Introduction
Human babesiosis is a vector-borne disease transmitted by ticks; the frequency of occurrence and geography of its cases have been increasing in recent decades (Yabsley and Shock, Reference Yabsley and Shock2013; Hildebrandt et al., Reference Hildebrandt, Zintl, Montero, Hunfeld and Gray2021). The causative agent of babesiosis is the protozoan blood parasites Babesia spp. (Apicomplexa: Babesiidae). Babesial parasites require both a competent vertebrate and invertebrate host to maintain transmission cycles and 2 types of reproduction: asexual and sexual. Sexual reproduction (sporogony) occurs in the invertebrate host and consists of fusion of gametes and formation of zygotes in the gut of the vector, followed by multiple fissions in various tick tissues, and culminating in the development of infective stages in the salivary glands. Infective sporozoites are injected into the bloodstream of a vertebrate host during tick feeding and invade red blood cells (RBCs), where they initiate cycles of asexual reproduction (merogony) by asynchronous binary fission, yielding a complex pleomorphic population of intraerythrocytic (IE) parasites (Sevilla et al., Reference Sevilla, González, Luque, Gray and Montero2018; Gray et al., Reference Gray, Estrada-Peña and Zintl2019; Conesa et al., Reference Conesa, Sevilla, Terrón, González, Gray, Pérez-Berná, Carrascosa, Pereiro, Chichón, Luque and Montero2020). Asexual stages cause clinical manifestations of the disease in the vertebrate host. In addition to merogony, gametogenesis occurs in the blood of the vertebrate host. The formation of gametocytes is a mandatory stage in the apicomplexan life cycle. It is necessary for further sporogony in invertebrate hosts.
From the first reported case of human babesiosis in 1956 (Skrabalo and Deanovic, Reference Skrabalo and Deanovic1957) to the present, about 60 cases have been published in 19 European countries (Hildebrandt et al., Reference Hildebrandt, Gray and Hunfeld2013, Reference Hildebrandt, Zintl, Montero, Hunfeld and Gray2021). Almost all cases of human babesiosis in Europe were caused by Babesia divergens and were observed in patients who had been splenectomized prior to infection (Centeno-Lima et al., Reference Centeno-Lima, do Rosário, Parreira, Maia, Freudenthal, Nijhof and Jongejan2003; Corpelet et al., Reference Corpelet, Vacher, Coudore, Laurichesse, Conort and Souweine2005; Mørch et al., Reference Mørch, Holmaas, Frolander and Kristoffersen2015; Asensi et al., Reference Asensi, González, Fernández-Suárez, Sevilla, Navascués, Suárez, Lauret, Bernardo, Carton and Montero2018; Kukina et al., Reference Kukina, Guzeeva, Zelya and Ganushkina2018). These cases are often fatal. However, in Europe, sporadic cases of babesiosis have been diagnosed in patients with an intact spleen (Martinot et al., Reference Martinot, Zadeh, Hansmann, Grawey, Christmann, Aguillon, Jouglin, Chauvin and DeBriel2011; Gonzalez et al., Reference Gonzalez, Rojo, Gonzalez-Camacho, Luque, Lobo and Montero2014; O'Connell et al., Reference O'Connell, Lyons, Abdou, Patowary, Aslam, Kinsella, Zintl, Hunfeld, Wormser, Gray, Merry and Alizadeh2017; Kukina et al., Reference Kukina, Zelya, Guzeeva, Karan, Perkovskaya, Tymoshenko and Guzeeva2019).
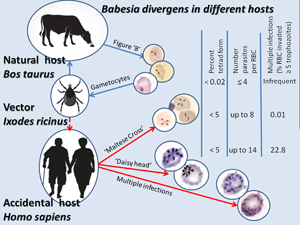
Babesia divergens is primarily specific to bovines and widespread throughout Europe with Ixodes ricinus as the vector. People are not natural hosts for B. divergens but can serve as accidental hosts. The trophozoite size, position inside the erythrocyte and morphological detail are dependent on the host species (Krylov, Reference Krylov1996; Zintl et al., Reference Zintl, Mulcahy, Skerrett, Taylor and Gray2003). In recent years, significant progress has been made in our understanding of the asexual reproduction of Babesia based on studying this process in human RBCs cultured in vitro (Rossouw et al., Reference Rossouw, Maritz-Olivier, Niemand, van Biljon, Smit, Olivier and Birkholtz2015; Cursino-Santos et al., Reference Cursino-Santos, Singh, Pham, Rodriguez and Lobo2016; Conesa et al., Reference Conesa, Sevilla, Terrón, González, Gray, Pérez-Berná, Carrascosa, Pereiro, Chichón, Luque and Montero2020). However, the development of the parasite in vitro and in vivo may vary somewhat, and some aspects of this process may be studied by using microscopy blood smears from patients. Unfortunately, the appearance of gametocytes in the blood is a crucial event that is still not fully understood. The characteristics of asexual reproduction of B. divergens in a splenectomized patient and a patient with an intact spleen are compared with a description of the division stages of parasites that had not been reported previously in infected humans.
Materials and methods
Patient 1 was a 58-year-old man, a huntsman. Post-traumatic splenectomy had been performed 12 years ago. Babesiosis was diagnosed only on the 7th day of illness, and therapy with clindamycin was started. Multisystem failure increased progressively and the patient died on day 10 of illness (Kukina et al., Reference Kukina, Guzeeva, Zelya and Ganushkina2018).
Patient 2 was a 74-year-old female whose spleen was intact; she was hospitalized after 10 days of fever. She developed jaundice, dyspnoea, fatigue and a decrease in diuresis. The patient was diagnosed with a severe course of babesiosis with multisystem failure. Therapy was started with quinine orally (650 mg per 8 h), clindamycin intravenously (1800 mg day−1), intubation, dialysis and plasmapheresis. The parasitaemia diminished gradually and resolved 12 days later. However, the patient developed a systemic inflammatory syndrome of an infectious nature, and after 3 months, she died. The cause of death was pneumonia (Kukina et al., Reference Kukina, Zelya, Guzeeva, Karan, Perkovskaya, Tymoshenko and Guzeeva2019).
In both cases, the diagnosis was obtained from blood smears. The morphological features of the parasites were described before the start of specific treatment. Numerous IE parasites were found, which were initially falsely identified as Plasmodium falciparum. Babesiosis was diagnosed only on days 7 and 10 for patients 1 and 2, respectively. Complex morphological characteristics [absence of haemozoin, pear-shaped trophozoites, paired pyriforms and tetrad forms (‘Maltese Cross’)] allowed us to identify the parasites as Babesia sp. The paired forms diverged at a wide angle (up to 180°), which is a characteristic feature of B. divergens. The piroplasm 18S ribosomal RNA sequence (MK510929, GenBank) from patient 2 was identical to B. divergens EU lineage (99.5–100% identity).
Results
Different morphological IE stages of asexual parasites were found in thin blood smears, namely a single ring trophozoite (Fig. 1A), paired pyriforms (club-shaped and 2 attached pear-shaped sister cells) (Fig. 1B), double trophozoites (2 round unattached cells) (Fig. 1E) and double paired pyriforms (2 sets of paired sister cells) (Fig. 1C). Less commonly, we observed tetrads or ‘Maltese Crosses’ (4 attached sister cells) (Fig. 1E), 4 trophozoites (4 round unattached cells) (Fig. 2A) and filamentous shapes (Fig. 2F). Moreover, in the blood smear of patient 2, we found parasites that divided into 6 and more merozoites; they are typically piriform and joined by their pointed ends, forming a ‘daisy head’ (Fig. 2B and C).

Fig. 1. Babesia divergens. Romanovsky-stained thin blood smear. Ring form (A); the paired forms ‘figure 8s’ diverge at a wide angle (up to 180°); club-shaped forms (B); double paired pyriforms (2 sets of paired sister cells) (C); simultaneous division into 4 daughter nuclei (D); tetrads or Maltese Crosses (4 attached sister cells) and 2 separated mononucleated rounded sister trophozoites (E); a presumable gametocyte (F).
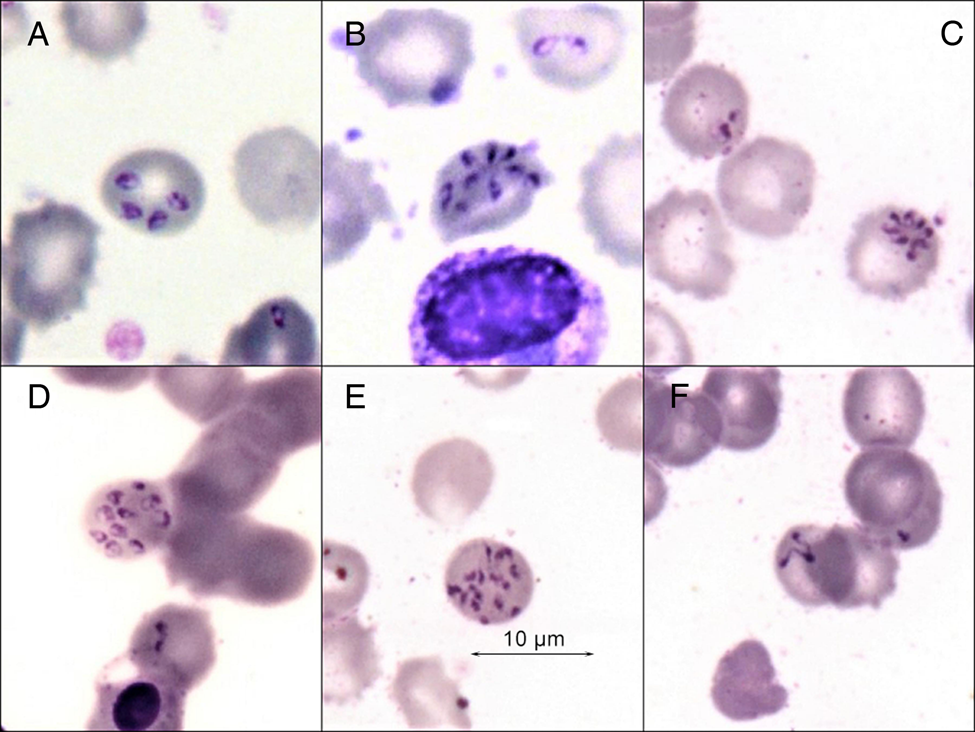
Fig. 2. Babesia divergens. Romanovsky-stained thin blood smear. Four sister trophozoites (A); super multiple fission – formation of a sextet and possibly more merozoites (‘daisy head’) (B, C); multiple invasions (D, E); filamentous forms, noted as crisis forms (F).
Both patients showed a similar per cent of paired pyriforms and tetrad forms considering all infected erythrocytes (iRBCs) (Table 1). However, the total number of trophozoites in the erythrocytes of patient 2 was higher than that of patient 1 (2.9 parasites per iRBC and 1.8 parasites per iRBC, respectively), due to multi-occupancy with practically equal parasitaemia (i.e. the per cent of iRBCs). Multi-occupancy was found in 22.8% of iRBC, with up to 14 merozoites inside the erythrocyte of patient 2 (Fig. 2D and E).
Table 1. Characteristics of patients by the level of infected erythrocytes

a Per cent of paired pyriforms/tetrad stages/multiple parasites in all infected erythrocytes.
Discussion
Unlike P. falciparum, Babesia sporozoites, which enter the bloodstream of the vertebrate host after a tick bite, immediately penetrate RBCs, develop into trophozoites and begin to divide (Krylov, Reference Krylov1996; Hunfeld et al., Reference Hunfeld, Hildebrandt and Gray2008). Based on in vitro studies, there are various pathways by which IE stages could develop (Cursino-Santos et al., Reference Cursino-Santos, Singh, Pham, Rodriguez and Lobo2016). In the first way, inside the host erythrocytes, a sporozoite becomes a ring trophozoite, proliferates (mononucleated rounded/ovoid trophozoite) and divides by binary fission (budding) (Fig. 1A–C). This process produces 2 daughter merozoites. Typically, all merozoites of B. divergens parasites are piriform. Finally, they egress from the RBC and invade intact erythrocytes, become ring trophozoites and initiate new cycles of infection. In vitro, the cycle of asexual reproduction from the merozoite penetration of the cell to the release of the next generation of parasites takes 4–6 h (Rossouw et al., Reference Rossouw, Maritz-Olivier, Niemand, van Biljon, Smit, Olivier and Birkholtz2015; Conesa et al., Reference Conesa, Sevilla, Terrón, González, Gray, Pérez-Berná, Carrascosa, Pereiro, Chichón, Luque and Montero2020). In the second way, daughter merozoites do not leave the host cell. They become ring trophozoites and begin the next cycle of asexual reproduction, sharing the same host cytoplasm as sister cells, a phenomenon that eventually results in multi-occupancy of the erythrocyte (Fig. 2A, D and E).
According to previous views, multi-occupancy is limited to 10–12 parasites per iRBC when B. divergens is cultured in vitro (Cursino-Santos et al., Reference Cursino-Santos, Singh, Pham, Rodriguez and Lobo2016). In natural hosts (B. taurus), multi-occupancy of an RBC is very rare: no more than 4 parasites and tetrads are very rare (only in 0.02% of infected erythrocytes). By contrast, in humans up to 5% of infected erythrocytes contain tetrads (Gorenflot et al., Reference Gorenflot, Brasseur, Precigout, L'Hostis, Marchand and Schrevel1991; Krylov, Reference Krylov1996; Zintl et al., Reference Zintl, Mulcahy, Skerrett, Taylor and Gray2003). The latter is undoubtedly consistent with the data obtained in both patients. Among the published cases of human babesiosis in Europe, there are no mentions of the extraordinarily high level of propagation of B. divergens (>8 IE parasites). The number of parasites in 1 erythrocyte did not exceed 8 on the presented photos of thin blood smears (Martinot et al., Reference Martinot, Zadeh, Hansmann, Grawey, Christmann, Aguillon, Jouglin, Chauvin and DeBriel2011; Gonzalez et al., Reference Gonzalez, Rojo, Gonzalez-Camacho, Luque, Lobo and Montero2014; Mørch et al., Reference Mørch, Holmaas, Frolander and Kristoffersen2015; O'Connell et al., Reference O'Connell, Lyons, Abdou, Patowary, Aslam, Kinsella, Zintl, Hunfeld, Wormser, Gray, Merry and Alizadeh2017). Even in fatal cases of patients who had undergone splenectomy, including patient 1, most iRBC did not have more than 8 parasites (Fig. 1C) (Centeno-Lima et al., Reference Centeno-Lima, do Rosário, Parreira, Maia, Freudenthal, Nijhof and Jongejan2003; Asensi et al., Reference Asensi, González, Fernández-Suárez, Sevilla, Navascués, Suárez, Lauret, Bernardo, Carton and Montero2018; Kukina et al., Reference Kukina, Guzeeva, Zelya and Ganushkina2018). A very high infection of erythrocytes in patient 2 (22.8% of iRBC contained 5–14 parasites) is described for the first time. Such an extraordinarily high rate of parasite reproduction had been unknown for B. divergens in either natural or accidental hosts (Fig. 2D and E). The same intensity of the division of rabbit Babesia sp. (strain NR774) was noted in an in vitro culture of human RBCs (Spencer et al., Reference Spencer, Goethert, Telford and Holman2006).
Observations in in vitro culture have shown that the tetrad forms (‘Maltese Crosses’) are formed from paired pyriform (‘figure 8’) by sequential division (Cursino-Santos et al., Reference Cursino-Santos, Singh, Pham, Rodriguez and Lobo2016; Conesa et al., Reference Conesa, Sevilla, Terrón, González, Gray, Pérez-Berná, Carrascosa, Pereiro, Chichón, Luque and Montero2020). It has previously been assumed that a tetrad of parasites is, in some, perhaps a simple superposition of 2 pairs of dividing individuals on top of each other. However, in Fig. 1D, it is clear that the division into 4 daughter nuclei occurred simultaneously. The discovery of the formation of a ‘daisy head’ as a result of simultaneous division of the parasite into 6 and possibly more merozoites all the more disproves the simple overlap of individuals.
There is a lack of data on gametogenesis because previous work has often been carried out on long-term cultures of parasites, which lose the ability to gametogenesis. The capacity of B. divergens to produce gametocytes has been shown (measured by expression of the bdccp genes) in several bovine strains after short-term cultivation (Jalovecka et al., Reference Jalovecka, Bonsergent, Hajdusek, Kopacek and Malandrin2016). We did not find data on the morphology of babesial gametocytes in blood smears stained by the Romanovsky–Giemsa method. Perhaps by analogy with Plasmodium, gametocytes could be single, large, vacuole-free cells with a large centrally located nucleus (Fig. 1F).
Babesia reproduction occurs asynchronously, so all developmental stages of the parasite may be seen simultaneously. RBCs can bear multiple parasites that may not necessarily be in the same step in the division's process (Fig. 1C and E). A very high infection of erythrocytes is evidenced by the presence of filamentous forms, noted as crisis forms during in vitro B. divergens cultivation when the culture medium is exhausted (Fig. 2F) (Gorenflot et al., Reference Gorenflot, Brasseur, Precigout, L'Hostis, Marchand and Schrevel1991). It can be assumed that the reason for the extraordinarily high level of multi-occupancy iRBCs and the formation of crisis forms of the parasite, in the described case, was an adverse condition, namely an increase in the concentration of metabolites in the blood due to the development of acute renal failure in the patient (Fig. 2B–F).
In conclusion, it is shown that in Babesia, in addition to simple binary fission, there are probably more complex variants of asexual reproduction. Simultaneous division of the parasite into 6 and, possibly, more merozoites (vs the usual 2, less often 4 merozoites), was found. This phenomenon had been unknown in both natural and accidental hosts. Under certain conditions, fission of parasite reproduction is possible without egressing the erythrocyte. This phenomenon results in unusually high multi-occupancy – up to 14 parasites developed in the affected erythrocytes. Some RBCs were so filled with trophozoites that they could not be counted.
Data
All research data are presented in the article. Photos of Romanovsky-stained thin blood smears can be obtained from the author for correspondence.
Author contributions
The authors contributed equally to the conceptualization, investigation (data collection), writing and editing of this article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.