Introduction
Craniospinal irradiation (CSI) is a technique used to treat several tumours of the central nervous system (CNS). This technique is utilised in preventing recurrence of the tumour due to dissemination in the subarachnoid space via cerebrospinal fluid.Reference Barrett, Dobbs, Morris and Roques1 Medulloblastoma is an example of a CNS tumour that is currently treated with postoperative CSI due to its tendency to recur. Before the introduction of postoperative CSI, the 5-year survival for patients with medulloblastoma was 0%.Reference Fossati, Ricardi and Orrechia2 After 1970, the 5-year survival rate for medulloblastoma increased to 53%.Reference Fossati, Ricardi and Orrechia2 This marked increase in survival was mainly caused by the introduction of postoperative CSI.Reference Fossati, Ricardi and Orrechia2 While CSI is effective in increasing survival, the long-term sequelae of this treatment can affect the patient’s quality of life for years to come. It is the need to improve quality of life after treatment that should be driving the adoption of new techniques.
There has been an increasing interest in the use of proton therapy instead of conventional radiotherapy for the treatment of a number of tumour sites, as proton therapy has a minimal exit dose, which can theoretically spare organs at risk (OARs) from high doses. Although previous reviews have concluded that there is little high-quality evidence to support the use of proton therapy as a major treatment modality, it should be acknowledged that a broad brush approach may not be appropriate and that proton therapy may have a strong indication in the treatment of certain tumours.Reference Lodge, Pijls-Johannesma, Stirk, Munro, de Ruysscher and Jefferson3 The purpose of this systematic review is to establish the potential role of proton therapy in the treatment of patients requiring CSI and evaluate the cost-effectiveness of this approach.
Methods
For the purposes of this systematic review, a search was performed across the Medline, Scopus, Cochrane Library and Inspec databases to ensure coverage of a wide range of relevant publications. The search term ‘proton therapy craniospinal’ was used to search for results pertaining to the use of proton therapy in CSI in the aforementioned databases. This search returned 142 results. A second search, using the term ‘cost analysis proton therapy accelerator’ was also made to search for the departmental implications of proton therapy. The second search returned 45 results.
Exclusion criteria
Studies concerning patients who had previously had the craniospinal axis irradiated were excluded. This is because previous irradiation could have already caused damage to the OARs and could have also limited the prescribed dose, thereby reducing the effectiveness of treatment; therefore, the results of studies would be less valid if they considered previously irradiated patients. Studies in which the entire craniospinal axis was not irradiated were excluded. Studies that featured hybrid (proton and photon fields) or did not directly compare proton therapy to conformal radiotherapy were excluded as differing methods and outcomes of different studies would frustrate direct comparisons between proton therapy and other modalities.
Critical appraisal process
Systematic appraisal of the sources was performed according to the Scottish Intercollegiate Guidelines Network (SIGN) critical appraisal checklists.4 The SIGN checklists are well-established critical appraisal tools that feature a range of lists for differing evidence formats.
Results
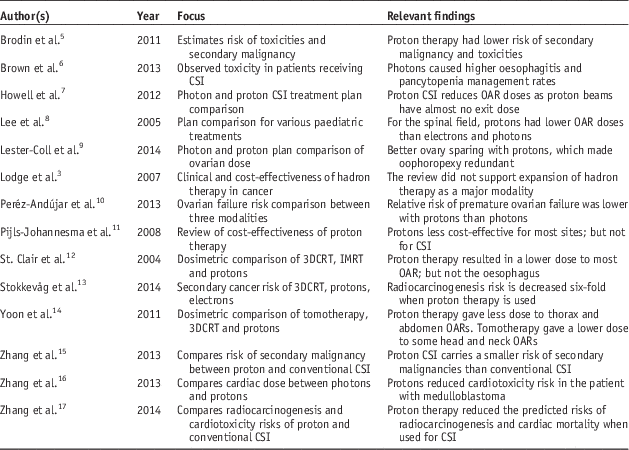
Out of the original 187 results, 16 publications remained after the exclusion criteria were applied. SIGN appraisal resulted in the rejection of two studies, leaving 14 publications, which form the basis for this literature review. One excluded publication was not relevant to the research question, while the other featured intervention bias. The included publications are summarised in Table 1.
Table 1 Publication characteristics

Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; CSI, craniospinal irradiation; IMRT, intensity-modulated radiation therapy; OAR, organs at risk.
Discussion
Organ sparing
One of the major disadvantages associated with conventional CSI is the large dose delivered to healthy tissue. Several studies have compared the use of proton therapy in CSI with conventional radiotherapy by comparing the doses to OARs. Other studies have analysed the incidence of various toxicities following proton therapy.
In the literature analysed in this review, proton therapy demonstrates a clear potential for organ sparing. In a 2004 study, proton therapy outperformed intensity-modulated radiation therapy (IMRT) and three-dimensional conformal radiation therapy (3DCRT) in terms of doses to the cochlea, pituitary gland, hypothalamus, temporomandibular joint, parotid salivary gland, pharynx, heart, lungs, stomach, kidneys and transverse colon.Reference St. Clair, Adams and Bues12 Proton therapy was superior for all OARs except for the oesophagus, due to the proximity of the oesophagus to the vertebral bodies.Reference St. Clair, Adams and Bues12 There were several strengths identified in this article; the prescriptions for all three modalities were consistent and the method clearly detailed how the treatments were planned. There were, however, several limitations, particularly that the reported OARs did not include the optic chiasm, brainstem, optic nerves, thyroid or lenses. The authors mentioned that the eyes were avoided with the IMRT plan, yet no data concerning optical structures were included. Other limitations included that only one patient was used for this study and no mention was made of whether or not the secondary neutron dose was taken into consideration.
Another similar study from 2012 analysed the doses to the optic chiasm, cochlea, brainstem, oesophagus, heart, kidneys, liver, lung and thyroid in 18 patients.Reference Howell, Giebeler and Koontz-Raisig7 The strengths of this study were the large cohort and depth of statistical analysis provided. The limitations of this study were the exclusion of secondary neutron dose from the calculation and frequent incomplete vertebral body irradiation. In addition this was the only study to use slightly angled lateral cranial fields to reduce optical structure dose. Overall, the publication was credible and the argument was well-structured. The findings of this article demonstrated that the OAR doses were lower for the oesophagus, heart, kidneys, liver, lungs and thyroid. The oesophageal dose had a high standard deviation due to the fact that not all patients had their vertebral bodies irradiated. Nonetheless, the oesophageal dose remained considerably lower with proton therapy. There was not as large a difference between the doses to the optic chiasm, cochlea and brainstem for the two modalities, meaning that proton therapy may not have benefit in the cranial field.
Lee et al.’sReference Lee, Bilton and Famiglietti8 study also supported the above findings, wherein the thyroid, lung, kidney, heart and liver doses were dramatically reduced with proton therapy.Reference Lee, Bilton and Famiglietti8 Unlike the aforementioned study, the oesophageal dose was not reported. The doses to the OARs for the cranial field were reduced with proton therapy, however, the reduction in dose was not as pronounced as in the spinal fields. While the secondary neutron dose was not accounted for in this paper, the results did suggest that the OAR doses were reduced with proton therapy.
The superior organ sparing of proton CSI was also demonstrated by Yoon et al.Reference Yoon, Shin and Kim14, who compared the doses to the oesophagus, stomach, liver, lung, pancreas, kidney, thyroid, lens and parotid salivary glands between proton therapy, 3DCRT and tomotherapy. In this study, proton therapy achieved lower average doses to the organs in the thorax and abdomen than 3DCRT or tomotherapy; however, tomotherapy was found to be superior when compared with proton therapy in sparing OARs in the head and neck as well as the kidneys. Tomotherapy was less effective than proton therapy in sparing the oesophagus from low doses, and more effective in sparing the oesophagus from high doses. Proton therapy achieved lower doses than 3DCRT for every OAR. These findings strengthen the suggestion that proton therapy was useful in reducing the risk of toxicities in thoracic and abdominal OARs. The main strength of this study was that it compared the doses between ten random patients and comparative dose–volume histograms for the OARs were shown.
The impact of proton therapy on the cardiac dose was discussed in the paper by Zhang,Reference Zhang, Howell and Homann16 wherein the authors concluded that in a medulloblastoma patient, the heart received less dose when treated with protons. The study had a very clear method and had taken into account the secondary dose from neutrons. The limitations were that it only used a single patient for each disease, and the method for predicting toxicity had many uncertainties.Reference Zhang, Howell and Homann16 This reduced the validity of the findings; however, they were supported by the author’s later article which also found that the risk of cardiac mortality was reduced when proton therapy was used.Reference Zhang, Howell, Taddei, Giebeler, Mahajan and Newhauser17 This second study improved on the previous study by having a total of 17 patients. The method was clearly detailed, and the secondary dose from neutrons had been considered again. The first study by Lee et al.Reference Lee, Bilton and Famiglietti8 also observed a reduction in the cardiac dose when proton therapy was used instead of 3DCRT. This reduction in cardiac dose decreased the risk of cardiac toxicity and mortality in later life, and could therefore potentially increase the patient’s quality and quantity of life.
There has also been interest in the use of proton therapy in the sparing of the ovaries. Lester-Coll et al.Reference Lester-Coll, Morse and Zhai9 reported that the dose to the ovaries reduced from 3·8 to 0·003 Sv when proton therapy was used. Peréz-Andújar et al.,Reference Peréz-Andújar, Newhauser, Taddei, Mahajan and Howell10 reported similar results, with proton therapy consistently outperforming 3DCRT and IMRT. While the studies had different prescriptions, both articles had taken various possible positions of the ovaries into consideration. Another strength of the article by Peréz-Andújar et al.Reference Peréz-Andújar, Newhauser, Taddei, Mahajan and Howell10 was that the dose from secondary neutrons had been considered, and thus the findings were more valid than the findings of Lester-Coll. Peréz-Andújar et al.Reference Peréz-Andújar, Newhauser, Taddei, Mahajan and Howell10 estimated that 3DCRT, IMRT and proton therapy would destroy 23·09, 15·86 and 7·71% of primordial follicles, respectively, in the patient’s ovaries.Reference Peréz-Andújar, Newhauser, Taddei, Mahajan and Howell10 In each of the other possible ovarian positions, a lower percentage of primordial follicles would be destroyed with proton therapy. These findings suggested that proton therapy offered an increased chance of fertility preservation compared with IMRT or 3DCRT.
Overall, proton therapy was found to result in superior OAR sparing, as seen in Figure 1. This graph shows the number of studies analysing each OAR, and also compares the efficacy of proton therapy to other modalities in organ sparing

Figure 1 Modalities offering greatest organs at risk sparing. Abbreviation: TMJ, temporomandibular joint.
Risk of toxicities
The risk of toxicities has been estimated in several studies. Brodin et al.Reference Brodin, Rosenschöld and Aznar5 estimated the risk of adverse effects and secondary malignancies between intensity-modulated proton therapy (IMPT), volumetric-modulated arc therapy and 3DCRT in paediatric medulloblastoma patients. The risk of secondary malignancies, pneumonitis, heart failure, xerostomia, blindness, hypothyroidism and ototoxicity were predicted to be markedly lower with IMPT. The strengths of this article included a strong literature base, a discussion of the rationale behind the models used and the consideration of secondary neutrons. As proton therapy has not been historically used for CSI, late toxicities may not yet have physically manifested in patients, and thus empirical measurement would be difficult.
The article by Brown et al.Reference Brown, Barney and Grosshans6 concluded that patients treated with protons experienced less upper gastrointestinal toxicity and lower rates of haematological toxicities. The study included 40 patients, all of whom were adults. In this study, none of the patients had the entire vertebral body treated, which reduced the risk of both oesophagitis and pancytopenia.Reference Brown, Barney and Grosshans6 This was realistic, as the vertebrae of adults have finished developing, and as a result the entirety of the vertebral body does not require treatment to prevent iatrogenic growth deformities. Consequently, these results are not necessarily valid for paediatric patients as they do require the entire vertebral body to be irradiated. While this study demonstrated that proton therapy reduced acute toxicities, further studies analysing toxicities among paediatric patients are also required to produce more conclusive results on gastrointestinal and haematological toxicities associated with proton CSI.
Risk of secondary malignancy
Another area of concern identified in the literature was the risk of secondary malignancy. This is of concern as paediatric cancer patient survival rates have increased dramatically, along with the potential of developing radiation related malignancies later in life. Several articles have analysed the risk of secondary malignancies following proton therapy and compared the risks between different modalities.Reference Brodin, Rosenschöld and Aznar5, Reference Stokkevåg, Engeseth and Ytre-Hauge13, Reference Zhang, Howell, Giebeler, Taddei, Mahajan and Newhauser15, Reference Zhang, Howell, Taddei, Giebeler, Mahajan and Newhauser17 All of the studies utilised a prescription of 23·4 Gy, while the article by Brodin et al.Reference Brodin, Rosenschöld and Aznar5 also analysed the risks associated with a prescribed dose of 36 Gy. Zhang et al., Brodin et al. and Stokkevåg et al.Reference Brodin, Rosenschöld and Aznar5, Reference Stokkevåg, Engeseth and Ytre-Hauge13, Reference Zhang, Howell, Giebeler, Taddei, Mahajan and Newhauser15 utilised linear, linear exponential and plateau dose–response models. Table 2 lists the results of these three studies as a percentage, but it is important to note that Brodin et al. and Zhang et al. provided percentage figures based on the results of all of the models, whereas Stokkevåg et al. only provided percentages for each model.Reference Brodin, Rosenschöld and Aznar5, Reference Stokkevåg, Engeseth and Ytre-Hauge13, Reference Zhang, Howell, Giebeler, Taddei, Mahajan and Newhauser15
Table 2 Percentage risk of secondary malignancy incidence at 23·4 Gy

Abbreviations: LNT, linear no-threshold model; LinExp, linear exponential model; 3DCRT, three-dimensional conformal radiation therapy; VMAT, volumetric-modulated arc therapy; DSPT, double scattered proton therapy; IMPT, intensity-modulated proton therapy.
The radiocarcinogenesis risk estimated by Zhang et al. was much higher than the other studies in the table due to the fact that it considered the probability of developing a secondary cancer over 100 years, whereas the other studies considered the risk over an average lifetime.Reference Brodin, Rosenschöld and Aznar5, Reference Stokkevåg, Engeseth and Ytre-Hauge13, Reference Zhang, Howell, Giebeler, Taddei, Mahajan and Newhauser15 The findings presented in Table 2 were supported by Yoon et al.,Reference Yoon, Shin and Kim14 who found that organ effective doses in the thorax and abdomen were lower with proton therapy than with 3DCRT and tomotherapy. This resulted in an approximate five-fold reduction in secondary malignancy risk.Reference Yoon, Shin and Kim14 Zhang et al.Reference Zhang, Howell, Taddei, Giebeler, Mahajan and Newhauser17 did not supply a percentage figure, but showed that the use of proton therapy reduced the risk of radiocarcinogenesis by six times. These studies unanimously agreed that proton therapy resulted in reduced secondary cancer incidence.
Cost-effectiveness
A further key issue identified in the literature is related to the cost-effectiveness of proton therapy. Two literature reviews relating to this topic did not suggest that the use of proton therapy as a major treatment modality was appropriate at the time of publication.Reference Lodge, Pijls-Johannesma, Stirk, Munro, de Ruysscher and Jefferson3, Reference Pijls-Johannesma, Pommier and Lievens11 The review by Lodge et al.Reference Lodge, Pijls-Johannesma, Stirk, Munro, de Ruysscher and Jefferson3 analysed 137 articles, and concluded that more publications were required to draw firm conclusions. Pijls-Johannesma et al.’sReference Pijls-Johannesma, Pommier and Lievens11 review only analysed five articles within the literature, but also concluded that further evaluations may provide a better insight into the cost-effectiveness of proton therapy. It was interesting to note that two of the articles identified in this literature review suggested that in the treatment of medulloblastoma, proton therapy was dominant in cost per quality adjusted life year gained. According to this paper, in 2005, proton therapy for CSI cost an additional €23,647 (£18,820) per patient, however, proton therapy was associated with an additional 0·683 quality adjusted life years on average per patient.
Conclusion
The majority of the reviewed evidence focused on medulloblastoma treatment in children, as medulloblastoma is a disease that primarily affects paediatric patients and indicates CSI. A number of key themes have been discussed in this systematic review, which include the risk of toxicities, secondary malignancies and cost-effectiveness arising from proton beam therapy. It is clear that the doses to OARs, as well as the risk of toxicities, are reduced with proton therapy, due to the minimal ventral dose delivered with proton CSI. The studies analysed in this review also unanimously agreed that there is a decreased risk of radiocarcinogenesis with proton CSI than with conventional radiation therapy. This potentially decreased risk of the development of a secondary malignancy is ideal, especially in paediatric patients.
The cost-effectiveness of proton therapy remains controversial, and the evidence does not suggest that proton therapy should be used as a major treatment modality. However, proton CSI is superior to other CSI modalities in terms of OAR doses and toxicities. Evidence suggests that the costs are outweighed by the reduced OAR doses, related toxicities and risk of radiocarcinogenesis associated with this modality, although further studies would help to strengthen this evidence. Further cost-effectiveness studies must consider the incidence of malignancies that indicate CSI, as the costs of building, maintaining and operating a facility capable of delivering proton therapy may be high considering that only a small portion of patients will benefit from this modality.
The benefits of this modality, especially the reduced risk of secondary malignancy, would be extremely valuable in the treatment of tumours that indicate CSI. The evidence within this review supports the use of proton therapy as a standard treatment for CSI.
Acknowledgements
The authors would like to thank Allison Dry from the Royal Brisbane and Women’s Hospital, Brisbane and Cathy Hargrave from the Princess Alexandra Hospital, Brisbane for their advice and support.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.