Introduction
Brown-Vialetto-Van Laere syndrome is a rare neurodegenerative disorder characterised mainly by progressive sensorineural deafness and childhood axonal sensorimotor neuropathy, with bulbar weakness, sensory ataxia, limb and axial weakness and, less commonly, involvement of cranial nerves II to VI.Reference Gallai, Hockaday, Hughes, Lane, Oppenheimer and Rushworth1,Reference Foley, Menezes, Pandraud, Gonzalez, Al-Odaib and Abrams2
The term Brown-Vialetto-Van Laere syndrome has more recently been assigned to a heterogenous group of conditions, some with prominent bulbar weakness and others with mainly limb weakness, all with sensorineural deafness as a common feature.Reference Bosch, Stroek, Abeling, Waterham, Ijlst and Wanders3,Reference McShane, Boyd, Harding, Brett and Wilson4 Brown-Vialetto-Van Laere syndrome and Fazio-Londe syndrome are both riboflavin transporter deficiency syndromes, a phenotypic continuum of motor, sensory, and cranial nerve neuronopathy, with hearing loss present in Brown-Vialetto-Van Laere syndrome only.
The aetiology of Brown-Vialetto-Van Laere syndrome has been uncovered in a large proportion of cases through identification of mutations in the riboflavin transporter genes SLC52A3 (formerly C20orf54)Reference Green, Wiseman, Crow, Houlden, Riphagen and Lin5 and SLC52A2,Reference Johnson, Gibbs, Megarbane, Urtizberea, Hernandez and Foley6 and treatment with high-dose oral riboflavin therapy has been shown to result in significant clinical and biochemical improvements in some of these patients, particularly in those with the SLC52A2 mutation.Reference Foley, Menezes, Pandraud, Gonzalez, Al-Odaib and Abrams2
In most cases of Brown-Vialetto-Van Laere syndrome, the first symptom is hearing loss, which was described as sensorineural, usually bilateral, progressive and severe.Reference Sathasivam7 Recent reports have identified that hearing loss in Brown-Vialetto-Van Laere syndrome is because of auditory neuropathy spectrum disorder rather than cochlear sensorineural loss.Reference Anderson, Schaefer, Henderson and Bruce8–Reference Menezes, O'Brien, Hill, Webster, Antony and Ouvrier10 Here, we present four cases of Brown-Vialetto-Van Laere syndrome in patients with mutations in SLC52A2 and SLC52A3 genes; these patients had hearing impairment characterised by auditory neuropathy spectrum disorder and had additionally had inner ear imaging and vestibular function assessed. Two of the cases underwent cochlear implantation, and all four received riboflavin treatment. Data was gathered retrospectively. This paper highlights further information on less well-known aspects of Brown-Vialetto-Van Laere syndrome, including onset and evolution of hearing loss, vestibular findings and imaging.
Clinical details
Case 1
A 4-year-old girl presented with a history of fluctuating hearing loss since the age of 18 months. She had difficulty hearing from the back seat of a car, needed instructions repeated when not facing the speaker and watched television at loud volume. She was much better in one-to-one situations in a quiet environment. There was a history of speech and motor developmental delay. Her birth and family history were unremarkable. Initial assessment showed evidence of bilateral glue ear and enlarged tonsils. Behavioural hearing assessment using play audiometry demonstrated mild conductive hearing loss and clinical evidence of otitis media with effusion. She underwent adenotonsillectomy and grommet insertion, which resulted in resolution of the middle-ear effusion and peaked traces on tympanometry; however, there was no improvement in her hearing difficulties. Her speech perception difficulties continued to be much more pronounced than would be expected from pure tone audiometry alone, which showed only mild hearing loss (Figure 1).

Fig. 1. Pure tone audiograms for the (a) right ear and (b) left ear for case 1 performed at four separate measurement intervals: (i) age 4 years (before starting riboflavin), (ii) age 6 years, 4 months (3 months after starting riboflavin), (iii) age 6 years, 6 months (5 months after starting riboflavin) and (iv) age 6 years, 10 months (9 months after starting riboflavin).
Over the next two years, she developed progressive neurological symptoms of four-limb weakness, ataxia and worsening hearing loss. At five years of age, she was found to have a sensory and motor axonal neuropathy with left optic nerve dysfunction on electrophysiology. She had poor speech clarity, significant difficulty in understanding speech and heavy dependence on lipreading but responded well to gestures, expressions and signing and had an appropriate level of vocabulary for her age. Transient evoked otoacoustic emission (OAE) testing showed responses across a range of frequencies. Stapedial reflexes were absent bilaterally. Auditory brainstem response (ABR) testing showed no response to 1, 2 and 4 kHz tone burst stimuli at 95–110 dBnHL.
Her hearing profile and audiological findings were consistent with a diagnosis of auditory neuropathy spectrum disorder. She underwent a trial of hearing aids with some benefit in improving understanding of speech and communication. Play audiometry performed at four and six months after hearing aid fitting showed progressive deterioration in her hearing reaching severe-to-profound levels of loss (Figure 1). At this point, her transient evoked OAE responses were absent. On speech discrimination testing, she was able to identify words at 45–50 dB with lipreading but not at 80 dB without lipreading.
Vestibular function was assessed with rotation chair testing using sinusoidal rotation at 0.16 Hz and impulsive rotation at 120 degrees/second, eliciting the presence of vestibular (lateral semi-circular canal) function bilaterally (Figure 2). Because of her limited co-operation, quantification of vestibular function was difficult. She was diagnosed with Brown-Vialetto-Van Laere syndrome shortly after her sixth birthday, following the finding of a SLC52A2 mutation and was commenced on riboflavin therapy. After nine months, her mother noticed an improvement in her hearing and lack of further progression in her neurological symptoms. She was a consistent hearing aid user and reported significant benefit in understanding speech. A repeat audiogram showed improvement to her hearing thresholds (Figure 1). Computed tomography (CT) of the petrous bones showed normal bony labyrinths, bony cochlear nerve canals and vestibular aqueducts. Magnetic resonance imaging (MRI) of internal auditory meati was planned and she was referred to the cochlear implant department; however, subsequently the family moved abroad, and she was lost to follow up.

Fig. 2. Vestibular function test results for case 1 showing: (a) impulsive rotation 120 degrees/second between 0–9 seconds, (b) impulsive rotation 120 degrees/second between 14–23 seconds and (c) sinusoidal rotation of 0.16 Hz.
Case 2
A six-month-old girl presented with sudden collapse and was admitted to paediatric intensive care with respiratory failure. She was fitted with a tracheostomy and required full assisted ventilation. She was also percutaneous endoscopic gastrostomy fed because of dysphagia. She developed progressive bilateral ptosis, facial and limb muscle weakness, and demonstrated significant motor developmental delay. Her birth and family history were unremarkable. She had passed the newborn hearing screen on OAE testing.
From the age of six months, her responses to sound were felt to be variable. When she was a year old, she would sometimes indicate that she had heard and recognised certain sounds using hand gestures. There was significant speech delay; however, she showed appropriate social and non-verbal communication skills for her age.
ABR was performed when she presented to the audiology department at the age of 18 months, which showed no response to 100 dBnHL click stimuli bilaterally; however, distortion product OAE testing showed clear responses present in both ears, leading to a diagnosis of auditory neuropathy spectrum disorder. Her observed behavioural responses were much better than indicated by the ABR, and her understanding of speech when combined with signing was felt to be good; however, she did not cooperate for behavioural hearing tests at this point, and she was monitored.
Following extensive investigations, she received a diagnosis of Brown-Vialetto-Van Laere syndrome with recessive SLC52A3 gene mutation at two years of age and was commenced on riboflavin therapy with noticeable improvements in her neuromuscular function following treatment; however, her hearing did not seem to improve.
At the age of 4 years, repeat ABR testing showed no recordable waveforms to 100 dBnHL clicks and 1 and 4 kHz tone pips bilaterally. Pure tone audiometry at five years of age showed moderate mixed hearing loss, and she was fitted with hearing aids with no significant benefit. She underwent grommet insertion at six years of age. Play audiometry six months after grommet insertion demonstrated severe-to-profound mixed hearing loss that was worse at low frequencies with moderate-to-severe bone conduction thresholds despite the right grommet being in situ (Figure 3). At this point cochlear implantation was under consideration. Further ABR testing at six years of age showed the presence of cochlear microphonics bilaterally, in the absence of responses to 100 dBnHL air conduction clicks, 1 and 4 kHz tone burst stimuli and 50 dBnHL bone conduction 4 kHz tone burst stimuli (Figure 4). Repeat audiogram aged seven years showed progressive sensorineural hearing loss with no responses to unmasked bone conduction stimuli at maximum levels (Figure 3). She was using British sign language as her main mode of communication.

Fig. 3. Pure tone audiograms for the (a) right ear and (b) left ear for case 2 performed at three separate measurement intervals: (i) age 5 years, 4 months, (ii) age 6 years, 7 months and (iii) age 7 years, 8 months.

Fig. 4. Auditory brainstem response testing in case 2 showing the presence of cochlear microphonics in (a) left and (b) right ears and absent responses on air conduction click on the (c) left and (d) right ears.
Vestibular function testing demonstrated no observable responses on impulsive rotation at 120 degrees/second and sinusoidal rotation at 0.16 Hz, indicating bilateral severe vestibular hypofunction (Figure 5).

Fig. 5. (a) Sinusoidal chair rotation at 0.16 Hz, (b) example of normal nystagmus responses to chair rotation and (c) rotation in case 2 showing no response.
An MRI scan of the internal auditory meatus showed hypoplastic cochlear nerves bilaterally. Both the facial nerves were also hypoplastic. The common VIII nerve and the vestibular divisions were normal in size. A CT scan of petrous bones showed normal inner ear structures. The patient received a unilateral cochlear implant at 10 years old with some benefit in gaining environmental sound awareness.
Case 3
A 15-year-old boy was assessed in the audiovestibular medicine clinic for a second opinion regarding hearing loss. His birth history was unremarkable, and he was born at full term. He had bouts of breathing difficulties shortly after birth and for the first few weeks, after which his breathing settled. His early motor developmental milestones were normal.
There had been longstanding concerns from the boy's parents regarding his hearing. His speech development was felt to be delayed. He was described as a quiet baby who did not always respond to sounds. He did not babble until 18 months and spoke his first words at approximately 3 years of age. His listening was felt to be poor, and he relied very heavily on lipreading. When he was four years old he received speech and language therapy and underwent adenotonsillectomy and grommet surgery, but he made very little progress with his speech and language skills and his hearing did not improve. Pure tone audiogram showed mild-to-severe low frequency mixed hearing loss, and he was fitted with hearing aids aged seven years. However, he did not get on with his hearing aids, and his parents and teachers were uncertain whether he gained any benefit from them.
When he was 10 years old, he was diagnosed with axonal motor and sensory neuropathy after developing rapidly progressive symptoms of ataxia and reduced function of his hands with respiratory deterioration. Due to swallowing difficulties, he became dependent on nasogastric tube feeding. His vision deteriorated, and he was noted to have bilateral optic atrophy. He developed bilateral tinnitus, and his hearing continued to deteriorate. His speech became less intelligible.
Pure tone audiogram performed at 15 years of age showed bilateral moderate hearing loss, and his aided soundfield thresholds were between 35 and 50 dBA. His score on the Manchester Junior Word list was 20 per cent at 70 dBA without lipreading but was 90 per cent with lipreading. As in case 1, his speech perception difficulties were significantly more pronounced than would have been expected from pure tone audiometry. Repeat audiometry two years later showed further deterioration (Figure 6). Transient evoked OAE were clearly present; however, stapedial reflexes were absent bilaterally. ABR testing using a click stimulus showed no wave present bilaterally at 102 dBnHL, with cochlear microphonics present. Cortical testing showed no clear responses to 1 and 4 kHz tone pip at 90 dBnHL bilaterally.

Fig. 6. Pure tone audiograms for the (a) right ear and (b) left ear for case 3 performed at: (i) age 15 years and (ii) age 17 years.
Vestibular investigations showed evidence of mild bilateral vestibular hypofunction on rotation chair testing, with no significant asymmetry or directional preponderance. Sinusoidal rotation at 0.05, 0.08 and 0.16 Hz showed poor gain and phase lead. Impulsive rotation at 120 degrees/second showed low time constant, particularly for stop rotations and low peak eye velocity gain (Figure 7). Central eye movement tests such as smooth pursuit, optokinetic responses and vestibulo-ocular reflex were normal. MRI and CT scans showed normal inner ear structures and normal cochlear nerves.

Fig. 7. Vestibular function test results for case 3, showing poor start and negligible stop responses to leftward impulsive rotation at 120 degrees/second.
The patient was diagnosed with auditory neuropathy spectrum disorder and fitted with a unilateral cochlear implant when he was 17 years old. Unfortunately, his progress with the cochlear implant was not as good as hoped, with no reported improvement in speech perception. Over the next few years, he became wheelchair-bound and his speech was increasingly slurred. He subsequently received genetic confirmation of Brown-Vialetto-Van Laere syndrome at the age of 22 years.
Case 4
A 13-year-old boy was referred from his local audiology unit for a cochlear implant assessment with a history of vision, gait and hearing difficulties. His birth and early developmental history were unremarkable, and there was no known family history of hearing loss. He first presented at age six years with vision difficulties as he complained that he was not able to see the whiteboard clearly in class. At the same time, he developed problems with his gait and was noted to have distal lower limb weakness. He was diagnosed with optic atrophy at age seven years and was registered as sight impaired.
At age eight years his mother noticed that his hearing had deteriorated, and he was frequently asking people to repeat themselves. He eventually had a hearing test aged 10 years and was diagnosed with auditory neuropathy spectrum disorder at his local audiology unit. His pure tone audiogram showed bilateral moderate sensorineural hearing loss (Figure 8). He was fitted with hearing aids but did not report significant benefit as he found that the hearing aids amplified environmental sounds and background noise but did not improve his understanding of speech. He stopped wearing his hearing aids after a few months and started using a radio aids system with variable benefit. He felt that his hearing fluctuated day to day. Over the next few months, he felt his hearing got significantly worse despite stable pure tone audiograms (Figure 8), and he became increasingly frustrated.

Fig. 8. Pure tone audiograms for the (a) right ear and (b) left ear for case 4 performed at: (i) age 10 years, (ii) age 11 years and (iii) age 13 years.
The patient received a diagnosis of Brown-Vialetto-Van Laere syndrome at age 10 years because of SLC52A2 mutation and was started on riboflavin treatment. By this point he was relying on a combination of verbal communication and signs or gestures, and his family had to raise their voice and repeat themselves in order to be understood. His ability to learn new vocabulary had become restricted because of his hearing difficulties.
His vision improved once he started riboflavin treatment, which helped with lipreading and using sign language. He felt some improvement in his functional hearing despite his audiograms over the next two years remaining fairly stable (Figure 8). However, although performance on a speech discrimination test in quiet showed deterioration between the ages of 9 and 10 years, there was no decline between 10 and 11 years after starting riboflavin.
It was felt that his functional hearing was much worse than would be expected from his audiogram, and he was referred for a cochlear implant assessment. His MRI and CT scans showed normal inner ear structures, with both cochlear nerves slightly small in calibre. Vestibular function tests have yet to be performed.
Discussion
Earlier descriptions of Brown-Vialetto-Van Laere syndrome cases mainly reported the presence of sensorineural deafness but did not elaborate further on details of audiological testing performed and the degree of hearing loss found.Reference Al-Twaijri and Shevell11,Reference Hawkins, Nevin and Harding12 More recent reports have identified that hearing loss in Brown-Vialetto-Van Laere syndrome is characterised by auditory neuropathy spectrum disorder. This was first described in 2015 whereby four siblings with Brown-Vialetto-Van Laere syndrome were shown on audiological investigations to have features of auditory neuropathy spectrum disorder with normal OAE and absent ABRs,Reference Chandran, Alexander, Naina and Balraj9 with subsequent studies reporting similar findings.Reference Anderson, Schaefer, Henderson and Bruce8,Reference Menezes, O'Brien, Hill, Webster, Antony and Ouvrier10 This report adds further information to the current understanding of the audiological profile of Brown-Vialetto-Van Laere syndrome. To date, the vestibular function of patients with Brown-Vialetto-Van Laere syndrome has yet to be described. Our cases therefore highlight some important audiovestibular clinical features in this condition, as summarised in Table 1. We recognise the limitations associated with retrospective reports and incomplete data availability.
Table 1. Summary of audiovestibular clinical features
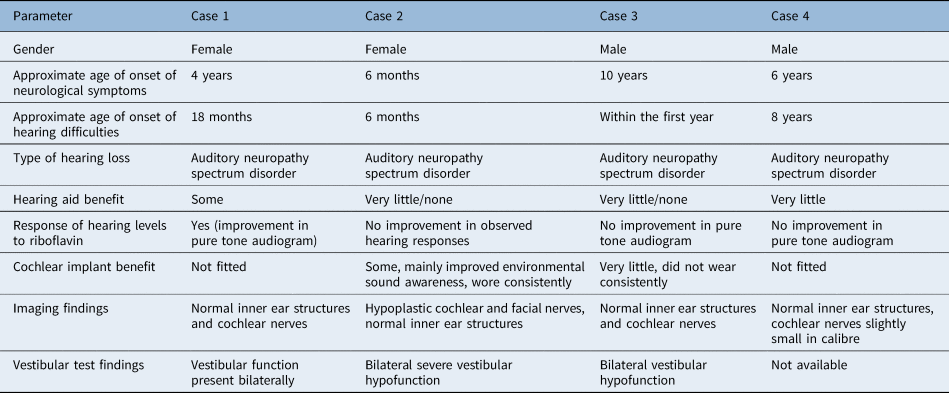
The diagnosis of auditory neuropathy spectrum disorder is based on a set of audiological findings, which are the presence of OAEs or cochlear microphonics with absent or grossly abnormal ABR. These findings suggest the presence of cochlear hair cell function but with absent or disordered neural responses. Auditory neuropathy spectrum disorder accounts for approximately 8 per cent of newly diagnosed permanent childhood hearing loss. Behavioural audiometric thresholds can fluctuate and range from normal to profound levels of hearing impairment. Auditory neuropathy spectrum disorder patients with normal hearing thresholds (as measured on behavioural audiometry) may still report hearing difficulties in the presence of noise. Speech perception difficulties are often more pronounced than would be expected from the audiometric thresholds alone.Reference Rance13 This pattern of fluctuating hearing loss was evident in our four cases and, for case 1 and 3 in particular, significant functional hearing difficulties were noted as out of proportion to audiometric thresholds.
Auditory neuropathy spectrum disorder has been described in sensory and motor neuropathies such as Charcot-Marie-Tooth syndrome and Friedreich's ataxia, which reflect postsynaptic neural conduction abnormalities leading to disrupted neural synchrony and auditory temporal processing deficit with the primary clinical consequence being impairment of speech perception.Reference Rance, Ryan, Bayliss, Gill, O'Sullivan and Whitechurch14,Reference Rance, Corben and Delatycki15 Neurophysiological studies have also identified Brown-Vialetto-Van Laere syndrome as a progressive axonal sensorimotor neuropathy with evidence of sensory neuropathy preceding the motor neuropathyReference Foley, Menezes, Pandraud, Gonzalez, Al-Odaib and Abrams2 and thus explaining a possible mechanism of damage to the auditory nerve leading to presentation of auditory neuropathy spectrum disorder in this condition. More recently, a post-synaptic pathology has been postulated as a causative factor for auditory neuropathy spectrum disorder in Brown-Vialetto-Van Laere syndrome, with riboflavin deficiency leading to auditory neuronal firing dysfunction and resulting auditory dys-synchrony.Reference Chandran, Alexander, Naina and Balraj9
There are minor differences reported between the profile of SLC52A2 and SLC52A3 mutations in Brown-Vialetto-Van Laere syndrome. It has been proposed that late onset disease might be more suggestive of SLC52A3 mutation and that abnormal gait or ataxia is often a presenting feature of SLC52A2 mutation but much less common with SLC52A3 mutation. SLC52A3 is primarily expressed in the intestine, testis and prostate, whereas SLC52A2 is more ubiquitously expressed though largely expressed in the brain and nerves.Reference O'Callaghan, Bosch and Houlden16 Whether these different expression profiles could explain the relative vulnerability of different tissues and variability in clinical presentation has yet to be clarified.
In our cases, there was significant variation in the age of onset of neuromuscular symptoms; however the onset of hearing loss was early for three of the cases, within the first 18 months. Our cases appear to present earlier with hearing loss compared with reports in the literature, with studies and review articles citing an average age of onset of symptoms including hearing loss as between 4.1 and 5.6 years,Reference Anderson, Schaefer, Henderson and Bruce8,Reference Menezes, O'Brien, Hill, Webster, Antony and Ouvrier10,Reference O'Callaghan, Bosch and Houlden16,Reference Jaeger and Bosch17 with a very wide range reported from 7 months to 19 years in one large study of 20 individuals.Reference Manole, Jaunmuktane, Hargreaves, Ludtmann, Salpietro and Bello18 Three of our cases demonstrated rapid deterioration in hearing thresholds to severe and profound levels within two years of their first audiogram, which supports previous reports of the progressive nature of hearing loss in Brown-Vialetto-Van Laere syndrome.Reference Anderson, Schaefer, Henderson and Bruce8,Reference Menezes, O'Brien, Hill, Webster, Antony and Ouvrier10,Reference Sinnathuray, Watson, Fruhstorfer, Olarte and Toner19 These same three cases had significantly delayed speech milestones. Our fourth case presented with hearing loss much later; however, the deterioration of his functional hearing was again rapid and by the time he had his hearing tested he had already developed significant hearing loss. The earlier presentation of his gait difficulties and peripheral weakness compared with hearing loss is in keeping with the current profiles described in the literature on SLC52A2. While auditory neuropathy spectrum disorder was the audiological finding in our cases, it was interesting that case 1 showed a variable audiological picture with loss of OAE responses over time. This may suggest an evolving clinical course in the audiological profile of Brown-Vialetto-Van Laere syndrome.
Our cases illustrated the variability in audiological response to riboflavin therapy, in contrast to the significant improvement in motor function and strength in all four subjects. Only case 1 demonstrated a clear response on audiometry to riboflavin treatment. It was interesting to note that there was a nine-month period post-treatment before the improvement in thresholds was measurable; a previous audiogram performed five months after commencing treatment showed continued deterioration in thresholds. This complements the existing literature describing the heterogeneity of the response in hearing to riboflavin. The fact that case 1 had an SLC52A2 mutation whereas case 2 had an SLC52A3 mutation may be a factor.
Although the improvement in neuromuscular function in response to riboflavin appears comparable between SLC52A2 and SLC52A3 cases, much less is known about the effect of treatment on hearing loss. Improvement in hearing thresholds on audiometry testing following riboflavin therapy has been reported mainly during the first year of treatment in those with recent-onset hearing loss, as reported by Menezes et al.Reference Menezes, O'Brien, Hill, Webster, Antony and Ouvrier10 in their study of seven children with Brown-Vialetto-Van Laere syndrome and auditory neuropathy spectrum disorder, all with SLC52A2 mutation. Two cases demonstrated improved hearing with treatment commenced within 12 months of onset of hearing loss. However, our case 1 showed hearing improvement many years after her hearing loss was first identified and within a year of commencing riboflavin treatment. There have been more recent reports of improved functional hearing in a few single cases, with equivalent numbers reporting no improvement.Reference Anderson, Schaefer, Henderson and Bruce8,Reference Garg, Kulkarni, Hegde and Shah20–Reference Shi, Shi, Yan, Wang, Yang and Xiong22 Although our case 4 did not show improvement in pure tone audiograms, the patient did report some improvement in functional hearing. Measurable improvement in hearing in response to riboflavin treatment has yet to be reported in Brown-Vialetto-Van Laere syndrome cases with SLC52A3 mutations.Reference Garg, Kulkarni, Hegde and Shah20,Reference Woodcock, Menezes, Coleman, Yaplito-Lee, Peters and White21
There is also significant variability in the vestibular profile of our three cases who underwent vestibular testing, with case 1 demonstrating the presence of lateral semicircular canal function, case 2 showing severe bilateral vestibular hypofunction and case 3 demonstrating mild symmetrical hypofunction. Vestibular dysfunction has been described in the wider auditory neuropathy spectrum disorder literature using a variety of methods. One study of 35 children with auditory neuropathy spectrum disorder reported the presence of vestibulopathy in 42 per cent of the cohort using rotational chair testing and clinical examination,Reference Nash, Veness, Wyatt, Raglan and Rajput23 whereas other studies utilising caloric and clinical examination described prevalence of between 50 and 100 per cent.Reference Starr, Picton, Sininger, Hood and Berlin24,Reference Sheykholeslami, Kaga, Murofushi and Hughes25 A recent study using vestibular evoked myogenic potential, caloric testing, video head impulse testing and suppression head impulse paradigm testing in a group of 22 children with auditory neuropathy spectrum disorder reported 91 per cent with abnormal cervical vestibular evoked myogenic potential, 86 per cent with abnormal ocular vestibular evoked myogenic potential, 70 per cent with abnormal rate on caloric testing, and most revealing, no overt corrective saccades on video head impulse testing and normal anti-compensatory saccades on suppression head impulse paradigm testing.Reference Hu, Chen, Zhang, Xu, Ma and Zhang26
In considering vestibulopathy in auditory neuropathy spectrum disorder, the auditory and vestibular systems are intrinsically linked and therefore the suggested post-synaptic pathology resulting from riboflavin deficiency in Brown-Vialetto-Van Laere syndrome would be expected to affect both systems. It is also possible that vestibular dysfunction could precede or occur in the absence of hearing loss, which suggests a rationale for vestibular testing in Brown-Vialetto-Van Laere syndrome with or without auditory neuropathy spectrum disorder. As our cases have only had rotational chair testing performed, we are not reporting the complete picture of their vestibular function and further testing is being considered, including vestibular evoked myogenic potential and video head impulse testing.
It is worth mentioning the presence of hypoplastic cochlear and facial nerves in case 2, which is difficult to explain from the standpoint of Brown-Vialetto-Van Laere syndrome alone as there is no reported association between cochlear and facial nerve hypoplasia and Brown-Vialetto-Van Laere syndrome from neuroimaging studies. The large majority of Brown-Vialetto-Van Laere syndrome cases have normal brain MRI scans, with rare descriptions of cerebellar, corpus callosum and optic nerve abnormalities, and T2-weighted hyperintensities in the brainstem and brainstem nuclei.Reference O'Callaghan, Bosch and Houlden16,Reference Jaeger and Bosch17 Although this could represent a separate pathology to Brown-Vialetto-Van Laere syndrome, we argue that this finding provides a case for considering imaging despite the diagnosis of Brown-Vialetto-Van Laere syndrome.
• Hearing impairment in Brown-Vialetto-Van Laere syndrome is characterised by auditory neuropathy spectrum disorder
• There is significant variability in the vestibular profile of Brown-Vialetto-Van Laere syndrome
• Audiological response to riboflavin therapy in Brown-Vialetto-Van Laere syndrome is variable, in contrast to clearer improvement in motor function
• There is a heterogeneous profile of benefit from cochlear implantation
• Serial behavioural audiometry should be conducted in Brown-Vialetto-Van Laere syndrome as part of systematic examination of riboflavin effects
• Comprehensive vestibular function testing should be studied in Brown-Vialetto-Van Laere syndrome
Finally, our cases demonstrate a heterogeneous profile of benefit from cochlear implantation. This again seems consistent with studies showing that the benefit of cochlear implantation in children with auditory neuropathy spectrum disorder in Brown-Vialetto-Van Laere syndrome was variable though largely positive.Reference Anderson, Schaefer, Henderson and Bruce8 The benefit of cochlear implantation in auditory neuropathy spectrum disorder is well documented and has been shown to be comparable with outcomes in other paediatric implant patients.Reference Buss, Labadie, Brown, Gross, Grose and Pillsbury27–Reference Ehrmann-Müller, Back, Kühn, Hagen and Shehata-Dieler29 More recently it has been suggested that the predicted outcomes from cochlear implantation in auditory neuropathy spectrum disorder depend on whether the pathology is pre- or post-synaptic, with pre-synaptic auditory neuropathy spectrum disorder benefitting comparably to those with cochlear sensorineural hearing loss whereas the outcomes for post-synaptic auditory neuropathy spectrum disorder are more variable.Reference Rance and Starr30 It has been proposed that the poorer performance in post-synaptic auditory neuropathy spectrum disorder could be because of the effects on neural transmission of the cochlear implant electrical signal.Reference Shearer and Hansen31 Taking these findings into consideration, the decision regarding cochlear implantation in Brown-Vialetto-Van Laere syndrome with auditory neuropathy spectrum disorder should be made taking into account any potential improvements in hearing thresholds following riboflavin therapy, in addition to other factors commonly assessed, such as audiological test results, accompanying disorders and speech development.
Conclusion
Our cases highlight the variability in the audiovestibular profile of Brown-Vialetto-Van Laere syndrome with SLC52A2 and SLC52A3 mutations included. We suggest that serial behavioural audiometry should be conducted in larger numbers of Brown-Vialetto-Van Laere syndrome cases as part of the systematic examination of the effects of riboflavin and that comprehensive vestibular function testing including vestibular evoked myogenic potential and video head impulse testing should be studied.
Competing interests
None declared











