Introduction
Animal numbers are driven by interactions between abiotic and biotic factors (Krebs, Reference Krebs2001; Begon et al., Reference Begon, Townsend and Harper2005). These factors do not work independently; rather, they interact to affect population dynamics. It has been suggested that abiotic conditions can modulate predatory impacts on herbivores (Chase, Reference Chase1996; Stiling & Rossi, Reference Stiling and Rossi1997; Fraser, Reference Fraser1998; Fraser & Grime, Reference Fraser and Grime1998). Weather conditions, especially rainfall, have been proven to be a major factor regulating arthropod populations (Watson & Carter, Reference Watson and Carter1983; Masters et al., Reference Masters, Brown, Clarke, Whittaker and Hollier1998; Frampton et al., Reference Frampton, van den Brink and Gould2000; Ovadia & Schmitz, Reference Ovadia and Schmitz2004). For example, rain dislodges aphids from plants or initiates inter-plant movement (Dhaliwal & Singh, Reference Dhaliwal and Singh1975; Zuniga, Reference Zuniga, Peters, Webster and Chlouber1991; Mann et al., Reference Mann, Tatchell, Dupuch, Harrington, Clark and McCartney1995; Narayandas & Alyokhin, Reference Narayandas and Alyokhin2006). A higher proportion of living aphids on the soil surface increases the potential of ground-dwelling predators to control aphid numbers (Griffiths et al., Reference Griffiths, Wratten and Vickerman1985; Sopp et al., Reference Sopp, Sunderland and Coombes1987; Losey & Denno, Reference Losey and Denno1998a). Several studies have demonstrated regulation of cereal aphids by ground-dwelling predators (reviewed in Symondson et al., Reference Symondson, Sunderland and Greenstone2002). Although within many of these studies the potential of abiotic effects, such as wind and rain, to affect predator-prey interactions has been discussed (Sunderland & Vickerman, Reference Sunderland and Vickerman1980; Dennis & Sotherton, Reference Dennis and Sotherton1994; Holland & Thomas, Reference Holland and Thomas1997a, Reference Holland and Thomasb; Sunderland et al., Reference Sunderland1997), we know of no previous studies that have specifically investigated the effects on herbivores of interactions between rainfall and ground-dwelling predators. Without assessing how abiotic factors shape predator-prey interactions, the efficiency of herbivore control in the field is difficult to predict.
One reason for the lack of studies investigating how abiotic factors modify predator prey-interactions is the difficulty in evaluating trophic links, especially where predators are small, nocturnal or subterranean. Even in well-controlled systems, such as microcosms, it is impossible to follow predator-prey interactions for extended periods. New techniques, particularly gut-content analysis using PCR and prey-specific primers, may allow unprecedented progress in quantifying who is feeding on whom without disturbing the system under study prior to predator collection (Symondson, Reference Symondson2002; Sheppard & Harwood, Reference Sheppard and Harwood2005; King et al., Reference King, Read, Traugott and Symondson2008). Recently, this approach has been developed to study predator-prey links specific to agricultural systems (Agustí et al., Reference Agustí, Shayler, Harwood, Vaughan, Sunderland and Symondson2003; Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005; Greenstone et al., Reference Greenstone, Rowley, Weber, Payton and Hawthorne2007; Juen & Traugott, Reference Juen and Traugott2007). The ability to determine which prey species have actually been consumed by a predator allows the ‘black box’ of trophic links in terrestrial ecosystems to be opened. Surprisingly, no microcosm studies employing these promising PCR-based approaches have yet been reported.
Here, we employed PCR-based gut content analysis in a microcosm experiment investigating the effects of rainfall on predator-prey interactions by (a) assessing the immediate effect of rain on aphid consumption by carabid beetles, Pterostichus melanarius (Illiger) (Coleoptera: Carabidae), and (b) determining the longer-term effects of rainfall and predation by P. melanarius on aphid population growth. We hypothesized that: (i) rainfall dislodges aphids from wheat plants, making them more accessible to ground-dwelling predators; (ii) more predators will have consumed aphids directly after rainfall compared to predators in no-rain treatments; and (iii) synergistic effects exist between rainfall and ground-dwelling predators due to higher availability and consumption of prey after rain.
Materials and methods
Adult P. melanarius were collected by pitfall trapping from a winter wheat field near Darmstadt, Germany, during May and June 2006. Beetles were transferred individually into plastic containers (diameter 9.5 cm; height 4.5 cm), filled with damp potting compost and maintained in a controlled environment (16°C; L:D 16:8) until the start of the experiment. Twice per week, one larva of Calliphora vomitoria L. (Diptera: Calliphoridae) was fed to each beetle to ensure the same nutritional status of the beetles. Prior to the experiment the beetles were starved for five days. A polyclonal population of the aphid Sitobion avenae (F.) (Homoptera: Aphididae) was cultured in glass containers on winter wheat at 24°C and L:D 16:8. Wheat plants were replaced regularly to keep aphid populations at low densities, to avoid development of alatae. To ensure similar reproductive rates between individuals, only late instar aphids were used in the experiments.
Experiments were conducted in a ventilated greenhouse in July 2006. Temperatures averaged 34.9±5.9°C and 20.3±2.4°C during the day and night, respectively. Experimental treatments were established in a 2×2 factorial design with the factors ‘Rain’ (yes/no) and ‘Predators’ (yes/no). Each treatment was replicated 16 times, resulting in 64 experimental pots. Circular microcosms (diameter 25 cm, height 25 cm) were three-quarters filled with potting compost covered with a 5 cm layer of field soil. The latter was taken from a ploughed arable field near Darmstadt in May 2006, sieved and heated to 60°C for 3 h prior to the experiment to kill soil-living invertebrates. The upper layer of field soil was intended to simulate the soil surface structure of an arable field.
Each microcosm was planted with ten wheat plants, two in the middle and eight in an outer circle. Before wheat ear development, each microcosm received approximately 50 aphids and three adult P. melanarius. To prevent aphids, as well as predators, from migrating into or out of the microcosms, mosquito nets (mesh size 1 mm, tightly sealed with clips and tape) covered the microcosms up to 75 cm in height. Additionally, the smooth inner surface of the microcosm walls prevented beetles from escaping. During the three-week experimental period, rain treatment microcosms were sprinkled with tap water (1 l m−2 min−1) for 5 min once per week (equivalent to 250 ml per microcosm), simulating a typical summer rain shower. For this, the nets were carefully opened on top and the water was applied with a sprinkler lance held 20 cm above the wheat plants. After sprinkling, the nets were immediately closed. All other microcosms received the same amount of water (0.25 l) directly on the soil surface. In the second week, 24 h after sprinkling the microcosms, one P. melanarius per microcosm was collected at random and frozen immediately at −24°C for subsequent gut content analysis. Three weeks after the start of the experiments, the nets were carefully removed and the aphids were counted on all ten wheat plants in the microcosms, respectively. To synchronise aphid counting the counts were performed simultaneously and blockwise by five people.
To evaluate the direct effects of the rain treatment on aphid dropping, four additional microcosms, identical to the ones described above, were sprinkled with 250 ml tap water only at the end of the first week for 5 min. Unlike the microcosms in the rain treatment of the main experiment, shortly before and immediately after sprinkling, aphids on ears and shoots were counted.
DNA extraction and PCR
For DNA extraction, each beetle foregut (crop) was removed and homogenised in 50 μl of PCR water (distilled and autoclaved water) by opening the foregut with a pipette-tip and vortexing. For each beetle, separate gloves were used to avoid sample-to-sample contamination. Twenty-five μl of the homogenate were utilised for DNA extraction using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The DNA was resuspended in 200 μl of manufacturer's elution buffer and stored at −24°C.
The primer pair S103 and A103, developed and tested for specificity in a previous study to detect S. avenae prey DNA in P. melanarius (von Berg et al., Reference von Berg, Traugott, Symondson and Scheu2008) was used to amplify a 231 bp fragment of the mitochondrial cytochrome oxidase I gene (COI) of S. avenae. PCRs were performed in 10 μl reactions containing 3 μl of extracted DNA, 0.25 mm dNTPs (Fermentas GmbH, St. Leon-Rot, Germany), 1 μm of each primer, 1 μl 10×buffer (Invitrogen GmbH, Karlsruhe, Germany), 3 mm MgCl2, 0.12 μg bovine serum albumin (BSA, 10 mg ml−1) and 1.5 U Taq DNA polymerase (Invitrogen). The DNA was amplified in an Mastercycler Gradient PCR machine (Eppendorf AG, Hamburg, Germany); cycling conditions were 2 min at 94°C, 40 cycles of 15 s at 94°C, 30 s at 61°C, 45 s at 72°C, and a final elongation step of 2 min at 72°C. PCR water as well as DNA from P. melanarius and S. avenae were included within each PCR to test for DNA carry-over contamination, false-negative and false-positive amplifications. PCR products were visualised on a 1.5% agarose gel stained with ethidium bromide.
Statistical analysis
Data on aphid numbers were analysed by two-factor analysis of variance (ANOVA) with the independent variables generalist predators (yes and no) and rain (yes and no). To improve homogeneity of variances, data were log10(x)-transformed. The direct effects of rain on aphid dropping were analysed by a paired t-test comparing aphid numbers before and after rain on shoots and ears. To test for differences in aphid dropping rates between wheat-shoots and wheat-ears, the percent decrease of aphid numbers after rain was calculated for shoots and ears, respectively. Data were arcsine square root transformed and compared by paired t-test. Molecular data were analysed using a chi-square test to analyse for differences in the rates of beetles testing positive for aphid DNA between the ‘rain yes’ and the ‘rain no’ treatments. Statistical analyses were calculated using Statistica 7.1 (StatSoft, Tulsa, USA).
Results
Aphid numbers (means±SE) were significantly reduced in the ‘rain yes’ treatment from 3394±290 to 2483±166 individuals per microcosm (F 1,53=6.40; P=0.01) (fig. 1). Aphid numbers were also reduced in the presence of P. melanarius, from 3119±237 to 2795±258 individuals per microcosm, but this decrease was not significant (F 1,53=2.55; P=0.12). There was no significant interaction between rain and P. melanarius. In the four microcosms used to determine the immediate effect of rain, aphid numbers decreased significantly, after rain, on ears and shoots from 140±8 to 105±11 and 81±28 to 56±12 individuals per microcosm, respectively (ears t=3.90, P<0.05; shoots t=26.34, P<0.001; paired t-tests) (fig. 2). Numbers of aphids dropping from ears and shoots differed significantly (t=−5.67; P=0.01; paired t-test) with ~32% more aphids dropping from shoots than from ears (fig. 2).

Fig. 1. Mean number of aphids per microcosm as affected by ground-dwelling predators (without predators black bars, with predators open bars) and rain (without rain black bars, with rain open bars). Significant differences between means are marked (**, P<0.01). Error bars are ±SE.
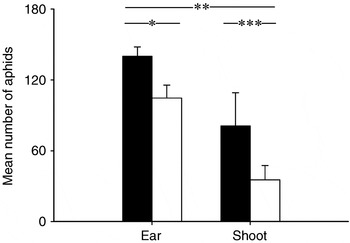
Fig. 2. Mean number of aphids per ear and shoot before (black bars) and after rain (open bars). Significant differences between means are marked (*, P<0.05; **, P<0.01; ***, P<0.001). Error bars are ±SE.
In the ‘rain yes’ treatment, 69% of the analysed beetles tested positive for aphid DNA compared to only 31% in the ‘rain no’ treatment. This difference was significant (χ2=4.50; P<0.05).
Discussion
We investigated the combined effect of a biotic factor, the predator Pterostichus melanarius, with an abiotic factor, rain, on aphid population dynamics in wheat. Combining an experimental microcosm approach with molecular analyses allowed us to determine directly how trophic interactions between ground beetles and aphids were affected by abiotic factors.
Rain significantly dislodged aphids from the wheat plants. On average, more than 40% of the aphids were displaced from ears and shoots after rain. Therefore, a high proportion of aphids was available on the soil surface as prey for P. melanarius. In fact, molecular gut content analysis identified more beetles containing aphid DNA in their guts in the rain treatment (69%) than without rain (31%). As the beetles for DNA analysis were sampled 24 h after the application of rain, this suggests that beetles consumed aphids which had been falling onto the soil surface. However, aphid numbers in the rain treatments were not significantly reduced by P. melanarius. DNA gut content analysis cannot distinguish between consumption of living prey by active predation and consumption of dead prey by scavenging (Foltan et al., Reference Foltan, Sheppard, Konvicka and Symondson2005; Juen & Traugott, Reference Juen and Traugott2005). Moreover, the PCR-based gut content analysis applied in the present study was qualitative and did not allow us to determine how many aphids each predator, testing positive for aphid DNA, had consumed. Therefore, both types of prey (live and dead) could have contributed to the high detection rates in the beetles and indeed both types of prey were accessible to the beetles.
Even 24 h after rain was applied, some of the dislodged aphids were active at the soil surface and, therefore, available as prey for P. melanarius. The time aphids survive off plants can exceed 24 h, as has been shown in laboratory experiments with the aphids Acyrthosiphon pisum (Harris) and A. kondoi (Shinji) (Homoptera: Aphididae) (Losey & Denno, Reference Losey and Denno1998b). The ability of aphids to survive under wet conditions can be remarkable; flooded Rhopalosiphum padi L. (Homoptera: Aphididae) survived at a rate of 98% if they floated and 82% if they became submerged (Araya & Fereres, Reference Araya and Fereres1991). However, high numbers of aphids dislodged from the wheat plants by rain must have died at the soil surface as rain significantly decreased aphid populations by 27%, indicating that rain is an important mortality factor for aphids. Mann et al. (Reference Mann, Tatchell, Dupuch, Harrington, Clark and McCartney1995) suggested that the mortality due to rain in S. avenae on wheat is about 25%. Dhaliwal & Singh (Reference Dhaliwal and Singh1975) reported 74% mortality in the wheat aphid Macrosiphum miscanthi (Takahashi) (Homoptera: Aphididae) due to dislodgement by rain. Therefore, after rain, dead aphids are likely to form part of the diet for generalist predators, such as P. melanarius. Presumably, P. melanarius consumed both living and dead aphids but at different ratios. This carabid is known to scavenge and has been shown to prefer fresh dead aphids over living ones (Foltan et al., Reference Foltan, Sheppard, Konvicka and Symondson2005). In our experiment, high numbers of dead aphids may have distracted P. melanarius from living aphid prey. The detection of aphid DNA in beetle guts within this treatment suggests that aphids had been falling to the ground without the influence of rain, as P. melanarius was thought to be unable to climb the wheat-plants (Griffiths et al., Reference Griffiths, Wratten and Vickerman1985). Consumption of these aphids would not have been detectable without a DNA-based technique, as the effect on aphid populations was not significant and would have been ignored.
Winder et al. (Reference Winder, Hirst, Carter, Wratten and Sopp1994) estimated aphid availability for ground-dwelling predators and suggested that aphid consumption in the field may often be limited simply by aphid availability. The authors concluded that total consumption would increase if aphid numbers increased. In fact, in our study, rain increased aphid numbers on the soil surface causing higher predation rates. Predators which do not scavenge, or have a strong preference for live prey, such as linyphiid (Fraser, Reference Fraser1982; Sunderland et al., Reference Sunderland, Crook, Stacey and Fuller1987) and lycosid spiders (von Berg et al., unpublished data), may contribute to synergistic effects caused by rain.
Adult aphids have been shown to have a higher risk of falling off plants than younger nymphs (Dewar et al., Reference Dewar, Dean and Cannon1982; Watson, Reference Watson1983; Cannon, Reference Cannon1984). Furthermore, more aphids are dislodged from shoots than from ears. Similarly, in our microcosms, the proportion of dislodged aphids from shoots (57%) was more than twice the number dislodged from ears (25%). Also, Dhaliwal & Singh (Reference Dhaliwal and Singh1975) found a higher dislodgement of aphids from wheat plants without ears than from those with ears. Sopp et al. (Reference Sopp, Sunderland and Coombes1987) suggested that the peak of predation on aphids by generalist predators in cereals, before wheat earing early in the season, is due to high numbers of aphids active on the soil surface. Rain may contribute to dropping of aphids early in the year, triggering positive synergistic effects leading to high predation rates by generalist predators. Indeed, rain before the end of wheat flowering may prevent aphid outbreaks in cereal fields (Watson & Carter, Reference Watson and Carter1983), but interactions between dropped aphids and ground-dwelling predators were not investigated in their study.
In conclusion, by applying artificial rain to a plant-herbivore-predator system, we demonstrated negative effects of rain on aphid population development. Moreover, by employing a DNA-based gut content analysis, we showed for the first time that rain can affect insect predator-prey interactions. Dislodgement of aphids not only directly increased aphid mortality but also increased aphid consumption rates by ground-dwelling predators. Our results suggest that weather conditions, such as wind and rain, can modify predator-prey interactions in the field, possibly triggering synergistic effects. The combination of a manipulative microcosm experiment with a DNA-based gut content analysis proved to be an effective strategy which we recommend.
Acknowledgements
The authors thank Ludwig Schwinn for providing access to fields for beetle collection and Helmut Wagner for providing greenhouse space for conducting the experiments. This work was funded by the German Research Foundation DFG Grant SCHE 376/14.




