Introduction
Recent studies of the frontal lobes and their relation to executive functions have led to a revamping of theoretical constructs and to a change in experimental approaches. This review summarizes the lessons learned, and the results obtained. Because these are “lessons,” and the review is “short,” the examples provided will primarily come from our own research with supportive evidence from other laboratories as appropriate.
Terms: Executive or Frontal Lobe Functions
Lezak defined executive functions as “those capacities that enable a person to engage successfully in independent, purposive, self-serving behavior” (Lezak, Reference Lezak1995, p. 42), and the index (p. 1006) identifies as specific executive functions initiation, planning, purposive action, self-monitoring, self-regulation, and volition. Other terms commonly used include inhibition and flexibility (shifting).
Impairments in these functions have been most commonly observed after frontal lobe damage, and the terms “executive dysfunction” and “frontal lobe dysfunction” have been often used interchangeably. There are problems with such a rigid equation. First, the frontal lobes are very large (estimated at 25–33% of the entire cortex) with over 15 Brodmann areas, each with architectonic specificity and many having specific connectivity with non-frontal regions (e.g., Alexander, Delong, & Strick, Reference Alexander, Delong and Strick1986; Petrides & Pandya, Reference Petrides and Pandya1994). Second, much of the classic literature on anatomical/functional correlations comes from clinicopathological studies of patients with significant cognitive deficits beyond just executive or with poorly localized lesions often extending beyond the frontal lobes. Third, many illnesses or injuries produce executive impairments with little to no demonstrable frontal injury—diffuse trauma, multiple sclerosis, vascular cognitive impairment, schizophrenia, even depression. In neither pairing—frontal lobe functions and the central executive nor frontal lobe damage and executive dysfunction—are the terms truly interchangeable.
A Shift in mental set
Clarifying the brain–behavior correlates between “executive functions” and the frontal lobes in adults therefore demands, at least in the initial stages of understanding brain–behavior relations, tethering of the psychological process to some defined anatomical region. Functional imaging and behavioral studies in non-focal lesion patients, and research in other populations such as patients with Alzheimer's disease, do not inform us of the necessary relation of an anatomical region with a cognitive function. To determine if the relationship is primary and necessary, studies must be based on patients with very carefully defined and limited focal frontal lobe lesions (see Stuss et al., Reference Stuss, Alexander, Shallice, Picton, Binns, MacDonald and Katz2005 for definitions).
Statistical Approaches to Patient Subclassification
A priori anatomical classifications such as frontal versus posterior, or unilateral frontal versus bi-frontal, have not been very successful in revealing specific frontal-behavioral relationships. One approach is “reverse engineering,” that is, discovering the principles of the functions of specific frontal regions through analysis of their structure and operations (for review of methods, see Stuss, Alexander et al., Reference Stuss, Alexander, Floden, Binns, Levine, McIntosh and Hevenor2002). Split-half division of all frontal patients based on their performance, for example, was successful in identifying that some but not all frontal patients had impaired recognition memory. Those who were impaired could in addition be divided into two subgroups: those with posterior inferior medial (e.g., septal region) damage who might be considered to have deficient limbic memory functioning affecting retrieval, and left lateral frontal patients whose impairment was more related to residual language deficits and impaired encoding (Stuss et al., Reference Stuss, Alexander, Palumbo, Buckle, Sayer and Pogue1994). There were two important lessons from this realization: tests (e.g., recognition memory) do not necessarily measure processes, and impairments in different processes can lead to similar test findings. The location of the lesions provides the clues to dissociating processes.
More sophisticated methods, such as the Classification and Regression Tree (CART) analysis (Breiman, Friedman, Olshen, & Stone, Reference Breiman, Friedman, Olshen and Stone1984), can provide finer anatomical group classifications. For example, using the number of words generated in a verbal fluency task as an independent measure, CART identified four separate groups of performance patterns. When the subjects in the groups were assessed, they fell into four different regional injury patterns: left lateral, right lateral, superior medial, and inferior medial (Stuss et al., Reference Stuss, Alexander, Hamer, Palumbo, Dempster, Binns and Izukawa1998; see also Stuss, Alexander et al., Reference Stuss, Alexander, Floden, Binns, Levine, McIntosh and Hevenor2002, for further description of the method).
The refinement of architectonic division within the frontal lobes provided by Petrides and Pandya (Reference Petrides and Pandya1994), the development of more process specific measurements (see below), and the availability of larger number of patients with well-defined circumscribed lesions led to development of an architectonic “hot-spotting” procedure (Stuss et al., Reference Stuss, Alexander, Shallice, Picton, Binns, MacDonald and Katz2005). For each functional outcome measure, the performance of all individuals with at least 25% involvement of a specific architectonic region was compared to that of all individuals who did not have damage to that region. All regions that led to significant impairment on that function were then considered to be areas necessary for the successful performance of that function.
Developing New Models of Frontal Lobe Functioning
There are influential models of the functions of the frontal lobe (e.g., Fuster, Reference Fuster2008; Godefroy, Cabaret, Petit-Chenal, Pruvo, & Rousseaux, Reference Godefroy, Cabaret, Petit-Chenal, Pruvo and Rousseaux1999; Grafman, Reference Grafman2002; Heilman & Watson, Reference Heilman and Watson1977; Knight, Reference Knight1991; Luria, Reference Luria1973; Mesulam, Reference Mesulam1985; Mirsky, Anthony, Duncan, Ahearn, & Kellam, Reference Mirsky, Anthony, Duncan, Ahearn and Kellam1991; Norman and Shallice, Reference Norman and Shallice1986; Paus et al., Reference Paus, Zatorre, Hofle, Caramanos, Petrides and Evans1997; Posner and Petersen, Reference Posner and Petersen1990; Shallice, Reference Shallice1982; Sturm & Willmes, Reference Sturm and Willmes2001; see Stuss & Knight, Reference Stuss and Knight2002, for details of other models). Some specifically emphasize a role in attention, with an anterior attentional system in the frontal lobe concerned with the “executive control” of attention, and a posterior system responsible for the spatial allocation of attention. We selected one as our starting point—the Supervisory Attentional System (SAS) model of Norman and Shallice (Reference Norman and Shallice1986).
One assumption guided our approach—there was no single basic frontal process. We searched for and reviewed all published papers up to 1994 that reported attentional impairments attributed to focal frontal lesions (see Stuss, Shallice, Alexander, & Picton, Reference Stuss, Shallice, Alexander and Picton1995). Allowing for different operational definitions from different researchers, we identified seven basic task types: sustaining, concentrating, sharing, suppressing, switching, preparing, and setting. Tasks, however, are not processes. Analysis of the demands of each task suggested that each might rely on one or some combination of the processes defined in our adaptation of the SAS model: energizing, monitoring, inhibiting, adjustment of contention scheduling, and logical analysis. Our hypotheses were (1) these processes could be experimentally defined, and (2) they would have different and specific correlations with regional frontal injuries. We spent the next 10 years examining these hypotheses.
A Different Approach to Assessment of Frontal Lobe Functions
It has been commonly held that the frontal lobes are necessary when tasks are complex, have novel demands or require considerable attention (Norman & Shallice, Reference Norman and Shallice1986; Stuss et al., Reference Stuss, Shallice, Alexander and Picton1995). The very inherent complexity, novelty, or effort required for a complex task may mean that different processes instantiated in different frontal and non-frontal regions may be involved. If the processes themselves are straightforward but supraordinate and domain general, they would operate on or modulate or control the execution of many other functions regardless of task difficulty. If this hypothesis is correct, then a more appropriate assessment of impairments would assess more basic processes. “Complexity” could be built into tests by requiring more integration of multiple processes or placing more time or context constraints on use of a process. We created such tests to assess these processes—a Feature Integration Task (Stuss, Binns, Murphy & Alexander, Reference Stuss, Binns, Murphy and Alexander2002) and the ROtman-Baycrest Battery for the Investigation of Attention (ROBBIA) (Stuss et al., Reference Stuss, Alexander, Shallice, Picton, Binns, MacDonald and Katz2005); we also analyzed traditional multidimensional neuropsychological tasks—word list learning, Stroop, and Wisconsin Card Sorting Test—to examine if the same processes are required (Stuss & Alexander, Reference Stuss and Alexander2007).
Evidence for fractionation of frontal lobe functioning
The conceptual heuristic guiding our research program is anatomically and functionally reductionist as a means of understanding the component processes associated with the frontal regions of the brain. Success has been partial. For some frontal regions, there is sufficient confidence to state that we are at least approximating the level of component processes. For other regions of the frontal lobes, however, research is still in the initial stages, and more general terms such as “functions” are more appropriate.
“Supervisory” Attention as a Framework: A Revamped Attentional Model
Three of the proposed processes (Energization, Monitoring, and Task Setting) and their correlations with regionally specific frontal injuries were readily identified in our studies on the role of the frontal lobes in attention. These results have been replicated across different patient groups, and different tasks (Stuss & Alexander, Reference Stuss and Alexander2007 for review).
i) Energization
Patients with superior medial (dorsomedial—primarily in areas 24, 9, and 6) damage had a unique cluster of deficits. They were significantly slower on all tasks that required speeded responses or time constrained suppression of responses. They could not sustain the beneficial effects of a warning signal over a 3-s period. They had a uniquely disproportionate decline in words during the last 45 s of a letter fluency task compared with the first 15 s. They underestimated a count of stimuli under both speeded and vigilant conditions, a deficit that worsened with task progression. Performance on all of these apparently disparate tasks is due to a failure of “energization,” that is, the process of initiation and sustaining any response (Alexander, Stuss, Picton, Shallice, & Gillingham, Reference Alexander, Stuss, Picton, Shallice and Gillingham2007; Alexander, Stuss, Shallice, Picton, & Gillingham, Reference Alexander, Stuss, Shallice, Picton and Gillingham2005; Picton et al., Reference Picton, Stuss, Alexander, Shallice, Binns and Gillingham2007; Shallice, Stuss, Alexander, Picton, & Derkzen, Reference Shallice, Stuss, Alexander, Picton and Derkzen2008; Shallice, Stuss, Picton, Alexander, & Gillingham, Reference Shallice, Stuss, Picton, Alexander and Gillingham2008; Stuss et al., Reference Stuss, Alexander, Hamer, Palumbo, Dempster, Binns and Izukawa1998, Reference Stuss, Alexander, Shallice, Picton, Binns, MacDonald and Katz2005; Stuss, Binns, et al., Reference Stuss, Binns, Murphy and Alexander2002).
ii) Executive functions
Two processes do fit a definition of executive functions.
iia) Monitoring
Patients with right lateral damage, primarily in areas 44, 45, 46, 9, 9/46, and 47/12, had increased individual variability, impaired variable foreperiod effect, and an increase of all types of errors, including false negatives. They also had difficulty keeping track of the count of stimuli under speeded conditions only. This combination suggested poor monitoring of ongoing performance on very different tasks (Picton, Stuss, Shallice, Alexander, & Gillingham, Reference Picton, Stuss, Shallice, Alexander and Gillingham2006; Shallice, Stuss, Alexander, et al., Reference Shallice, Stuss, Alexander, Picton and Derkzen2008; Stuss et al., Reference Stuss, Alexander, Shallice, Picton, Binns, MacDonald and Katz2005; Stuss, Binns, et al., Reference Stuss, Binns, Murphy and Alexander2002).
iib) Task setting
Patients with comparable left lateral damage had increased false positives (poor criterion setting) in any task (e.g., Stroop, word list learning, etc.) usually most prominent in the initial stages of learning (ROBBIA concentrate and ROBBIA suppress) (Alexander, Stuss, & Gillingham, Reference Alexander, Stuss and Gillingham2009; Alexander et al., Reference Alexander, Stuss, Shallice, Picton and Gillingham2005, Reference Alexander, Stuss, Picton, Shallice and Gillingham2007; Floden, Vallesi, & Stuss, Reference Floden, Vallesi and Stuss2011; Shallice, Stuss, Picton et al., Reference Shallice, Stuss, Picton, Alexander and Gillingham2008). Task setting requires both the processes of “if-then” logic and “adjustment of contention scheduling.”
Other Functions of the Frontal Lobes
Other research has suggested association of other functions with different regions of the frontal lobes. Whether there are component processes underlying these functions, and what they might be, remain to be determined.
Behavioral/emotional self-regulation
Damage to the ventromedial cortex (VMPFC—areas 32, 25, 24, 14, 13, 12, 11) results in difficulty with integrating the motivational, reward/risk, emotional, and social aspects of behaviors more than with the executive functions required to implement a behavior. Performance on commonly used neuropsychological tests of executive functioning is normal. The tasks required to demonstrate these difficulties are experimental and often unstructured—deception, empathy, and gambling tasks (Bechara, Damasio, Damasio, & Lee, Reference Bechara, Damasio, Damasio and Lee1999). All involve reward/risk processing of sorts, for the individual or for others. The tasks are complex and await identification of the fundamental processes, some perhaps “executive” (Manes et al., Reference Manes, Sahakian, Clark, Rogers, Antoun, Aitken and Robbins2002).
Metacognition/integration
There is a final category of function—higher-order processing—that is much harder to define and to measure but which seems exquisitely related to frontal lobe integrity. This function is integrative and coordinating—orchestrating the energization, motivation, emotional perspective, and executive capacities that are necessary to accomplish complex, novel tasks. Damage to polar regions (10s and 10i) impairs these integrative/gateway functions (Burgess, Gilbert, & Dumontheil, Reference Burgess, Gilbert and Dumontheil2007), although how to dissemble this putative function from the effects of VMPFC lesions is not certain. The tests for this category are also experimental and somewhat indirect—understanding humor, behaving from the perspective of another, recognizing the differences between what one knows from what one believes or remembers, amongst others. They are, thus, metacognitive.
Four Frontal Categories: Relation to Development and Connectivity
In summary, there are at least four categories of frontal lobe functioning (Energization, Executive, Emotion/Behavioral Regulation, Metacognition), each related to a different region within the frontal lobes. An early and simplified version of this model was suggested by Stuss and Benson (Reference Stuss and Benson1986). Somewhat different proposals for a fractionated frontal system also exist (e.g., Godefroy et al., Reference Godefroy, Cabaret, Petit-Chenal, Pruvo and Rousseaux1999; Koechlin, Basso, Pietrini, Panzer, & Grafman, Reference Koechlin, Basso, Pietrini, Panzer and Grafman1999; Shallice & Burgess, Reference Shallice and Burgess1996).
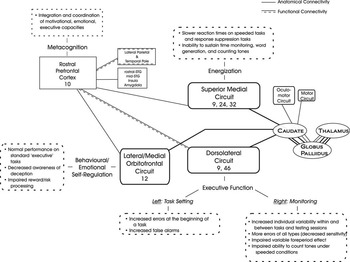
There is other support for this model of discrete functional categories within the frontal lobes. Comparative anatomical studies and mapping of human brain development have identified two main frontal systems—a lateral one with primarily bidirectional connections to and from posterior cortices (executive) and an inferior/medial one with prominent limbic connections (emotional) (Pandya & Yeterian, Reference Pandya and Yeterian1996). These two systems are “energized” by the superior medial region. The frontopolar region—both phylogenetically and ontogenetically late developing—integrates the executive and the emotional processes. Of the exquisitely mapped, vertically segregated frontal–subcortical circuits (Alexander et al., Reference Alexander, Delong and Strick1986), three align with our categories of energization, executive, and emotional. The frontopolar region (integrative function) does not have major frontal–subcortical connections precisely because its role is integrating processes within the frontal lobes and with other regions (Petrides & Pandya, Reference Petrides and Pandya2007). See Figure 1.

Fig. 1 This figure illustrates the frontal cortical—basal ganglia—thalamic circuits, supporting the fractionation of the frontal functional regions. Area 10 is not part of this circuitry, and is schematically presented in its polar location to suggest its integrative functions. For an expanded explanation of the anatomical and functional connections of Area 10 with other brain regions, see Gilbert, Gonen-Yaacovi, Benoit, Volle, & Burgess (Reference Gilbert, Gonen-Yaacovi, Benoit, Volle and Burgess2010) and Petrides and Pandya (Reference Petrides and Pandya2007). The figure also serves as a summary of the findings. STG = superior temporal gyrus; Right/Left = cerebral hemispheres.
Brain Systems and Networks
Our goal was to understand and fractionate the functions of the frontal lobes. For each frontal cortical functional region, there is a connection with a specific basal ganglia area, continuing to a defined-thalamic region. Are the functions of the connected regions the same? This question needs to be pursued using similar operational definitions of processes as those outlined in the frontal patients. However, the demonstration of a parallel functional separation within the subcortical regions will be difficult, because of the smaller size of these areas. For example, in several of our studies (Stuss et al., Reference Stuss, Alexander, Hamer, Palumbo, Dempster, Binns and Izukawa1998, Reference Stuss, Levine, Alexander, Hong, Palumbo, Hamer and Izukawa2000), patterns of performance after basal ganglia damage were similar to frontal patterns but, other than left–right differences, further distinctions could not be isolated. One interesting approach has been the use of deep brain stimulation in the subthalamic nucleus to demonstrate an alteration in a frontotemporal network related to the performance of a verbal fluency task (Schroeder et al., Reference Schroeder, Kuehler, Lange, Haslinger, Tronnier, Krause and Ceballos-Baumann2003).
Similar questions could—and should—be raised about the functional similarities and dissimilarities in other frontal networks. We have pursued the question related to frontocerebellar connectivity. If characterization of patients is strict, and patients are studied in a chronic stage of recovery with lesions limited to the cerebellum, the functional similarity is quite limited and specific (Alexander, Gillingham, Schweizer, & Stuss, in press; Schweizer, Alexander, Gillingham, Cusimano, & Stuss, Reference Schweizer, Alexander, Gillingham, Cusimano and Stuss2010). The potential specific role of white matter pathways also needs to be investigated.
Understanding the role of specific brain regions within the frontal lobes is not phrenology; analysis of the simple tasks and how different regions, frontal and non-frontal, are required depending on task demands and difficulty, identifies these nodes as parts of flexible and dynamic networks (Stuss, Reference Stuss2006). More importantly, it provides a foundation for investigating and understanding the role of separate circuits, and the integration between and among circuits. The frontal lobes may play a key role in such integration but there is suggestion that integration and restructuring of neural assemblies can occur in different regions (Haber & Calzavara, Reference Haber and Calzavara2009).
Conclusion
Moving from multidimensional, clinical tasks to controlled experimental processes to reliably correlated brain regions has provided replicable evidence of fractionated frontal lobe functioning. Within the SAS model of attention, the processes of energization, monitoring, and task-setting (if-then logic and contingent responding) have been consistently identified and correlated with specific brain regions. It is highly likely that the appropriate experimental paradigm will reveal more frontal lobe processes, likely associated with other frontal brain regions.
Our summary paragraph in the 1995 study, rephrased as follows, remains relevant today.
The frontal lobes do not equal a central executive. Executive functions represent only one functional category within the frontal lobes. These frontal functions are domain general, possibly because of the extensive reciprocal connections with virtually all other brain regions, integrating information from these regions. Further integration of these processes with emotional and motivational processes allows the most complex behaviors (Alexander, Reference Alexander2006; Grafman, Reference Grafman2002).
Future horizons
There are three: (1) Can experimental neuropsychology identify and extract additional basic processes—executive, emotional, others—as the raw material for understanding integrated brain functioning in the most complex tasks, including “consciousness”? (Stuss & Benson, Reference Stuss and Benson1986; Stuss, Picton, & Alexander, Reference Stuss, Picton and Alexander2001); (2) Can experimental neuroscience create methodologies for observing these processes in their normal operation through imaging? (a journey already started; e.g., Brass & von Cramon, Reference Brass and von Cramon2004; Floden et al., Reference Floden, Vallesi and Stuss2011; Sturm & Willmes, Reference Sturm and Willmes2001; Vallesi, McIntosh, Alexander, & Stuss, Reference Vallesi, McIntosh, Alexander and Stuss2009; Vallesi, McIntosh, Shallice, & Stuss, Reference Vallesi, McIntosh, Shallice and Stuss2009); (3) Can understanding these fundamental processes provide a framework for pharmacotherapeutic or behavioral treatments and assessments when the processes are impaired? (Cicerone, Levin, Malec, Stuss, & Whyte, Reference Cicerone, Levin, Malec, Stuss and Whyte2006; Levine, Turner, & Stuss, Reference Levine, Turner and Stuss2008; Wang et al., Reference Wang, Ramos, Paspalas, Shu, Simen, Dogue and Arnsten2007).
Acknowledgments
The following are gratefully acknowledged: all the co-authors on the various publications for their essential contributions to all aspects of the research program, especially M.P. Alexander, T. Shallice, T. Picton; funding agencies, in particular Canadian Institutes of Health Research, the McDonnell Foundation, the Centre for Stroke Recovery; my research lab, in particular lab manager S. Gillingham; M.P. Alexander and D.P. Stuss for comments on an earlier draft, and S. Gillingham for figure preparation. There are no conflicts of interest to report.