Introduction
The ‘Global parasite project’ proposed by Carlson et al. (Reference Carlson, Hopkins, Bell, Doña, Godfrey, Kwak, Lafferty, Moir, Speer, Strona, Torchin and Wood2020a, Reference Carlson, Dallas, Alexander, Phelan and Phillips2020b) is an internationally coordinated effort to revolutionize the process of cataloguing parasite diversity with an ambitious goal of describing 50% of species in the next decade. Systematically collecting parasite data would help to establish current parasite biodiversity baselines, identify rare species and monitor future changes in parasite biodiversity (Gehman et al., Reference Gehman, Satterfield, Keogh, McKay and Budischak2019). Estimations suggest a global total of 100 000–350 000 species of helminth endoparasites of vertebrates, of which 85–95% are unknown to science (Carlson et al., Reference Carlson, Dallas, Alexander, Phelan and Phillips2020b). In the case of digeneans (Platyhelminthes: Trematoda), this estimation is 44 262 species but only 14% have been described (Carlson et al., Reference Carlson, Dallas, Alexander, Phelan and Phillips2020b). Despite the vast diversity of this group, they are also one of the less protected, even when the global impact of losing some of those parasites could lead to severe consequences to ecosystems. Parasites often play key roles that are usually underestimated for being considered dangerous, when the fact is that parasite diversity benefits ecosystems and decrease community-level disease risk (Lafferty et al., Reference Lafferty, Allesina, Arim and Briggs2008). For example, digeneans have an important role in ecosystems as ecosystem engineers, by contributing to biomass flow, food web connectivity, population control altering predator–prey interactions and driving the evolution of other species (Lafferty et al., Reference Lafferty, Allesina, Arim and Briggs2008; Sato et al., Reference Sato, Watanabe, Kanaiwa and Niizuma2011; Dunne et al., Reference Dunne, Lafferty, Dobson and Hechinger2013; among others). They can also be used as bioindicators of environmental natural fluctuations, abundance and diversity of biotic communities, climate changes, or anthropogenic impact, as they are sensitive to changes in habitat quality and land use (Lafferty, Reference Lafferty1997; Huspeni et al., Reference Huspeni, Hechinger, Lafferty and Bortone2005). Monitoring studies have shown that surrogate species can provide good information for the diversity of taxa present in an environment (Lindenmeyer et al., Reference Lindenmeyer, Barton and Pierson2015; Moore et al., Reference Moore, Gittman, Puckett, Wellman and Blakeslee2020), even indicating the presence of the more elusive ones as diverse as shorebirds (Byers et al., Reference Byers, Blakeslee, Linder, Cooper and Maguire2008), terrapins (Byers et al., Reference Byers, Altman, Grosse, Huspeni and Maerz2010), small fishes and benthic invertebrates (Hechinger et al., Reference Hechinger, Lafferty, Huspeni, Brooks and Kuris2007). However, digenean species’ identification and their diversity in the ecosystems must be known before being able to reveal their true role.
In studies with mollusc hosts, the temporal and spatial distributions of the hosts involved in the complex life cycles and the characteristics of the host's habitat (including biotic and abiotic factors) produce variations in the diversity and the structure of larval digenean communities (Kuris and Lafferty, Reference Kuris and Lafferty1994; Huspeni et al., Reference Huspeni, Hechinger, Lafferty and Bortone2005; Thieltges et al., Reference Thieltges, Fredensborg and Poulin2009). Additionally, habitat features over large and small spatial scales can also directly or indirectly influence transmission dynamics, and thus, the recruitment success of parasites to their hosts (Sousa and Grosholz, Reference Sousa and Grosholz1991). Particularly, local factors play a major role in determining the richness and abundance of larval digeneans in mollusc hosts (Byers et al., Reference Byers, Blakeslee, Linder, Cooper and Maguire2008).
In the last 20 years, much progress has been made to increase the knowledge of the diversity of digeneans in the lotic and lentic environments of Argentina, as well as their temporal and spatial dynamics in several mollusc hosts (Flores et al., Reference Flores, Semenas and Veleizan2010, Reference Flores, Brant and Loker2015; Merlo and Etchegoin, Reference Merlo and Etchegoin2011; Fernández et al., Reference Fernández, Hamann and de Núñez M2013; Parietti et al., Reference Parietti, Merlo and Etchegoin2013, Reference Parietti, Merlo and Etchegoin2020, Reference Parietti, Merlo and Etchegoin2021; Merlo et al., Reference Merlo, Parietti and Etchegoin2019; among others). However, due to the large number of water bodies that cover the Argentine territory, we are still far away from having adequate knowledge of the diversity of digeneans and the factors that determine their distribution that allows us to perform robust macroecological and biogeographical studies. Therefore, the main objective was to study the diversity of larval digeneans of Heleobia parchappii (Mollusca: Cochliopidae) in a small shallow lake (Nahuel Rucá). In addition, given the natural features and low human impact in this environment, the temporal and spatial dynamics of larval digenean assemblage was studied within the lake. These studies are fundamental to reduce the gap in the knowledge of digenean diversity and to establish in the future the true role of this group of parasites in freshwater ecosystems.
Materials and methods
Study area
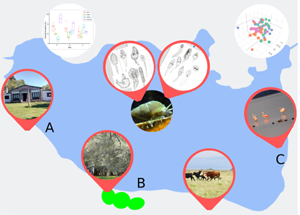
Nahuel Rucá shallow lake is located in the Pampean region of Argentina (37°37′S–57°25′W) (Fig. 1). It has a surface area of about 245 ha, and a mean depth of 1.5 ± 0.16 m (Isla and Gaido, Reference Isla, Gaido and Iribarne2001), and is 9.6 km from the Argentinian Sea. It has the contribution of a tributary, Dulce Stream, and flows through an artificial channel into the Sotelo Stream (effluent). It has reed beds (Schoenoplectus californicus) at the intersection between the streams and the lake, and its borders and along its coastline, which makes this water body important breeding, resting and feeding area for a large number of local and migratory birds species (Josens et al., Reference Josens, Pretelli and Escalante2009). In addition, five molluscs were found in this lake H. parchappii, Biomphalaria peregrina, Pomacea canaliculata, Musculium argentinum and Uncancylus concentricus (Tietze and De Francesco, Reference Tietze and De Francesco2010; Personal observation). However, H. parchappii is the only species present throughout the lake and with abundances ranging from 240 to 1478 ind. h−1 (Merlo et al., Reference Merlo, Parietti and Etchegoin2016). Nahuel Rucá is a particularly unstable environment, with a varied rainfall (10–268 mm monthly) and air temperature range (2–28°C) (personal data). It also suffered a total drought at the end of 2008 and the beginning of 2010. These abiotic features are particular and common to water bodies of the Pampean region (Sosnovsky and Quirós, Reference Sosnovsky and Quirós2006).

Fig. 1. Map showing locations of the Nahuel Rucá Shallow Lake in closeness to Mar Chiquita Coastal Lagoon in Argentina. In the satellite photograph, the three sampling sites are indicated.
Three sampling sites were selected from the lake. Site A at the extreme southwest, near the Sotelo stream (effluent), and characterized by having a coastline free of vegetation and with reed beds arranged in a semi-circular shape located 10 m from the water edge, forming a structure similar to a bay. At the central point of the lakeshore is located the site B with an abundant number of reeds arranged parallel to the coastline. And a site C, located in the extreme northwest of the lake near Dulce creek, with similar physiognomy to site A in terms of the disposition of the reeds, and it is an area used by birds as a roost (Josens et al., Reference Josens, Pretelli and Escalante2009).
Sample collections
Specimens of H. parchappii were collected from one sampling trip per season during autumn, winter and spring 2017 and during summer 2018. The samplings were carried out in the second month of each season during the morning (e.g. May in autumn, August in winter, etc.) and all three sites (A, B and C) were sampled in the same sampling trip. Five replicate samples per trip and site were taken. Snails were collected along a 200 m transect parallel to the coastline with the aid of sieves (0.1 mm × 0.1 mm) and placed into plastic containers of 1.5 L capacity for transportation. Water temperature and depth were measured at every trip per season in each site. In the laboratory, the shell length of each mollusc was measured to the nearest 0.05 mm using a Leica DM 500 stereomicroscope. Sixty randomly selected molluscs from each replicate and each season were isolated individually in 45 ml plastic cups and maintained under a 12–12 light-dark photoperiod for 48 h to stimulate shedding of larval digenean (cercariae). Finally, all molluscs were dissected under a stereomicroscope and all organs and tissues were examined to detect the presence of sporocysts, rediae, developing cercariae and metacercariae (Curtis and Hubbard, Reference Curtis and Hubbard1990). Emerged cercariae were studied alive, under a light microscope, and were identified according to Yamaguti (Reference Yamaguti1975), Martorelli (Reference Martorelli1986a), Martorelli and Etchegoin (Reference Martorelli and Etchegoin1996), Etchegoin (Reference Etchegoin1997), Etchegoin and Martorelli (Reference Etchegoin and Martorelli1998) and Merlo et al. (Reference Merlo, Parietti and Etchegoin2014).
Statistical analysis
Three indices were used to analyse and compare the temporal and spatial dynamics of the community of larval digeneans in H. parchappii: (a) species richness (S) which represents the total number of species in a sample (Magurran, Reference Magurran1988); (b) prevalence by species (number of snails parasitized by that species/number of collected snails × 100) and (c) overall prevalence (number of parasitized snails/number of collected snails × 100).
Generalized Linear Models (GLMs) with a Negative Binomial probability distribution model and the function glm from the MASS package for R (Venables and Ripley, Reference Venables and Ripley2002) were used to explore differences between seasonal and spatial overall prevalence. A total of five variables were considered for model selection in GLM: three continuous and explanatory variables: snail's size, water temperature and water depth; and two variable categories: season and site. Model selection in GLM involved the hypothesis testing (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009), starting with all three exploratory variables, and removing the water depth variable in the final model as it was not significant. Post-hoc comparisons were made between the sites and seasons.
A Bray–Curtis similarity matrix was computed at the infra-community level for the parasite assemblage in H. parchappii by site and season and was subjected to a permutational multivariate analysis of variance (PERMANOVA), using the function adonis from the vegan package for R (Oksanen et al., Reference Oksanen, Blanchet and Friendly2016). Due to the large differences in parasite abundance across parasite species, data were square-root transformed prior to multivariate analyses to down-weight the importance of very abundant species, such that less dominant species also played a role in determining similarity among samples. Since PERMANOVA is sensitive to differences in multivariate dispersion between groups (sensu homogeneity of variances), the same models were tested for differences in dispersion using multivariate homogeneity of group dispersions PERMDISP. To visualize possible temporal and spatial patterns in the composition of parasite assemblages, non-metric multidimensional scaling (nMDS) of the similarity matrix were performed between all infra-communities. Average values were then visualized in an nMDS using as many dimensions as needed to closely match the original distance matrix (correlation coefficient of ρ = 0.99).
Analyses were done using the statistical programming language R, version 2.15.2. (R Development Core Team, 2018).
Results
Between autumn 2017 and summer 2018, a total of 2871 individuals of H. parchappii were analysed and 23 species of larval digenea belonging to 12 families were recorded (Table 1). The greatest species richness was found in summer for site B and winter for site C (both S = 13), and the lowest species richness was found in summer for site C (S = 6) (Table 1). Of the total species types of digenea, only three were found during the four seasons and at the three sites: Notocotylidae sp. 1 (Notocotylidae), Levinseniella cruzi (Microphallidae) and Microphallus simillimus (Microphallidae). On the contrary, six were registered in one season and one site. Cercaria Haploporidae sp. 2 (Haploporidae) was registered in the winter of site A. In site B, Xiphidiocercaria sp. 2 (Ochetosomatidae) was registered in autumn, and cercariae [Xiphidiocercaria sp. 3 and Xiphidiocercaria sp. 4 (Plagiorchiidae)] in summer. In site C cercariae Schistosomatidae sp. 1 (Schistosomatidae), Furcocercaria sp. 2 (Schistosomatidae) and Pleurolophocercaria IV aff. Pygiodicpssis crassus (Heterophydae) were registered in winter.
Table 1. List of species or morphological type of larval digeneans that parasitize Heleobia parchappii in Nahuel Rucá shallow lake

The second intermediate host and definitive host according to bibliography available are also given. Ref: A, autumn; W, winter; Sp, spring; S, summer.
By identifying the larval stages at the family level and based on published life cycle data, it was possible to establish the possible definitive hosts that would contribute to forming the assemblages of larval digenea of H. parchappii in the Nahuel Rucá Lake. Birds and mammals, with a 66.6% contribution, are the main definitive hosts of the digenea that parasitize H. parchappii, followed by fishes (18.8%), and to a lesser extent, 8.7% use reptiles, and 1.5% amphibians as definitive hosts. Only one species [Genarchella genarchella (Hemiuridae)] has all monoxene life cycle in the mollusc host.
Significant effects of the water temperature and host's size on the prevalence, and interaction between sites and seasons were found (water temperature: P = 0.004, host's size: P = 0.03, interaction: P < 0.00005, Fig. 2). Between sites, the prevalence of spring was lower at A than C (P = 0.004). In contrast, the prevalence in summer was higher at A than C (P = 0.006). No differences were found in the overall prevalence between sites for autumn and winter, and between sites A and B in spring and summer (P > 0.05, for all cases). The post hoc analysis within each site yielded differences between the site A prevalence in spring compared to autumn (P = 0.0001), winter (P = 0.004) and summer (P = 0.0000009). However, at sites B and C, no seasonal differences were observed (P > 0.05, for all cases).

Fig. 2. Seasonal and spatial variation of the overall prevalence of larval digeneans that parasitize Heleobia parchappii. Boxes represent means and vertical bars denote maxim and minimum values.
The results of PERMANOVA showed an effect of sites and seasons on the parasite community structure [season × site: F = 1.94, P (perm) = 0.0013]. Pairwise tests agreed in general with nMDS ordinations (Fig. 3). In the analysis of pairs between sites by season, differences were observed between autumn of sites A, B and C [A vs B: P (perm) = 0.0053; A vs C: P (perm) = 0.0047; B vs C: P (perm) = 0.006], between winter and spring of sites B and C [winter B vs C: P (perm) = 0.023; spring B vs C: P (perm) = 0.026], and between summer of sites A and C [P (perm) = 0.03]. While in the seasonal analysis within each site, different patterns were observed. Site A presented differences between autumn and the other all seasons [autumn vs winter: P (perm) = 0.017; autumn vs spring: P (perm) = 0.015; autumn vs summer: P (perm) = 0.008], and winter was different from summer [P (perm) = 0.0073]. In site B, autumn presented differences from spring and summer [autumn vs spring: P (perm) = 0.02; autumn vs summer: P (perm) = 0.034]. Site C presented differences between almost all seasons [autumn vs winter: P (perm) = 0.012; autumn vs spring: P (perm) = 0.018; spring vs winter: P (perm) = 0.022; spring vs summer: P (perm) = 0.009; summer vs winter: P (perm) = 0.047], only summer was similar to autumn [P (perm) = 0.062]. A proportion of these differences can be attributed to differences in the dispersions of parasite communities in the lake [PERMIDISP: F = 5.99, P (perm) = 0.0007].

Fig. 3. Non-metric multidimensional scaling (NMDS) ordination plot based on Bray–Curtis similarity on parasite prevalence. (A) By seasons and sites. (B) By season. (C) By sites.
Discussion
This study examined temporal and spatial changes in composition and prevalence in larval digenea assemblages of H. parchappii. Our results indicate a high diverse parasite fauna with 23 species. The species richness remains constant throughout the lake with 16–17 species per site, but temporal and spatial differences were observed in the species composition. Also, the overall prevalence showed spatial variation between two of three sites and temporal variation only was observed at one site.
The larval digenean assemblage of H. parchappii has been studied in other shallow lakes, urban and peri-urban streams in Argentina, and 12–13 digenean species were found. The low specific richness found in those environments was mainly associated with heavily human-impact ecosystems due to recreation and agriculture (Merlo et al., Reference Merlo, Parietti and Etchegoin2019; Parietti et al., Reference Parietti, Merlo and Etchegoin2020). The shallow lake studied in this work is a very unstable environment, but with a lower human impact over it. A native Celtis tala forest is located on its southeast coast, and a little area of the southwest coast is used for small-scale cattle grazing. Site A is the site with the most human impact because it is close to the house and stable of a small family and a couple of workers that live there. Also, this lake is located in the vicinity of Mar Chiquita Coastal Lagoon Basin, a biosphere reserve under the UNESCO Man and Biosphere Program since 1996. The low human disturbance in this environment may explain the larger diversity registered in this lake, in contrast with the other environments already studied in the region (Merlo et al., Reference Merlo, Parietti and Etchegoin2019; Parietti et al., Reference Parietti, Merlo and Etchegoin2020).
Similar parasite diversity was registered in other molluscs species of the same genus, such as 22 species in Heleobia conexa in Mar Chiquita lagoon (Etchegoin, Reference Etchegoin1997; Merlo and Etchegoin, Reference Merlo and Etchegoin2011) and 15 species (Alda and Martorelli, Reference Alda and Martorelli2014) and 22 species (Parietti et al., Reference Parietti, Merlo and Etchegoin2013) in Heleobia australis from estuarial habitats. Both the Nahuel Rucá lake, the Mar Chiquita lagoon and the estuarial habitats studied by these authors are sites extremely connected with the marine environment, wetter, greener and more productive, which leads to the great biodiversity of possible definitive hosts and consequently a high diversity of parasites.
All digenea life cycle types are present in the digenean assemblage studied (three-host life cycle, two-host life cycle and monoxene life cycle). Thirteen species presented a typical three-host cycle (57%), 8 out of 23 species (35%) a two-host life cycle and even a monoxene life cycle was registered in Nahuel Rucá Lake. The two-host life cycles are not very frequent in marine trematodes given that extra intermediate hosts enhance the dispersion of the larval stages and, consequently, the number of possible definitive hosts (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003), but represented an important proportion in this assemblage in H. parchappii.
Particularly, three of the species identified in this work are well adapted to varied environmental conditions. Notocotylidae sp. 1 (Notocotylidae), M. simillimus (Microphallidae) and Levinseniella cruzi (Microphallidae) were registered in all seasons and all sites, the two first have a two-host life cycle, and the third a typically three-host life cycle. Also, these three species were recorded in the same host in other freshwater ecosystems (Martorelli, Reference Martorelli1986a, Reference Martorelli1986b, Reference Martorelli1986c; Merlo et al., Reference Merlo, Parietti and Etchegoin2019; Parietti et al., Reference Parietti, Merlo and Etchegoin2020), in other molluscs species of the same genus, as H. conexa in Mar Chiquita lagoon (Martorelli, Reference Martorelli1986a, Reference Martorelli1986b, Reference Martorelli1986c; Etchegoin and Martorelli, Reference Etchegoin and Martorelli1998, Merlo and Etchegoin, Reference Merlo and Etchegoin2011) and H. australis in estuarial habitats (Alda and Martorelli, Reference Alda and Martorelli2014; Parietti et al., Reference Parietti, Merlo and Etchegoin2013), and even in other mollusc genus, as B. peregrina in a temporary pond near to Mar Chiquita lagoon (Parietti et al., Reference Parietti, Merlo and Etchegoin2021). In Notocotylidae sp. 1 (Notocotylidae) the cercariae are expelled from the snail host and encyst upon aquatic plants, shells and other objects, then definitive hosts can get infected by feeding on plants that carry the metacercariae (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). The life cycle characteristics of this species could facilitate habitat use with different abiotic conditions and it presented probably low host-specificity (Parietti et al., Reference Parietti, Merlo and Etchegoin2021). Levinseniella cruzi and M. simillimus belong to the Microphallidae family, a trematode group originally from coastal environments. However, a successive transitional series from a typical (marine) three-host life cycle to a two-host one (freshwater) can be observed when the mollusc acts as the first and second intermediate host (Deblock, Reference Deblock1977).
Prevalence and species composition in the lake showed temporal-spatial dynamics similar to that found in the assemblage that parasitizes H. australis on a small scale in Mar Chiquita coastal lagoon (Parietti et al., Reference Parietti, Merlo and Etchegoin2013). This variability could be the result of the habitat used by the definitive host, mainly birds. More than half of the digenean species registered used birds as definitive hosts in Nahuel Rucá. Several bird species are registered in the literature as definitive hosts of the digenean species registered in this lake (see Table 1). Also, an important increase of migratory birds can be observed during spring and summer in Mar Chiquita coastal lagoon (Ferrero, Reference Ferrero and Iribarne2001) and due to the proximity between the lake and the lagoon, migratory birds, together with local breeding species of shorebirds, use the lake as a resting and feeding area, contributing to a temporal increase in the influx of digenean-infective stages, similar to what was observed in a pond also close to the Mar Chiquita lagoon (Parietti et al., Reference Parietti, Merlo and Etchegoin2021). In particular, birds used the reed beds in spring as nesting areas near site C, which is coincident with the higher overall prevalence values registered in this site. Lately, in summer, birds moved south into the lake to feed and perching near site A, coincident with the rise in overall prevalence there (Josens et al., Reference Josens, Pretelli and Escalante2009). For their part, fishes, being the 18.8% of digenean hosts, could be homogenizing the internal variances in the lake. Species from Heterophydae and Echinostomatidae family used small fishes as the second intermediate host, with Cnesterodon decemmaculatus and Jenynsia lineata cited in the literature (Rauque et al., Reference Rauque, Viozzi, Flores, Vega, Waicheim and Salgado-Maldonado2018; Taglioretti et al., Reference Taglioretti, Rossin and Timi2018; among others and see Table 1). Also, species from Acanthostomidae, Haploporidae and Hemiuridae have fishes as the definitive host, with Astyanax fasciatus, Pimelodus sp., Astyanax sp., Cynopotamus sp., Moenklausia doceana and Roeboides sp. cited in the literature (Ostrowsky de Nuñez et al., Reference Ostrowsky de Nuñez, Arredondo and de Pertierra2017 and see Table 1). All these fish species are registered in Nahuel Rucá shallow lake, in addition to Rhamdia quelen, species without parasitological studies so far.
It is interesting to mention that one digenean species registered in this lake completed its life cycle in reptiles, as the Ochetosomatidae family (Xiphidiocercaria sp. 2), and three others in reptiles and other vertebrate groups, as the Acanthostomidae family (Cercaria Acanthostomidae sp. 1) and the Plagiorchiidae family (Xiphidiocercaria sp. 3 and Xiphidiocercaria sp. 4). All four species were presented mainly in spring and summer. Cercaria Acanthostomidae sp. 1 is present in all three sites because, probably, used also fishes as the definitive host, and, as we mentioned, fishes unify the presence and abundance of the digenean in the lake. Site B is the only one with the presence of the four digenean species that used reptiles as definitive hosts. This place is located 200 m from the C. tala forest, a native American forest, on its southern edge of distribution. Reptiles probably used the C. tala forest as a refuge. Non-native plant species might generate habitat modifications that increase the predation pressure for reptiles (more pray visibilities, fewer refugees, more vantage points to potential predators). Contrary, predation risk is lower in native forests in lizards from avian predators (Stellatelli et al., Reference Stellatelli, Block, Vega and Cruz2015). Two freshwater turtle species were registered associated with this forest in the region, Hydromedusa tectifera and Phrynops hilarii, and a brown lizard species, Tupinambis merianae (Haene, Reference Haene, Merida and Athor2006). The proximity of the forest to the body of water in B could favour the contact between definitive and intermediate hosts allowing the digenean species to complete its life cycle. There is no record of reptile parasites in the bibliography for the area. Trematodes may be usefully employed as ecological indicators even in little studied environments since the taxonomic identification to family level is usually sufficient to identify both types of second intermediate and definitive hosts (Huspeni et al., Reference Huspeni, Hechinger, Lafferty and Bortone2005; Hechinger et al., Reference Hechinger, Lafferty, Huspeni, Brooks and Kuris2007; Duan et al., Reference Duan, Al-Jubury, Kania and Buchmann2021). Consequently, these digenean families registered in Nahuel Rucá may indirectly indicate the presence of reptiles using the lake. Taking into account that an important part of the reptile species is at conservation risk (Prado et al., Reference Prado, Waller, Albareda, Cabrera, Etchepare, Giraudo, González Carman, Prosdocimi and Richard2012), our work provides an idea of the possible hosts that may habitat in the area.
Another aspect to highlight is the register of the Cercaria Lecithodendriidae sp. 1 that belongs to a Lecithodendriidae family that was rarely mentioned in Argentina and are parasites of bats and occasionally of birds (Milano, Reference Milano2016). Adults of Lecithodendriidae were identified in bats in the Pampean province (Lunaschi, Reference Lunaschi2002) and dragon-fly naiads (Arthropoda: Odonata) are mentioned as the second intermediate host (Hall, Reference Hall1958). It is estimated that 8 bat species present a distribution that overlaps with the study region but only 2 species were observed in the study area (Barquez et al., Reference Barquez, Díaz, Montani and Pérez2020). In Argentina, 67 species of bats have been registered and bats are particularly susceptible to anthropogenic changes because of their low reproductive rate, longevity and high metabolic rates, and out of 1001 species of bats, almost a quarter are globally threatened and listed by the IUCN (Voigt and Kingston, Reference Voigt and Kingston2016). Given the few records of bats in Nahuel Rucá lake, and that this digenean species has not been found in other water bodies of the region, we suggest an interesting possible host–parasite association to be considered and developed in future studies. However, further studies are needed to determine whether this species could use other definitive hosts.
Multi-host parasite abundance can act as a surrogate for trophic complexity in structured habitats for the presence of their hosts, as is the case of digenean species. Their life cycle incorporates multiple invertebrate and vertebrate taxa, mainly due to the trophic transmission to the vertebrate definitive host, although there are many transmission variants. Except for the direct infection of definitive hosts by the cercariae (e.g. in bird schistosomes) or the monoxene life-cycles [e.g. G. genarchella (Hemiuridae)], all transmission strategies registered in this assemblage in Nahuel Rucá (three and two-host cycles) involve the trophic transmission to the definitive host, and thus provide information on trophic interactions and energy flow within the ecosystem. Also, the large number of transmission events from aquatic organisms to birds indicates a substantial energy flow from freshwater to terrestrial systems via these predation events. Parasites are usually not directly visible in an ecosystem, while their hosts are considered naturally regarded as the biome that inhabits an ecosystem. For this reason, parasites have traditionally been omitted from the majority of ecological studies (Poulin, Reference Poulin2007). As mentioned previously, parasites are deeply embedded in larger food webs and active elements of the ecological processes that shape and structure ecological communities, energy flow and the biodiversity of complex ecosystems (Lafferty et al., Reference Lafferty, Allesina, Arim and Briggs2008; Sato et al., Reference Sato, Watanabe, Kanaiwa and Niizuma2011; Dunne et al., Reference Dunne, Lafferty, Dobson and Hechinger2013; among others). This local trematode diversity could reveal information on definitive host occurrence, host's habitat use and trophic interactions within ecosystems, and is also an excellent ecosystem health indicator (Huspeni et al., Reference Huspeni, Hechinger, Lafferty and Bortone2005; Hechinger et al., Reference Hechinger, Lafferty, Huspeni, Brooks and Kuris2007). Conservation politics for parasites and their hosts are extremely necessary for this environment, due to its pristine characteristics and its closeness to a UNESCO reserve.
A recent legal ordinance enables the construction of an Estate project in the middle of the Mar Chiquita natural reserve that, according to experts and environmentalists, would generate very serious ecological consequences, such as a possible ‘increased risk of flooding due to disturbance of wetlands’, the ‘elimination, reduction and fragmentation of the Pampean grassland’, as well as an ‘alteration of the quality and quantity of water, both underground and surface’. This could have a serious impact on the biodiversity of the Mar Chiquita lagoon, and due to its proximity, over the Nahuel Rucá lake diversity. Our work supports the idea that this area is of great importance in biodiversity, and could be endemic areas of some species of digenean that use reptiles, amphibians and bats as hosts, groups that are at risk of conservation. The protection of these environments, and even the expansion of their geographic range, are fundamental pillars in the policies for the conservation of wild flora and fauna.
Author contributions
All authors equally contributed to the work of conceiving and designing the study, conducting data gathering, performing statistical analyses, and writing the article.
Financial support
Financial support was received from ANPCyT (PICT 2017-1819 to Etchegoin JA) and from Universidad Nacional de Mar del Plata (Grant number 15/E935 EXA997/20 to Etchegoin JA).
Conflict of interest
None.
Ethical standards
Not applicable.