this review showed that during the H1N1 pandemic several misconceptions and unconfirmed beliefs were apparent. During future outbreaks of (emerging) infectious diseases, health authorities should regularly update their messages and include actual information on the number of (fatal) cases, chance of becoming infected, what is (un)known, and the benefits of preventive measures. Second, decreasing trends were observed in perceived severity, perceived anxiety, and intention to receive vaccination. This was probably caused by the fact that initially, the media representatives of (inter)national health institutes predicted a worse-case scenario with large numbers of fatal cases, based on influenza pandemics in the past and early reports concerning the pandemic potential of the H1N1 virus. During future outbreaks of infectious diseases, it is important that risk communicators be aware of the way they present their message in the media. Furthermore, they should present a range of scenarios, not only worst-case but also more balanced, positive scenarios. This is important to prevent misconceptions, to increase realistic risk perceptions and actual preventive behavior, and to build trust in public health authorities. A recommendation for risk communication policy is that research on risk perception and behavioral responses of the general public during outbreaks of infectious diseases be embedded in existing communication and preparedness and response plans. For further research, it is important to study how the results of research on public perception and behavior can be translated into risk communication and how to build, maintain, and restore public trust during different outbreak scenarios. Finally, few studies in this review used a theoretical framework (eg, a behavioral theory). We strongly recommend the use of health behavior theories when conducting studies on public perceptions and behavioral responses during outbreaks of infectious diseases. This approach is more likely to provide profound insights into perceptions, behaviors, and their underlying correlations. Moreover, the use of health behavior theories in studies on public perceptions and behavioral responses would greatly facilitate the development of effective public health interventions that counter the effect of an outbreak.
Conclusions
This review showed that public perceptions and behaviors are not stable and can evolve over a short period of time. Public misconceptions were apparent regarding modes of transmission and preventive measures. To prevent misconceptions during future outbreaks, it is important that health authorities provide up-to-date information about the virus and possible preventive measures. Therefore, health authorities should continuously monitor public perceptions and misconceptions. Because public perceptions and behavioral responses varied between countries during the pandemic, risk communication should be tailored to the specific circumstances of each country.
Funding
This work was performed as part of a project funded by the European Commission within the Seventh Framework Programme. The project is entitled “E-com@eu, Effective Communication in Outbreak Management: development of an evidence-based tool for Europe” (278763). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Acknowledgement
The authors thank Wichor Bramer of the Medical Library, Eramus MC, University Medical Center Rotterdam, for his guidance in developing the search strategy and support during the literature search.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/dmp.2014.160





