1. Introduction
Native sulphur occurrences are commonly closely associated with evaporites and authigenic carbonates (e.g. Dessau, Jensen & Nakai, Reference Dessau, Jensen and Nakai1962; Davis & Kirkland, Reference Davis and Kirkland1979; Pierre & Rouchy, Reference Pierre and Rouchy1988; Youssef, Reference Youssef1989). Examples of this paragenesis are found in cap rocks of salt diapirs (Ruckmick, Wimberly & Edwards, Reference Ruckmick, Wimberly and Edwards1979) and strata-bound deposits like those of northern Iraq (Jassim, Raiswell & Bottrell, Reference Jassim, Raiswell and Bottrell1999), the Delaware basin (Hentz & Henry, Reference Hentz and Henry1989), the Carpathian foredeep (Böttcher & Parafiniuk, Reference Böttcher and Parafiniuk1998) and the Mediterranean area (Dessau, Jensen & Nakai, Reference Dessau, Jensen and Nakai1962; Anadón, Rosell & Talbot, Reference Anadón, Rosell and Talbot1992; Rouchy et al. Reference Rouchy, Taberner, Blanc-Valleron, Sprovieri, Russell, Pierre, Di Stefano, Pueyo, Caruso, Dinarés-Turell, Gomis-Coll, Wolff, Cespuglio, Ditchfield, Pestrea, Combourieu-Nebout, Santisteban and Grimalt1998; Ortí, Rosell & Anadón, Reference Ortí, Rosell and Anadón2003; Ziegenbalg et al. Reference Ziegenbalg, Brunner, Rouchy, Birgel, Pierre, Böttcher, Caruso, Immenhauser and Peckmann2010). Both native sulphur and associated authigenic carbonate minerals are commonly considered as products of bacterial sulphate reduction (Feely & Kulp, Reference Feely and Kulp1957; Dessau, Jensen & Nakai, Reference Dessau, Jensen and Nakai1962; Davis & Kirkland, Reference Davis and Kirkland1979; Peckmann, Paul & Thiel, Reference Peckmann, Paul and Thiel1999; Ziegenbalg et al. Reference Ziegenbalg, Brunner, Rouchy, Birgel, Pierre, Böttcher, Caruso, Immenhauser and Peckmann2010). In this process, the heterotrophic bacteria oxidize locally abundant crude oil, methane or more pristine organic matter and produce sulphide ions, facilitating subsequent biological or abiological sulphide oxidation to native sulphur (Machel, Reference Machel, Wessel and Wimberly1992). Another common consequence of bacterial sulphate reduction is an increase in alkalinity, which results in the precipitation of carbonate minerals (Castanier, Le Métayer-Levrel & Perthuisot, Reference Castanier, Le Métayer-Levrel and Perthuisot1999).
Depending on the time of formation of authigenic minerals, Ruckmick, Wimberly & Edwards (Reference Ruckmick, Wimberly and Edwards1979) distinguished between syngenetic and epigenetic processes. Syngenesis takes place during sedimentation and early diagenesis. Notably, precipitation of carbonate minerals induced by sulphate-reducing bacteria has been described in modern hypersaline environments as well as in laboratory experiments (Lalou, Reference Lalou1957; Vasconcelos & McKenzie, Reference Vasconcelos and McKenzie1997; van Lith et al. Reference van Lith, Warthmann, Vasconcelos and McKenzie2003; Wright & Wacey, Reference Wright and Wacey2005). Epigenesis, on the other hand, takes place after deposition during late diagenesis. In the case of epigenetic mineral formation, sulphate, the electron acceptor for the involved heterotrophic bacteria, is delivered by dissolution of sulphate minerals by meteoric waters.
Because bacterial sulphate reduction is dependent on a high supply of sulphate ions, it is favoured in marine rather than in lacustrine environments. In lacustrine settings, methanogenesis tends to act as the main process of organic matter remineralization (Cerling, Bowman & O'Neil, Reference Cerling, Bowman and O'Neil1988; Talbot & Kelts, Reference Talbot, Kelts and Katz1990). Nevertheless, some examples of carbonate mineral formation induced by sulphate reduction in lacustrine deposits are known, including lakes in SW Australia (Wright, Reference Wright1999) and the eastern USA (Riccioni, Brock & Schreiber, Reference Riccioni, Brock and Schreiber1996). Sulphur-bearing authigenic carbonates have been reported from the lacustrine Teruel basin in Spain (Anadón, Rosell & Talbot, Reference Anadón, Rosell and Talbot1992), where weathering of Triassic marine evaporites provided the sulphate for bacterial sulphate reduction (Utrilla et al. Reference Utrilla, Pierre, Ortí and Pueyo1992). A similar occurrence of native sulphur and associated authigenic carbonates is known from the Late Miocene Hellín basin in SE Spain (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). This restricted basin was typified by lacustrine conditions with only episodic inflow of marine waters (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). Although dolomite and native sulphur have been suggested to derive from bacterial sulphate reduction using sulphate that originated from the dissolution of Triassic gypsum (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990), these authigenic minerals have not been studied in detail to date.
Here we present a petrographic and isotopic study on the evaporitic sedimentary sequence of the Hellín basin, focusing on authigenic carbonates and native sulphur. Our study confirms that sulphate-reducing bacteria can be major players in the early diagenesis of lacustrine settings as well, provided that sufficient sulphate ions are available.
2. Geological setting and material
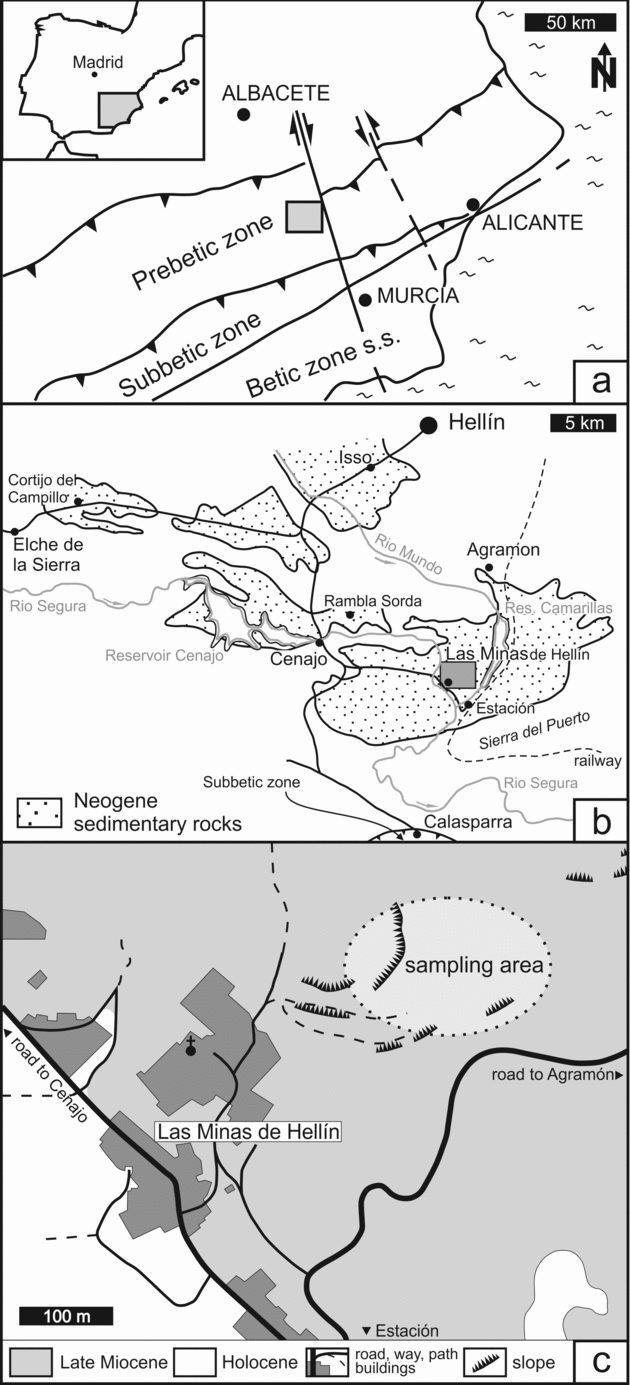
The Hellín basin of SE Spain is an intramontane basin in the external part of the Betic Chain (Fig. 1a). Cretaceous limestones and Triassic evaporites represent the basement of the basin (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). Basin development is attributed to subsidence related to a system of transform faults of an extensional regime, which was active during the Neogene; subsidence terminated in the Late Tortonian (Giese et al. Reference Giese, Reutter, Jacobshagen, Nicolich, Berckhemer and Hsü1982; Sanz de Galdeano, Reference Sanz de Galdeano1990; Krijgsman et al. Reference Krijgsman, Garcés, Agusti, Raffi, Taberner and Zachariasse2000; Jolivet et al. Reference Jolivet, Augier, Robin, Suc and Rouchy2006). Initially the basin was filled by marine sediments dominated by marls and marly carbonate beds. The last marine sedimentation occurred in the Middle Tortonian (Calvo et al. Reference Calvo, Elizaga, Lopez Martinez, Robles and Usera1978). A late phase of N–S-oriented compression started in the Tortonian and led to regional uplift of the crust (Rouchy et al. Reference Rouchy, Taberner, Blanc-Valleron, Sprovieri, Russell, Pierre, Di Stefano, Pueyo, Caruso, Dinarés-Turell, Gomis-Coll, Wolff, Cespuglio, Ditchfield, Pestrea, Combourieu-Nebout, Santisteban and Grimalt1998; Jolivet et al. Reference Jolivet, Augier, Robin, Suc and Rouchy2006). Subsequently, folding of Mesozoic to Cenozoic rocks ended in the development of several local swells that divided the Hellín basin into several sub-basins (Fig. 1b). Formation of a swell to the south close to Calasparra led to the successive isolation of the Hellín basin from the marine environment (Calvo & Elizaga, Reference Calvo, Elizaga, Ortí and Salvany1989; Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). The change to lacustrine conditions resulted in the accumulation of evaporites and carbonate deposits of the Las Minas de Hellín Formation, which are overlain by carbonate deposits, marls and diatomites (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). The evaporites, mainly represented by gypsum, derived from the dissolution of Triassic evaporites; continued dissolution of gypsum of the Triassic basement (Garcia Domingo et al. Reference Garcia Domingo, Lopez Olmedo, Jerez Mir and Gallego Coiduras1980) is reflected by karst phenomena in the Hellín basin (Navarro Hervás & Rodríguez Estrella, Reference Navarro Hervás and Rodríguez Estrella1985). In the sub-basins of Cenajo and Las Minas, the Las Minas de Hellín Formation contains native sulphur, which was mined until 1960.
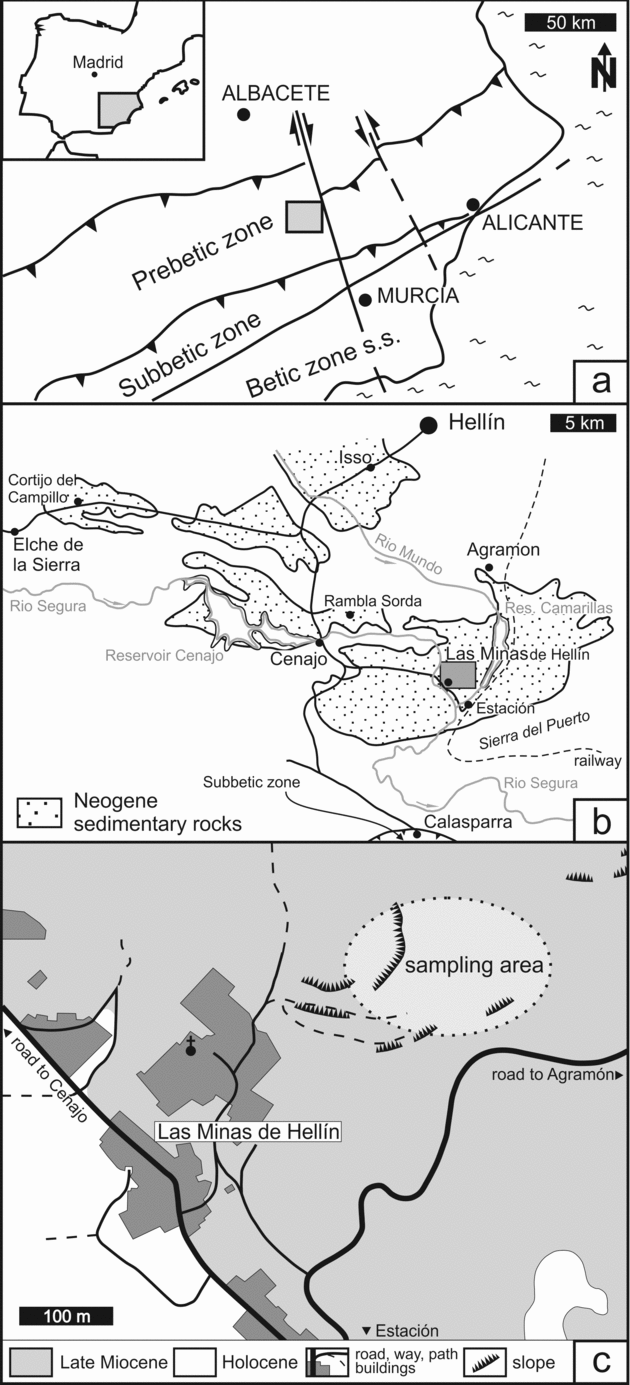
Figure 1. Study area. (a) Location of the Hellín basin in tectonic context of the Betic Chain; modified after Calvo & Elizaga (Reference Calvo, Elizaga, Ortí and Salvany1989). (b) Sub-basins of the Hellín basin with Neogene sediments; modified after Calvo & Elizaga (Reference Calvo, Elizaga, Ortí and Salvany1989). (c) Location of the sampling area in the former sulphur mine of Las Minas.
For this study, carbonate and gypsum rocks containing native sulphur were collected from the mining area northeast of the small miners’ village of Las Minas (Fig. 1c). Sections of evaporites and carbonates are well exposed, but strongly weathered; the samples studied here derived from the upper part of the stratigraphic interval described by Servant-Vildary et al. (Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). Strata-bound nodules of native sulphur are predominantly embedded in laminated gypsum beds (Fig. 2a) and to a lesser degree in carbonate beds (Fig. 2b). A similar sedimentary sequence is found in the Las Minas de Hellín Formation of the Cenajo sub-basin. The aggregates of native sulphur in the Cenajo sequence resemble those of the Las Minas sequence, although spherical nodules of sulphur have only been recognized in carbonate deposits from Cenajo (Fig. 2c).
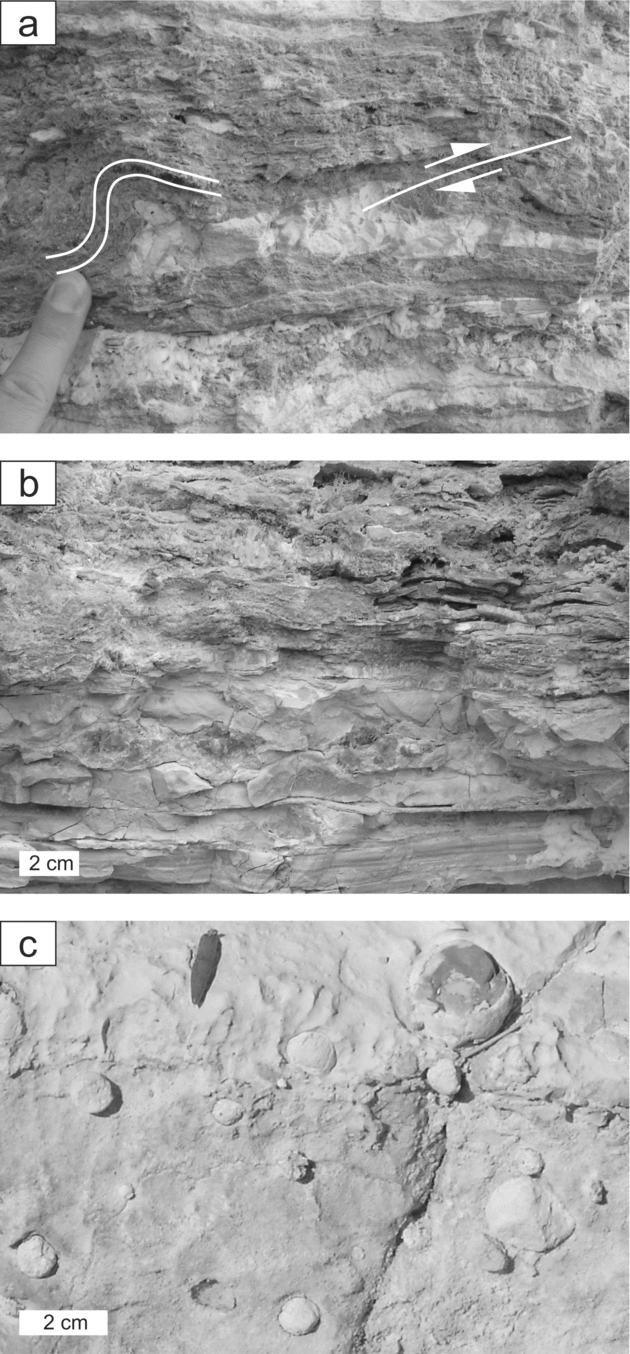
Figure 2. Native sulphur. (a) Sulphur enclosed in secondary gypsum; overlying laminar gypsum beds are bent and faulted (see indication), Las Minas. (b) Sulphur enclosed in carbonate deposits, Las Minas. (c) Sulphur nodules embedded in a carbonate bed, Cenajo. For a colour version of this figure see online Appendix at http://journals.cambridge.org/geo.
3. Methods
Thin-sections were studied with transmitted light and fluorescence microscopy on a Zeiss Axioskop 40 A Pol, equipped with an Axio-Cam MRc digital camera (lamp: HBO 50, filter: BP 365 FT 395 LP 397 and BP 450–490 FT 510 LP 515). Thin-sections were partly stained with combined potassium ferricyanide and alizarin red solution (Füchtbauer, Reference Füchtbauer1988). Scanning-electron microscopy with qualitative element recognition was performed with a Zeiss Supra 40 REM equipped with an Oxford EDX-detector (Inca Penta FETx3).
Mineralogy was determined with a PANalytical X'Pert Pro diffractometer using a Ni-filtered Cu-anode, equipped with a multichannel detector (X'cellerator). Mineral content was estimated semi-quantitatively using the peak areas. Proportions of magnesium to calcium in dolomite were calculated after Lumsden (Reference Lumsden1979) by quartz-corrected d-values. A LECO CS 200 was used for determination of total organic carbon content.
For stable isotope analyses, mineral phases were drilled from the surface of slabs with a hand-held micro drill. Measurements of carbon and oxygen isotopes (sample weight: 0.02 to 0.10 mg) were performed with a Finnigan MAT 251 mass spectrometer using the Kiel carbonate device type ‘Bremen’ against natural CO2 from Burgbohl/Rheinland. A Solenhofen limestone was used as an internal standard, which was calibrated against the international standard NBS 19. Values are reported in the δ-notation relative to the Vienna Pee Dee Belemnite (VPDB) standard. Long-time standard deviation (1σ) for this measurement was ± 0.05‰ for δ13C and ± 0.07‰ for δ18O values. Oxygen isotope values of dolomite were not corrected for different fractionation compared to calcite during precipitation (McKenzie, Reference McKenzie1981) or during the analytical procedure (Sharma & Clayton, Reference Sharma and Clayton1965) because of an undetermined admixture of minor calcite.
For oxygen isotope analysis of sulphate, gypsum was dissolved with sodium chloride solution. After acidification of the solution, sulphate was precipitated as barium sulphate by addition of barium chloride. Sulphur isotopes of gypsum were measured either on gypsum without pre-treatment or on barium sulphate precipitated as above. Samples of sulphur received no pre-treatment for sulphur isotope measurements. Sulphur and oxygen isotope measurements were performed with a Thermo Finnigan mass spectrometer Delta V in a continuous flow setup.
For sulphur isotope analysis, 0.3 mg of barium sulphate, 0.4 mg of calcium sulphate or 0.05 mg of native sulphur (with 1 mg of V5O5 as catalyst, respectively) were weighed into tin capsules and combusted in a Euro EA Elemental Analyser (EuroVector, Milan, Italy). The sulphur isotope measurements were calibrated with reference materials NBS 127 and IAEA-SO-6. The sulphur isotopic composition is reported in the δ-notation relative to the Vienna Cañon Diablo Troilite (VCDT) standard. The standard errors (1σ) were less than ± 0.2‰ for δ34S values.
For oxygen isotope analysis, 0.16 mg of barium sulphate was weighed into silver capsules. Under the presence of graphite and glassy carbon, oxygen from sulphate was transferred to carbon monoxide within an elemental analyser (TC/EA, Thermo Fisher Scientific). NBS 127, IAEA-SO-5 and IAEA-SO-6 were used as references for calibration. The oxygen isotopic composition is reported in the δ-notation relative to Vienna Standard Mean Ocean Water (VSMOW). The standard errors (1σ) were less than ± 0.3‰ for δ18O values.
4. Results
4.a. Petrography
4.a.1. Carbonate lithology
Sampled carbonate beds consist predominantly of dolomite with varying amounts of gypsum, calcite and accessory minerals, including clay minerals, feldspars and quartz. Only a few carbonate beds contain more calcite than dolomite. The MgCO3 content of dolomite is generally high (47 to 50%). The total organic carbon content of carbonate deposits is low to moderate (0.2 to 0.7%).
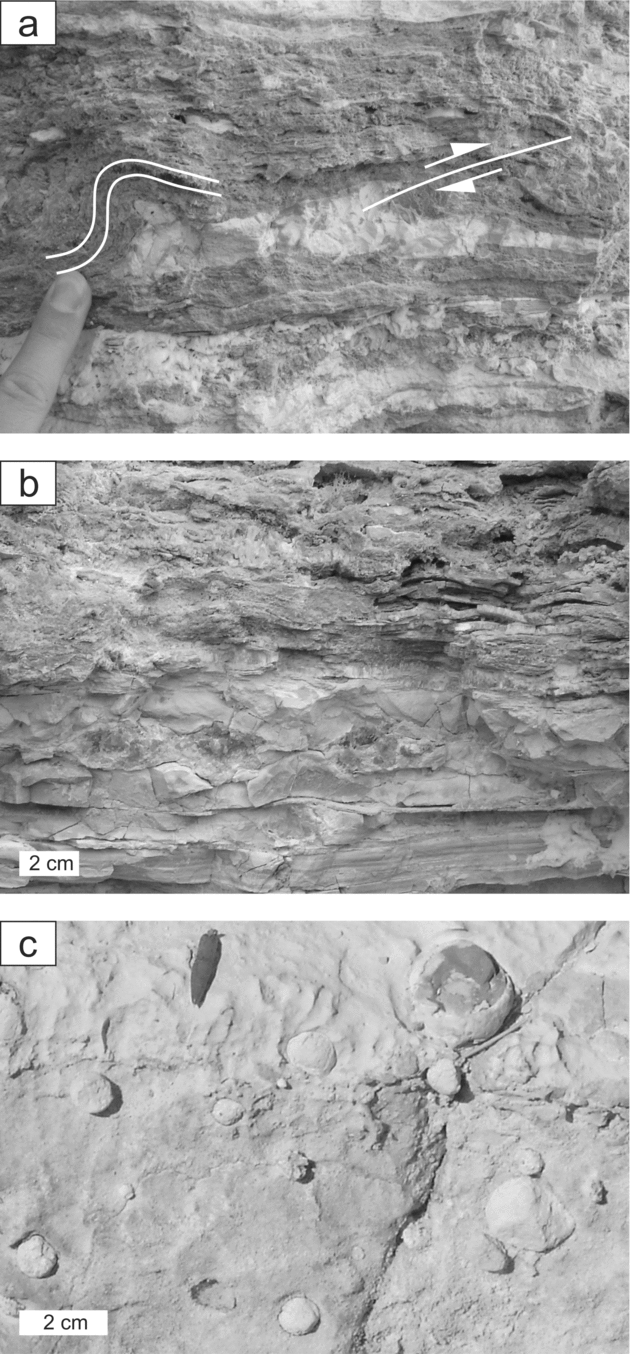
Most of the carbonate deposits exhibit a distinct lamination, which can be grouped into three types (Fig. 3). (1) A very regular, varve-like rhythmic lamination is characterized by thin laminae, approximately 0.5 mm in thickness that consist of light-coloured dolomitic microspar and dark organic-rich dolomicrite, respectively (Fig. 3a, b). Where sulphur nodules occur, the lamination is bent and occasionally faulted (Figs 2a, 3a–c). The dolomicrite is partly dissolved or replaced by gypsum, especially in the surroundings of sulphur nodules. Less commonly dolomitic microspar is replaced by gypsum. (2) A second type of lamination is less regular, resembling lamination in microbial mats (Fig. 3d). The carbonate laminae, which differ in thickness, show an uneven surface and wedge out laterally. They are interbedded with discontinuous clay layers. The predominant mineral is dolomite. (3) A third type of lamination comprises dolomite and gypsum laminae of irregular thickness, ranging from a few to 300 μm, accompanied by late diagenetic calcite and chert laminae (Fig. 3e). Plant fragments are common in this lithology.

Figure 3. Laminated carbonate beds. (a) Carbonate bed with varved lamination, which is bent around a sulphur nodule; arrowheads indicate laminae of secondary gypsum; scanned slab. (b) Detail of (a), carbonate laminae and small sulphur nodule (arrowhead); plane-polarized light. (c) Detail of (a), bent lamination around a sulphur nodule (S), which is partly dissolved and replaced by diagenetic gypsum (arrowheads); plane-polarized light. (d) Mat-like lamination; plane-polarized light. (e) Irregular lamination with two chert laminae (arrowheads); plane-polarized light.
Beside laminated carbonate beds, dense beds of dolomicrite occur, which are up to a few centimetres in thickness. These beds commonly reveal a clotted (Fig. 4a) or peloidal microfabric. Former cavities, now filled by authigenic minerals, are abundant in this lithology (Fig. 4a). In places the dolomicrite is alternating with dolomitic spar (Fig. 4b). Dolomicritic peloids are up to 30 μm in diameter and are partly recrystallized to microspar along their margins. Peloids tend to form dense fringes around sulphur nodules, which are commonly partly or completely replaced by gypsum (Fig. 4c). Blocky calcite spar with crystals up to 200 μm in diameter is an accessory phase in this lithology.

Figure 4. Carbonate deposits. (a) Clotted dolomicrite to microspar; plane-polarized light. (b) Irregular alternation of dolomicrite and microspar; plane-polarized light. (c) Peloidal carbonate with a former sulphur nodule replaced by gypsum surrounded by a dark rim (black arrowheads). White arrowheads point to large peloids; crossed nicols.
In different types of dolomitic beds spheroidal dolomite is observed in rock-forming quantities. The pore space left by spheroids is filled by gypsum (Fig. 5a). In some of the vugs, interlayers and surroundings of spheroids, submicroscopic aggregates of clay minerals are present (Fig. 5b). The fluorescent spheroids reveal a dark core of unknown composition surrounded by concentric rings of dolomite (Fig. 5c, d). Spheroids vary between 5 and 10 μm in diameter and are composed of irregularly arranged crystals (Fig. 5e, f).

Figure 5. Spheroidal dolomite. G – gypsum. (a) Spheroidal dolomite with gypsum; plane-polarized light. (b) Spheroidal dolomite and associated clay minerals (Cl); SEM micrograph of thin-section; circle displays area of EDX-measurement, spectrum with counts from 0 to 5 keV, gold peak from coating. (c) Spheroidal dolomite with gypsum; plane-polarized light. (d) Same detail as (c); note autofluorescence of spheroids; fluorescence image. (e) Aggregate of spheroidal dolomite; SEM micrograph. (f) Close-up view of two spheroids; SEM micrograph.
4.a.2. Native sulphur
Native sulphur occurs as large bright orange to yellow crystals with sharp crystal boundaries (Fig. 6a, b); the crystals are translucent in transmitted light. Sulphur is commonly closely associated with spheroidal dolomite and vugs or fissures filled by secondary gypsum (Fig. 6a, b). Weathered sulphur is bright yellow to whitish in colour (Fig. 2a), shows a powdery consistency, is blurry in transmitted light and is abundantly surrounded by secondary gypsum (Fig. 6c).

Figure 6. Native sulphur. C – carbonate; D – dolomite; G – gypsum; S – native sulphur. (a) Well-preserved sulphur surrounded by spheroidal dolomite (arrowheads); gypsum-filled fissure in the lower part of the photomicrograph; plane-polarized light. (b) Same detail as (a), note fluorescence of spheroidal dolomite (arrowheads); fluorescence image. (c) Slightly weathered aggregate of sulphur with a rim of gypsum (arrowhead); plane-polarized light. (d) Minor accumulation of sulphur in a laminated carbonate bed; plane-polarized light.
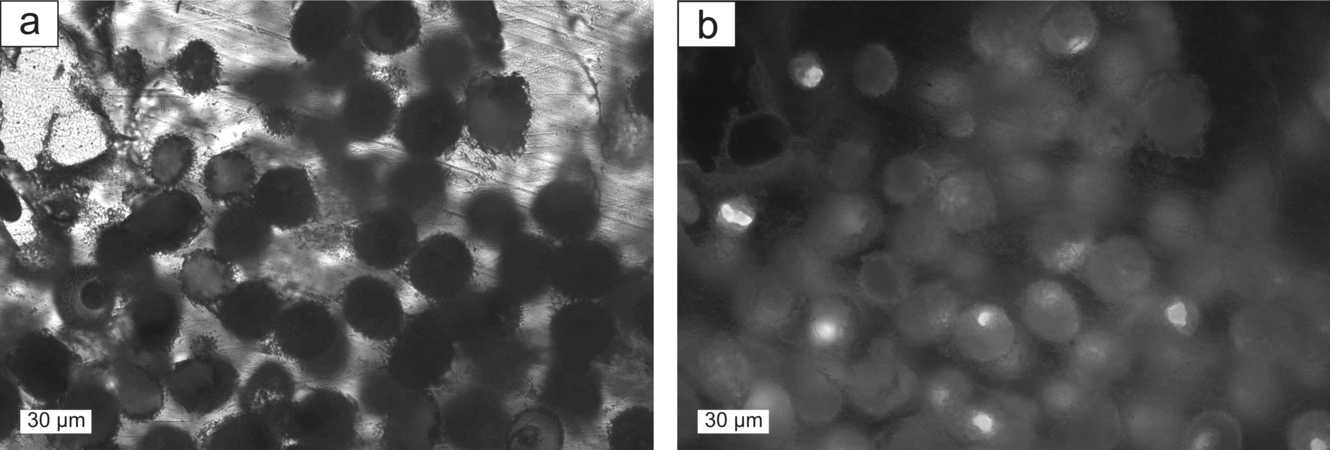
In carbonate beds with a varve-like lamination, sulphur aggregates tend to be particularly round (Fig. 3a–c). In carbonate beds of the types 2 and 3 described in the previous Section, lamination is partly interrupted by sulphur nodules. Native sulphur also appears as amoeboidal aggregates (Fig. 6c), possibly filling former cavities, and as tiny accumulations finely dispersed in the host rock (Fig. 6d). Very regular, fluorescent globules with a diameter of approximately 20 μm were recognized in one sample of well-preserved sulphur (Fig. 7).
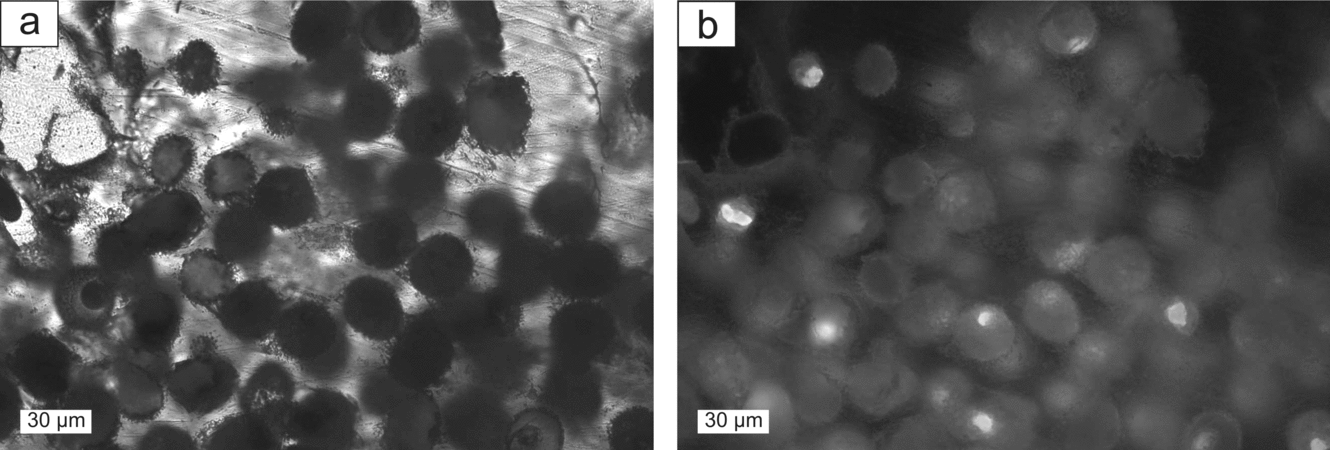
Figure 7. (a) Putative fossilized bacteria enclosed in sulphur; plane-polarized light. (b) Same detail as (a); fluorescence image.
4.a.3. Gypsum
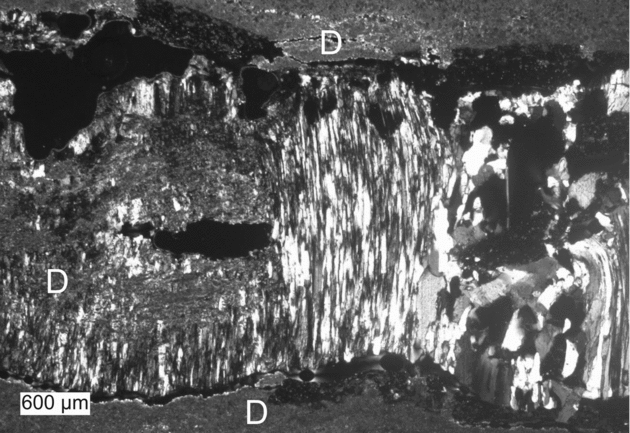
Gypsum is ubiquitous in the studied sedimentary rocks. In some carbonate beds, gypsum is finely dispersed in the rock matrix. Elsewhere, it represents a secondary mineral, growing as large crystals perpendicular to bedding. Secondary gypsum, which has been described in detail by Servant-Vildary et al. (Reference Servant-Vildary, Rouchy, Pierre and Foucault1990), commonly replaces sulphur (Figs 3c, 4c, 6c) and dolomitic microspar. It also fills fissures and vugs in carbonate deposits (Fig. 8). Growth of palisade-like gypsum commonly disturbed the primary lamination. The secondary gypsum of the Las Minas de Hellín sequence closely resembles diagenetic gypsum from the Miocene lacustrine sequence of the Teruel basin in NE Spain (cf. Ortí, Rosell & Anadón, Reference Ortí, Rosell and Anadón2010).
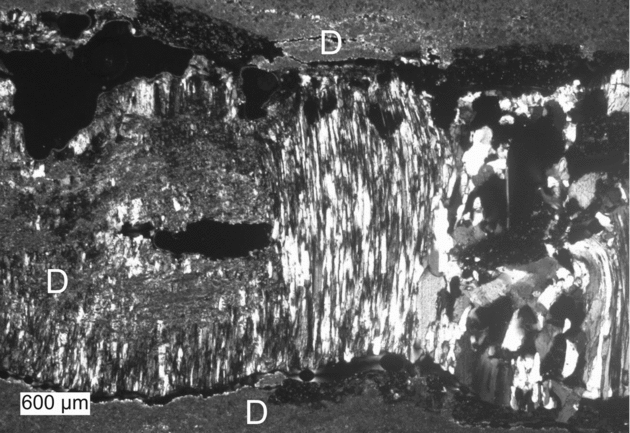
Figure 8. Fissure filled with fibrous gypsum (centre), blocky gypsum (right) and minor dolomite (D) derived from the rock matrix.
4.b. Stable isotopes
4.b.1. Carbon and oxygen isotopes of carbonates
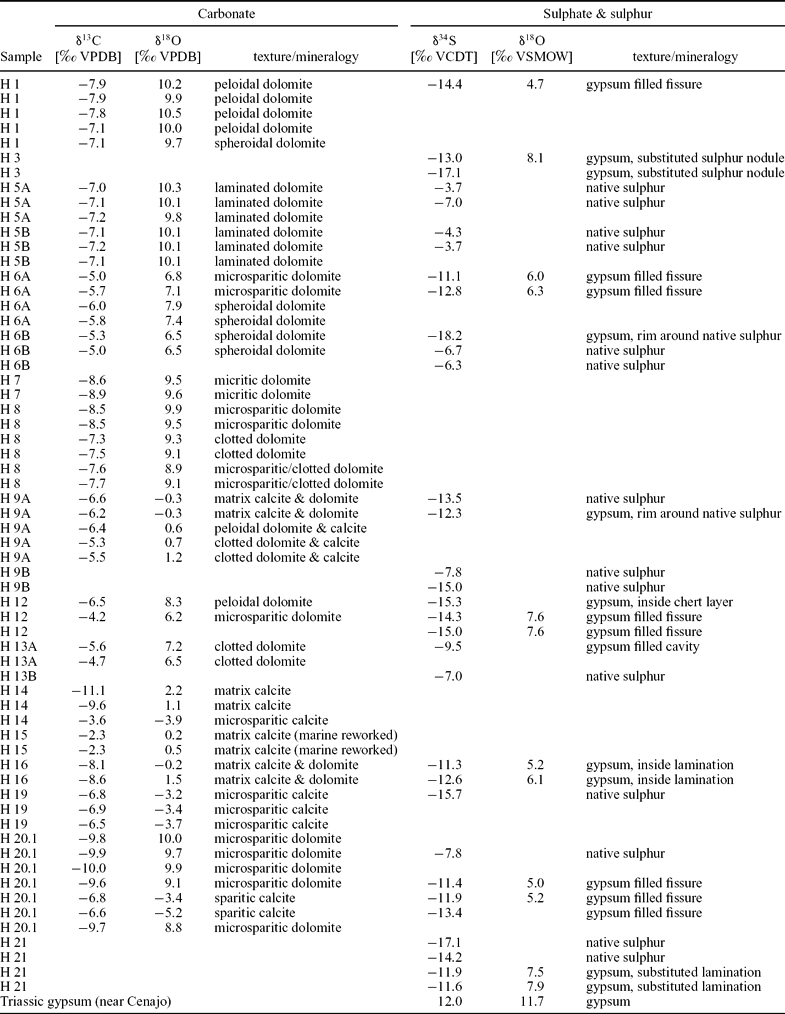
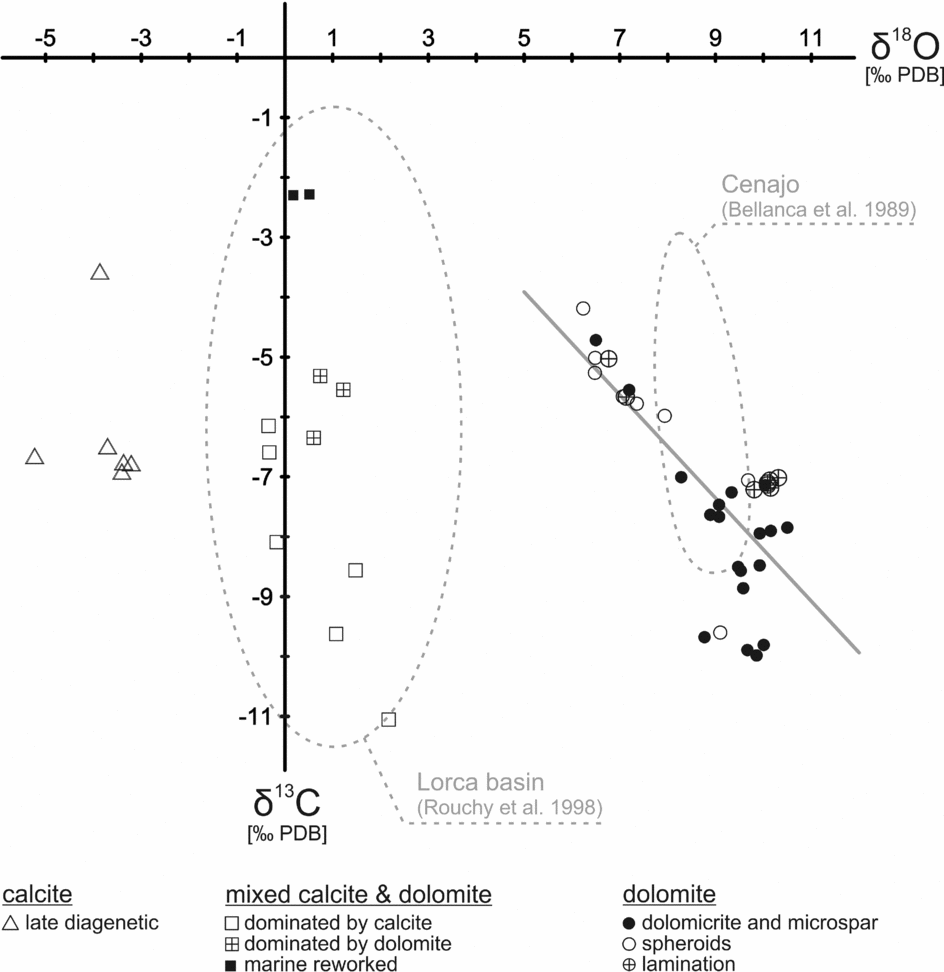
Oxygen isotope values scatter widely from −5.2 to +10.5‰ (Table 1; Fig. 9). Dolomite is more enriched in 18O than calcite. Spheroidal dolomite shows slightly lower δ18O values (+6.5 to +9.7‰) than other varieties of dolomite (+6.8 to +10.5‰). A group of samples with a mixed dolomite and calcite composition exhibits oxygen isotope values close to 0‰. The lowest oxygen isotope values are found for late diagenetic calcite (−5.2 to −3.2‰).
Table 1. Stable isotopes in carbonate deposits, native sulphur and secondary gypsum of Las Minas de Hellín and one sample of Triassic gypsum from near Cenajo
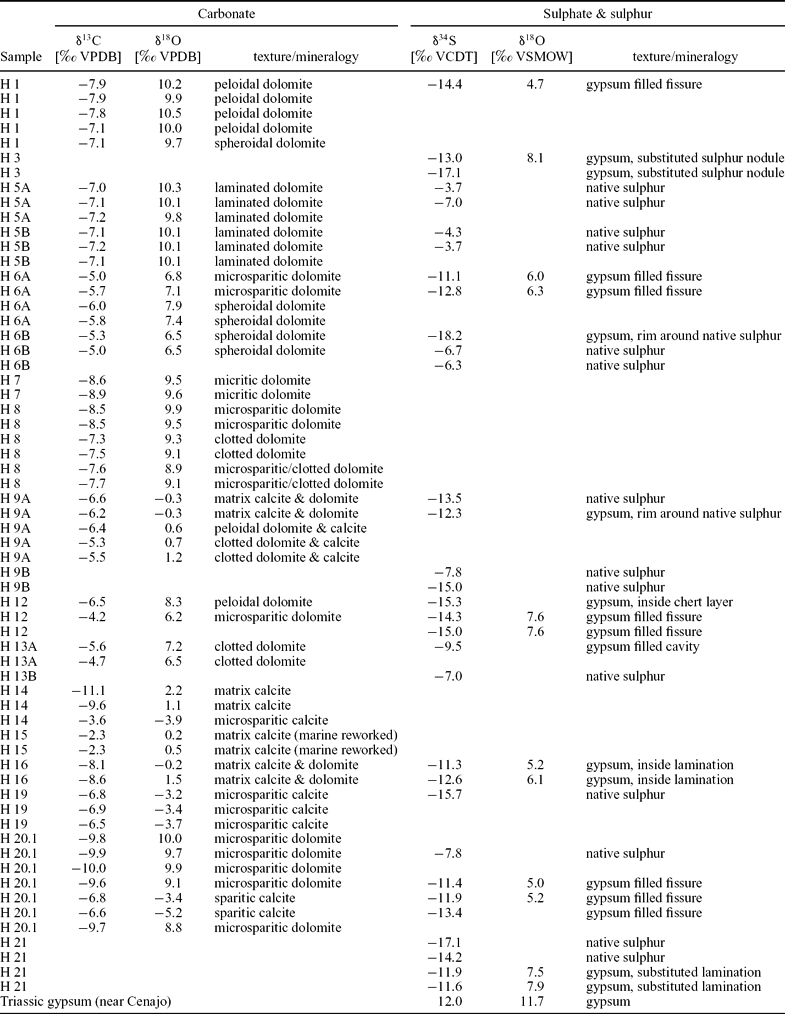
Figure 9. Stable carbon and oxygen isotope values of carbonate deposits of Las Minas de Hellín. Trend line indicates linear regression of dolomite values. Fields defined by dotted lines indicate composition of carbonates from the Lorca and Cenajo basins.
Most carbon isotope values range from −11.1 to −3.6‰. Two samples with slightly higher values (−2.3‰) probably reflect reworked marine carbonate. The δ13C values of samples with a mixed calcite and dolomite composition (−5.3 to −11.1‰) as well as the δ13C values of dolomite (−4.2 to −10.0‰) scatter widely. The δ13C values of dolomite are negatively correlated with the δ18O values, corresponding to a correlation coefficient of r = −0.75 (Fig. 9).
4.b.2. Oxygen isotopes of gypsum and sulphur isotopes of gypsum and native sulphur
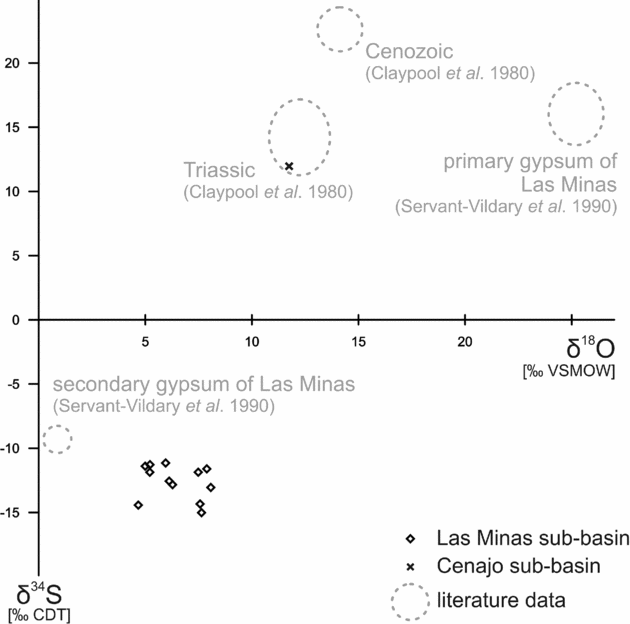
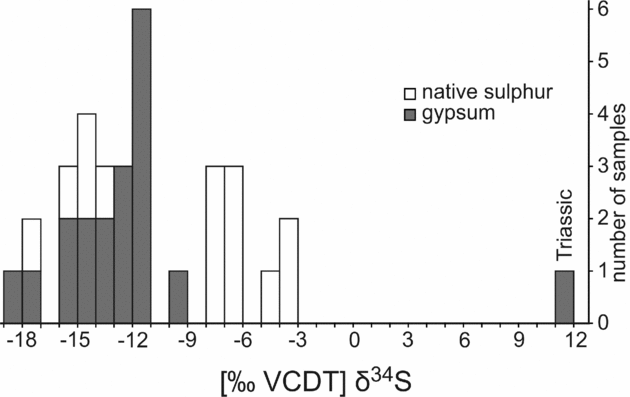
Oxygen isotope values of gypsum range from +4.7 to +8.1‰ (Table 1; Fig. 10). Native sulphur and secondary gypsum show much lower δ34S values (−18.2 to −3.7‰; Table 1; Figs 10, 11) than primary gypsum of the Las Minas sequence (+13 to +19‰; Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990; Fig. 10). Sulphur isotope values of native sulphur fall into two groups (Fig. 11). One group is less depleted in 34S (−7.8 to −3.7‰), whereas the other group shows very low δ34S values (−17.1 to −13.5‰), which are in the same range as values of secondary gypsum (−18.2 to −9.5‰). Triassic gypsum sampled from the periphery of the Cenajo sub-basin exhibits a positive sulphur isotope value (+12.0‰), in accord with the data of Claypool et al. (Reference Claypool, Holser, Kaplan, Sakai and Zak1980).
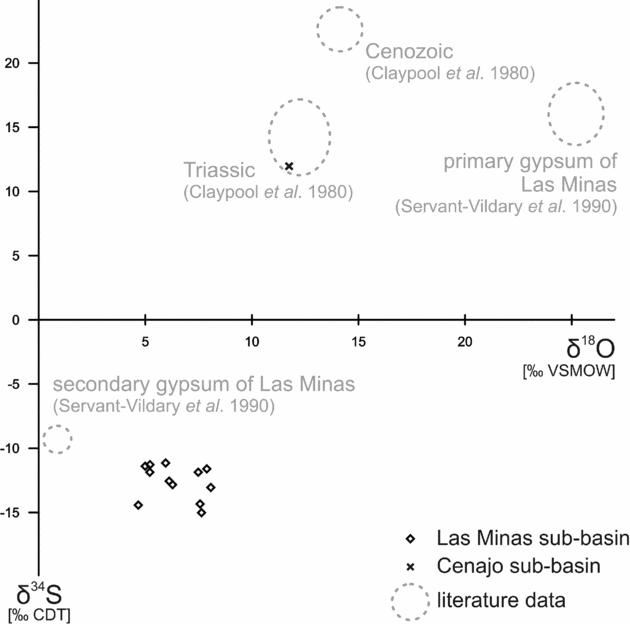
Figure 10. Stable sulphur and oxygen isotope values of secondary gypsum from Las Minas de Hellín and Triassic gypsum from Cenajo. Triassic and Cenozoic marine sulphate for comparison.
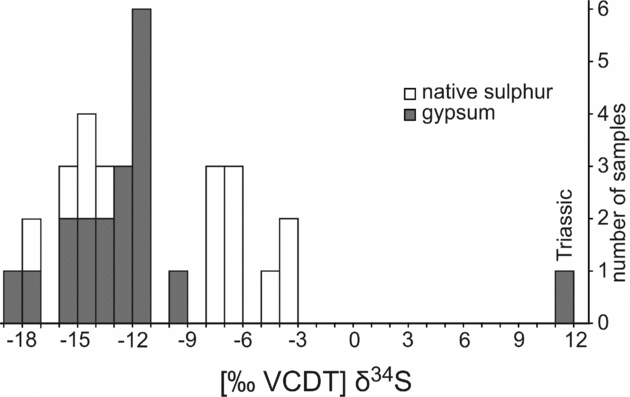
Figure 11. Stable sulphur (δ34S) isotope values of Neogene secondary gypsum and native sulphur from Las Minas de Hellín and Triassic gypsum from Cenajo.
5. Discussion
5.a. Formation of authigenic carbonate minerals
In lacustrine environments, carbonate minerals can precipitate from dissolved inorganic carbon (DIC) in the upper water column and accumulate at the lake bottom (Stabel, Reference Stabel1986; Talbot & Kelts, Reference Talbot, Kelts and Katz1990). The carbon isotopic composition of dissolved carbonate in a lacustrine environment is influenced by several factors including the amount and the composition of inflowing water, residence time and lake size (Talbot & Kelts, Reference Talbot, Kelts and Katz1990; Lee, McKenzie & Sturm, Reference Lee, McKenzie and Sturm1987). Furthermore, the δ13C values of DIC can shift towards higher values owing to primary production inside the lake and evaporation (Talbot, Reference Talbot1990). In closed lakes, δ13C values of primary carbonates usually range from –5 to +3‰ (McKenzie, Reference McKenzie and Stumm1985; Talbot, Reference Talbot1990; Talbot & Kelts, Reference Talbot, Kelts and Katz1990; Tenzer, Meyers & Knoop, Reference Tenzer, Meyers and Knoop1997). The Miocene Hellín lake was most likely hypersaline, because Late Miocene climatic conditions favoured evaporation in the Mediterranean area (van Dam & Weltje, Reference van Dam and Weltje1999; García-Alix et al. Reference García-Alix, Minwer-Barakat, Suárez, Freudenthal and Martín2008) and abundant Miocene evaporites are present in the Hellín basin. The variable δ18O values of the studied carbonates reflect the strong influence of inflow and evaporation, which is typical for hydrologically closed lakes (cf. Talbot & Kelts, Reference Talbot, Kelts and Katz1990).
Apart from formation in the water column, early diagenesis in lacustrine sediments can induce carbonate precipitation as well. As a consequence of anaerobic remineralization of organic matter, in particular bacterial sulphate reduction, carbonate alkalinity commonly increases (Abd-El-Malek & Rizk, Reference Abd-El-Malek and Rizk1963a, Reference Abd-El-Malek and Rizkb; van Lith et al. Reference van Lith, Warthmann, Vasconcelos and McKenzie2003). The resulting authigenic carbonate minerals are characterized by low δ13C values depending on the source of fresh organic matter or fossil hydrocarbons (Irwin, Curtis & Coleman, Reference Irwin, Curtis and Coleman1977; Peckmann & Thiel, Reference Peckmann and Thiel2004). The δ13C values of higher land plants (as low as −34‰) tend to be lower than that of freshwater algae (−23‰; Smith & Epstein, Reference Smith and Epstein1971). Because of the preferential uptake of 12C-enriched compounds during microbial remineralization, the produced carbonate is even more depleted in 13C than the source material (e.g. Lee, McKenzie & Sturm, Reference Lee, McKenzie and Sturm1987). The carbon isotopic composition of the Hellín basin carbonate deposits (as low as −11‰; Fig. 9), thus, reflects a combination of lake water DIC and carbonate alkalinity produced by the remineralization of organic matter. Dolomite from the Cenajo sub-basin of the Hellín basin and the Lorca basin reveal similar δ13C values, which have been attributed to bacterial sulphate reduction (Bellanca et al. Reference Bellanca, Calvo, Censi, Elizaga and Neri1989; Rouchy et al. Reference Rouchy, Taberner, Blanc-Valleron, Sprovieri, Russell, Pierre, Di Stefano, Pueyo, Caruso, Dinarés-Turell, Gomis-Coll, Wolff, Cespuglio, Ditchfield, Pestrea, Combourieu-Nebout, Santisteban and Grimalt1998).
Additional evidence for a microbial origin of the Hellín basin dolomites stems from the anticorrelation between δ13C and δ18O values (Fig. 9). During phases of enhanced evaporation the sulphate concentration in the lake water increased, allowing sulphate ions to penetrate deeper into the sediment, which consequently expanded the sulphate reduction zone (cf. Kasten & Jørgensen, Reference Kasten, Jørgensen, Schulz and Zabel2000). When this happened, sulphate reduction overprinted carbonate beds by inducing the interstitial precipitation of 18O-enriched and 13C-depleted dolomite, with 18O enrichment reflecting a high degree of evaporation and 13C depletion corresponding to a high contribution of carbonate derived from bacterial sulphate reduction coupled to oxidation of organic matter, crude oil or methane.
An anoxic milieu in the lacustrine sediments of the Hellín basin, which is required for sulphate reduction, agrees with the lack of bioturbation as revealed by the undisturbed lamination in the sulphur-bearing strata. The absence of replacive growth supports an early origin of dolomite (cf. Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). The biological origin of the studied dolomite – resulting from microbially induced precipitation and not from dolomitization – is evidenced by microfabrics such as mat-like lamination, aggregates of clotted or peloidal dolomicrite and particularly spheroidal dolomite. The strong fluorescence of the spheroids indicates a high content of organic matter (cf. Dravis & Yurewicz, Reference Dravis and Yurewicz1985). Notably, spheroidal dolomite has been suggested to result from the bacterial remineralization of organic matter (Lalou, Reference Lalou1957; Gunatilaka, Reference Gunatilaka1989; Ayllón-Quevedo et al. Reference Ayllón-Quevedo, Souza-Egipsy, Sanz-Montero and Rodríguez-Aranda2007; Sanz-Montero, Rodríguez-Aranda & García del Cura, Reference Sanz-Montero, Rodríguez-Aranda and García del Cura2009), indicating that this fabric is typical for microbial dolomite. Similar fabrics have been observed in microbial dolomite forming in modern lagoonal environments (Vasconcelos & McKenzie, Reference Vasconcelos and McKenzie1997; Wright, Reference Wright1999). The shape of spheroids is believed to reflect microenvironments of mineral precipitation, where cells and extracellular polymeric substances act as nucleation sites (Vasconcelos et al. Reference Vasconcelos, McKenzie, Bernasconi, Crujic and Tien1995; Burns, McKenzie & Vasconcelos, Reference Burns, McKenzie and Vasconcelos2000; van Lith et al. Reference van Lith, Warthmann, Vasconcelos and McKenzie2003; Ayllón-Quevedo et al. Reference Ayllón-Quevedo, Souza-Egipsy, Sanz-Montero and Rodríguez-Aranda2007; Dupraz et al. Reference Dupraz, Reid, Braissant, Decho, Norman and Visscher2008).
High sulphate concentrations were found to inhibit dolomite precipitation (e.g. Baker & Kastner, Reference Baker and Kastner1981; for an opposing view see Sánchez-Román et al. Reference Sánchez-Román, McKenzie, de Luca Rebello Wagener, Rivadeneyra and Vasconcelos2009). Dolomite forms under surface conditions when bacterial sulphate reduction effectively reduces sulphate concentration (Baker & Kastner, Reference Baker and Kastner1981; Vasconcelos & McKenzie, Reference Vasconcelos and McKenzie1997; Wright & Wacey, Reference Wright and Wacey2005). Sulphate-reducing bacteria are believed to favour dolomite formation by the liberation of magnesium ions, which are efficiently complexed by sulphate ions (Slaughter & Hill, Reference Slaughter and Hill1991; Vasconcelos et al. Reference Vasconcelos, McKenzie, Bernasconi, Crujic and Tien1995; Vasconcelos & McKenzie, Reference Vasconcelos and McKenzie1997). A magnesium to calcium ratio of 5:1 to 10:1 has been found to be necessary for dolomite precipitation (e.g. Folk & Land, Reference Folk and Land1975; Warren, Reference Warren2000). In lacustrine environments such high Mg:Ca ratios can be achieved (1) by consumption of calcium ions during gypsum precipitation (Folk & Land, Reference Folk and Land1975; Hardie, Reference Hardie1987), (2) by precipitation of calcite (Land, Reference Land1998) or (3) during early diagenesis of clay minerals with a release of magnesium ions (Kahle, Reference Kahle1965; Weaver & Beck, Reference Weaver and Beck1971). Whereas the latter process has been suggested to have played a significant role during the precipitation of dolomite spheroids in Kuwait (Khalaf, Reference Khalaf1990), the first factor and, to a lesser degree, the second factor probably favoured dolomite formation in the Hellín basin.
Variations in lake water chemistry are a possible cause for the changes from calcite to dolomite lithologies in the sedimentary strata of the Hellín basin (cf. Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). Such a scenario has been described for the Devonian Orcadian basin and the modern Lagoa Vermelha, where dolomite formation is believed to be linked to periods of enhanced evaporation, and calcite formation supposedly takes place during periods of enhanced freshwater input (Janaway & Parnell, Reference Janaway and Parnell1989; Vasconcelos & McKenzie, Reference Vasconcelos and McKenzie1997). High δ18O values of Hellín basin dolomites (+6.2 to +10.5‰) confirm that dolomite precipitated in an environment typified by evaporation (cf. Talbot & Kelts, Reference Talbot, Kelts and Katz1990), as the highest calculated value for calcite would still be +7.1‰ after the correction for different fractionation factors during precipitation (ε18Odolomite−calcite = +2.6‰; Vasconcelos et al. Reference Vasconcelos, McKenzie, Warthmann and Bernasconi2005) and for fractionation during the analytical procedure (ε18Odolomite−calcite = +0.8‰ at 25°C; Sharma & Clayton, Reference Sharma and Clayton1965). Carbonate deposits with a mixed composition of calcite and dolomite and δ18O values close to 0‰ probably reflect periods of dilution of the lake by freshwater, whereas calcite spar with negative δ18O values precipitated from meteoric waters during late diagenesis.
5.b. Formation of native sulphur and secondary gypsum
Microbial sulphate reduction releases hydrogen sulphide, which is further oxidized to native sulphur in some settings (e.g. Machel, Reference Machel, Wessel and Wimberly1992). Sulphate-reducing bacteria discriminate against 34S, resulting in the formation of hydrogen sulphide with low δ34S values (e.g. Kaplan & Rittenberg, Reference Kaplan and Rittenberg1964; Canfield, Reference Canfield, Valley and Cole2001). Similar to other basins in Spain like the Teruel basin, the Ebro basin or basins in the eastern Betics (Anadón, Rosell & Talbot, Reference Anadón, Rosell and Talbot1992; Utrilla et al. Reference Utrilla, Pierre, Ortí and Pueyo1992; Playà, Ortí & Rosell, Reference Playà, Ortí and Rosell2000), Triassic evaporitic rocks with an average δ34S value of +12‰ are present in the drainage area of the Hellín basin (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). During Miocene time, the Triassic evaporites were dissolved by meteoric waters and the resultant sulphate ions were subjected to bacterial sulphate reduction in the Hellín basin. The primary gypsum of the lacustrine sedimentary sequence of the Hellín basin is slightly enriched in 34S compared to Triassic gypsum (+13 to +19‰; Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990), reflecting the removal of isotopically light sulphate by bacterial sulphate reduction. The oxidation of sulphide to sulphur involves only a negligible fractionation effect for sulphur isotopes (Canfield, Reference Canfield, Valley and Cole2001; Hoefs, Reference Hoefs2004). Therefore, the low δ34S values of native sulphur from the Hellín basin (−17.1 to −3.7‰) reflect the isotopic composition of sulphide produced by bacterial sulphate reduction. The accordant fractionation of sulphur isotopes between sulphate and sulphide (14 to 30‰) is well within the known range of bacterial sulphate reduction (Hartmann & Nielsen, Reference Hartmann and Nielsen1969; Goldhaber & Kaplan, Reference Goldhaber, Kaplan and Goldberg1974; Bolliger et al. Reference Bolliger, Schroth, Bernasconi, Kleikemper and Zeyer2001; Detmers et al. Reference Detmers, Brüchert, Habicht and Kuever2001).
The oxidation of hydrogen sulphide to native sulphur occurs abiologically or biologically (Machel, Reference Machel, Wessel and Wimberly1992). One observation suggests that at least part of the native sulphur of the Hellín basin results from biological oxidation. The fluorescent globules enclosed in native sulphur (Fig. 7) probably represent fossilized bacterial cells. They resemble the sulphide-oxidizing bacteria Thiomargarita sp. or Achromatium sp. (pers. comm. H. N. Schulz-Vogt, 2009). Thiomargarita sp. have been exclusively described from marine sediments (Schulz & Schulz, Reference Schulz and Schulz2005). Their metabolism is based on oxidation of sulphide with oxygen or nitrate (Schulz & Jørgensen, Reference Schulz and Jørgensen2001; Schulz, Reference Schulz2002). Achromatium spp., however, prefer lacustrine conditions and gain energy by the oxidation of sulphide to sulphate with native sulphur as an intermediate (Gray et al. Reference Gray, Howarth, Pickup, Gwyn Jones and Head1999). The bending of sediment layers around sulphur nodules points to a syngenetic formation of sulphur and, thus, agrees with biological oxidation of hydrogen sulphide.
Most of the gypsum in the Las Minas de Hellín Formation is obviously of a diagenetic origin, resembling diagenetic gypsum of the Miocene Teruel basin (cf. Ortí, Rosell & Anadón, Reference Ortí, Rosell and Anadón2010). Such an origin is revealed by the replacement of sulphur nodules or carbonate laminae and the infilling of late fissures. The oxidation of reduced sulphur species produces sulphuric acid, which causes a drop in pH leading to carbonate dissolution (Boudreau, Reference Boudreau1991; Walter et al. Reference Walter, Bischof, Patterson and Lyons1993; Ku et al. Reference Ku, Walter, Coleman, Blake and Martini1999; Pirlet et al. Reference Pirlet, Wehrmann, Brunner, Frank, Dewanckele, van Rooij, Foubert, Swennen, Naudts, Boone, Cnudde and Henriet2010). The gypsum that tends to precipitate under these conditions replaces carbonate and native sulphur in the sedimentary sequence (Servant-Vildary et al. Reference Servant-Vildary, Rouchy, Pierre and Foucault1990). The fractionation effect for sulphur isotopes during the precipitation of gypsum (1.6‰) is small (Nakai & Jensen, Reference Nakai and Jensen1964; Thode & Monster, Reference Thode, Monster, Kirkland and Evans1973; Claypool et al. Reference Claypool, Holser, Kaplan, Sakai and Zak1980). The low δ34S values (–18.2 to –9.5‰) of diagenetic gypsum consequently confirm its origin from the oxidation of native sulphur. This secondary gypsum is also characterized by low δ18O values, reflecting the uptake of oxygen derived from meteoric waters (cf. Taylor & Wheeler, Reference Taylor and Wheeler1984; Brunner et al. Reference Brunner, Bernasconi, Kleikemper and Schroth2005).
6. Conclusions
The native sulphur and authigenic dolomite present in the lacustrine evaporitic sequence of the Late Miocene Las Minas de Hellín Formation of the Hellín basin in SE Spain formed as a consequence of bacterial sulphate reduction. The high sulphate concentration of the Miocene lake led to the deposition of primary lacustrine gypsum and served as an electron acceptor for bacterial remineralization of organic matter. A microbial origin of authigenic dolomite is confirmed by its δ13C values as low as −10‰ and textural evidence including spheroids, clotted and peloidal fabrics, as well as mat-like lamination. Microfossils preserved in native sulphur indicate that hydrogen sulphide produced by the sulphate-reducing bacteria was at least partially oxidized biologically, resulting in sulphur formation. The putative bacterial fossils resemble the sulphide-oxidizing bacteria Thiomargarita and Achromatium. A significant amount of the native sulphur in the Las Minas de Hellín Formation was later oxidized to 34S-depleted secondary gypsum (δ34S values as low as −18‰), still reflecting the sulphur isotope composition of the biogenic sulphur (δ34S values as low as −17‰).
Acknowledgements
We thank Sebastian Flotow (Bremen) for thin-section preparation, Brit Kockisch (Bremen) for TOC measurements, Thomas Max (MPI Bremen) for help with sulphur and oxygen isotope analysis, Monika Segl (Bremen) for carbon and oxygen isotope analysis, Christoph Vogt (Bremen) for XRD measurements, Petra Witte (Bremen) for help with REM analysis and Heide Schulz-Vogt (MPI Bremen) for comments on sulphide-oxidizing bacteria. Comments by Federico Ortí (Barcelona) and an anonymous referee helped improve the manuscript. This project was supported by the DFG Research Centre ‘The ocean in the Earth system’, the international graduate college EUROPROX and the Max Planck Society.