Perventricular device closure of trabecular ventricular septal defects is a successful therapeutic modality but remains challenging particularly in high-risk patients and complex anatomies.Reference Zhu, Tao, An, Luo, Gan and Lin1,Reference Gray, Menon and Johnson2 Since the recent marketing of the new KONAR-MF™ ventricular septal defect occluder, interventionists have been reporting the promising technical advantages of this device with a focus on its smart design, procedural flexibility, and ability to accommodate to various anatomies.Reference Schubert, Kelm, Koneti and Berger3–Reference Tanidir, Baspinar, Saygi, Kervancioglu, Guzeltas and Odemis5 Herein, we report the first perventricular implantation of this device to treat a residual apical ventricular septal defect following repair of complex transposition of the great arteries.
Case presentation
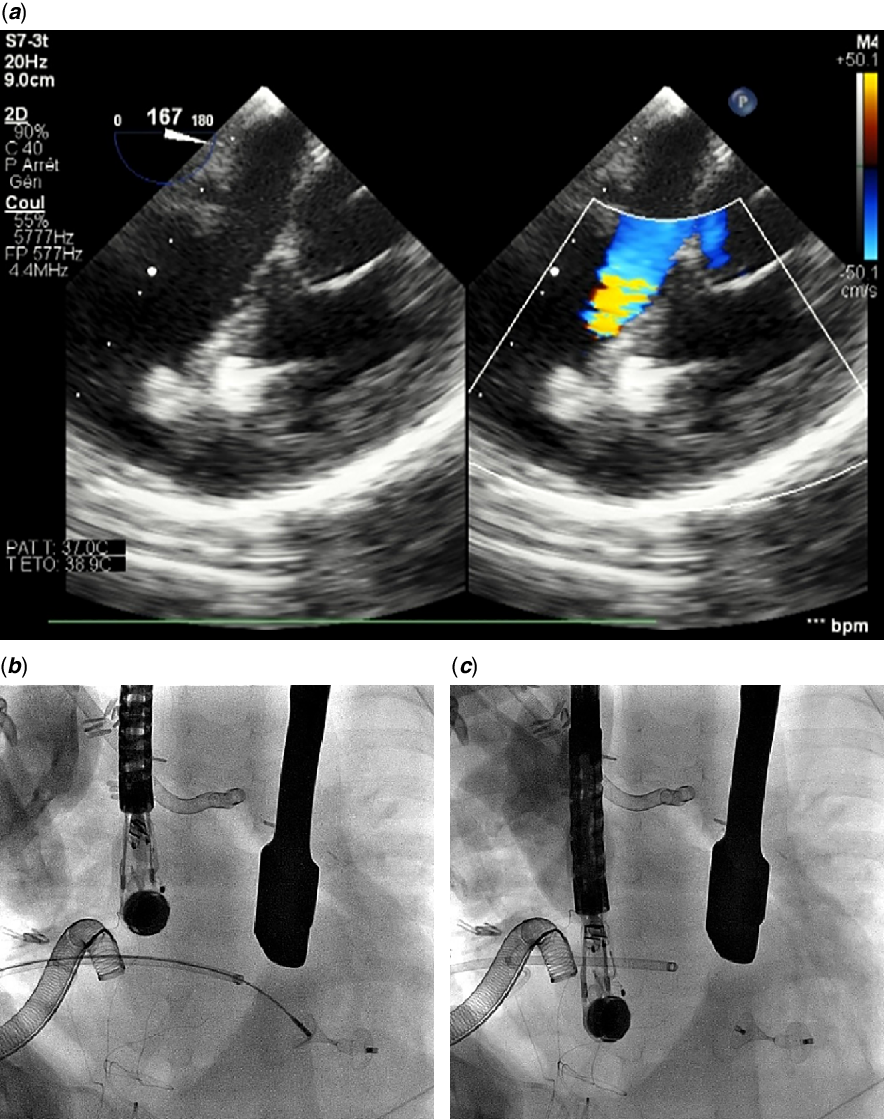
A neonate with prenatal diagnosis of d-transposition of the great arteries, multiple trabecular septal defects, and pulmonary stenosis underwent balloon atrial septostomy at birth for severe hypoxemia before he was palliated with a Blalock–Taussig shunt at five weeks of life. His post-operative ICU stay revealed a Wolff–Parkinson–White syndrome over episodes of supraventricular tachycardia and was complicated with chylothorax and necrotising enterocolitis until he was safely discharged at nine weeks of age. Three months later, right pulmonary artery stenosis at the insertion site of the surgical shunt motivated pulmonary arterioplasty and another Blalock–Taussig shunt implantation. Subsequently, a REV procedure with an attempt to address all ventricular defects was performed at 11 months of age. Surgery was complex because the most apical defect turned out to be challenging and an integrated valved conduit had to be used to reconstruct the right ventricular outflow tract with repeated arterioplasty of pulmonary arteries. Weaning of bypass was complicated with recurrent arrhythmias leading to haemodynamic instability. Per-operative ultrasound confirmed a 4 mm residual defect located in the apex with significant left-to-right shunting and elevated filling pressures in both ventricles (Fig 1). The patient was put on central veno-arterial extracorporeal membrane oxygenation and sternal closure was delayed. Repetitive ultrasound assessments confirmed deleterious consequences of the defect, and the patient was unable to come off circulatory support. Classical surgery was not thought to be the most appropriate treatment as the access to defect was difficult. Transfemoral closure was theoretically feasible, but vascular access practicality at a bodyweight of 6.5 kg was unpropitious, and open chest made the perventricular access as the most convenient strategy. The procedure was performed in a fully specified hybrid operating facility. The puncture site was identified with finger pressure under transoesophageal echocardiography guidance. A pledgeted purse-string was positioned to the right ventricular free wall, which was then punctured using an 18G needle and cannulated by introducing the first 3–4 cm of a 5-F short introducer. After multiple attempts with different types of catheters, the defect was crossed using a 4-F modified (cut tip at a 120° angle) pigtail catheter (Cordis Corp., Florida, United States) together with an angled 0.035″ Radifocus® hydrophilic Guidewire M (Terumo Corp., Tokyo, Japan) that had to be manoeuvred into the left ventricular outflow tract for system stability (Fig 2). The wire was then replaced with a stiff Type, angled 0.035″ Radifocus® Guidewire M (Terumo Corp., Tokyo, Japan) over which a standard 80-cm-long, 5-F SteerEase™ delivery sheath (Lifetech, Shenzhen, China) was easily advanced into the aorta. A previously loaded 5/3-mm KONAR-MF™ device, connected to the delivery cable from its right side (Fig 3) was advanced to the tip of the sheath under fluoroscopy. The left-side waist diameter (D2) guided the device selection and was chosen 1 mm larger than the defect size. Under ultrasound guidance, the entire assembly was slowly pulled back to the mid-left ventricular cavity. The sheath was retracted until the left disk was completely deployed. The entire assembly was then withdrawn as one unit onto the interventricular septum. The waist of the device and subsequently the right retention disk were uncovered and deployed in place. The device was released once residual leak, device position, and valvular regurgitations were assessed on ultrasound (Fig 4). The delivery sheath was removed and the purse-string tightened. The overall procedure time was 40 minutes and required ten minutes of fluoroscopy. The patient was transferred back to the cardiac ICU. Confirmatory imaging of the implanted device was made all over the post-operative period (Fig 5). However, the patient died after one week from a septic choc.

Figure 1. Ultrasound views. ( a ) 4-mm residual muscular VSD in a small baby after surgical repair of a TGA complex variant. Note the single left ventricular entry ( b ) and multiple right ventricular trabeculated exists ( c ). TGA = transposition of the great arteries; VSD = ventricular septal defect.

Figure 2. Fluoroscopic view. Perventricular access with catheter and guidewire combination passing through the defect into the left ventricular outflow tract.

Figure 3. KONAR-MF™ VSD occluder (Lifetech, Shenzhen, China). ( a ) A small model of the device (with no PTFE membrane inside) connected to the delivery cable from its right side. ( b ) Schematic illustration of the device configuration: D, left retention disk diameter; D2, waist diameter (left ventricular side); D1, waist diameter (right ventricular side); L, 4-mm waist length. PTFE = polytetrafluoroethylene; VSD = ventricular septal defect.

Figure 4. ( a ) TOE imaging with colour-flow Doppler mode showing an unreleased 5/3-mm KONAR-MF™ device in place with no residual shunt. Fluoroscopic view showing stable positioning before ( b ) and after release ( c ). TOE = transoesophageal echocardiography.

Figure 5. Ultrasound control showing stable device position 48 hours after deployment.
Discussion
The hybrid approach to close trabecular defects following surgical repair of complex cardiac anatomies is driven by their anatomical position and accessibility.Reference Bearl and Fleming6 We crossed the defect from the right side because manipulating the delivery sheath through the left ventricle without haemodynamical instability was likely to be impossible in our case. This approach was technically more challenging since we had to pass across tight right ventricular trabeculations while using angled tip guidewires to mitigate the risk of injuring cardiac tissues.Reference Pedra, Pedra and Chaccur7,Reference Yin, Zhu, Lin and An8 However, right-side access allowed us to oppose the 10-mm left retention disk firmly across the septum, thus establishing complete occlusion of the single left ventricular hole while avoiding disk protrusion in the diminished left ventricular cavity near the apex. A perpendicular approach from the ventricular free wall to the ventricular septum is required for fast deployment without distorting the anatomy.Reference Zhu, Tao, An, Luo, Gan and Lin1 We accepted a suboptimal angle of puncture since relocating the access site was more likely to induce ventricular dysfunction and arrhythmia. We overcame this incident by directing a modified catheter with proper wire combination into the desired position.
Pre-operative imaging data should be carefully scrutinised by all the operators to plan the equipment inventory and to reduce all recognised risks.Reference Bartel and Tuzcu9 We performed the intervention in a specified hybrid operating room to maximise patient security. In critical scenarios, fluoroscopy should be available for additional guidance, especially when ultrasound quality may not always be optimal in very small infants. Transoesophageal echocardiography is the most optimal real-time guidance for this type of intervention. However, it may give a less surgical orientation of the defect compared to the epicardial scan with which the desired angle of the wire passage can be mimed by the probe.Reference Pedra, Pedra and Chaccur7
The KONAR-MF™ is the new promising multi-functional occluder with many reported promising technical features.Reference Schubert, Kelm, Koneti and Berger3–Reference Tanidir, Baspinar, Saygi, Kervancioglu, Guzeltas and Odemis5 The self-expanding soft and compliant device is hybridly designed between single- and double-disc foregoing concepts. The two retentions discs are connected by an expanding cone-shaped waist, articulated solely from on its right side, offering high conformability to various anatomies. In complex anatomies, the right disk may not entirely conform in place due to trabeculations, moderator band, and limited chamber size. However, as expected, due to the astute specifications of the device, we easily managed to conform the right disk with the least tension and distortion possible omitting the need for several attempts of deployments reported with other occluders.Reference Pedra, Pedra and Chaccur7 Device sizes are categorised according to waist diameters D2/D1 (Fig 3). In total, eight available sizes are available starting from 5/3 mm and up to 14/12 mm. The diameter D of the left retention disk is 4 mm larger than D2 for even devices and for odd devices 5 mm larger.Reference Haddad, Daou and Saliba4 Both side deliverability allowed high procedural flexibility. The short 4-mm waist precluded disks protrusion and interference with surrounding structures. It is also noteworthy that the sheath was easily manoeuvred across the challenging non-perpendicular pathway without any kinking. The slim delivery cable minimised all unwanted mechanical damages. It can be also easily shortened to the desired length using a surgical plier offering a major advantage in situations where a short sheath has to be used instead of the standard long delivery system.
Conclusion
With the promising advantages of the new multi-functional occluder, perventricular closure of a surgically challenging trabecular defect can be easily achieved as planned when investment in infrastructure is maximised and even in a challenging scenario. The careful multi-disciplinary discussion required for this intervention cannot be overstated.
Author contributions
R.H. collected clinical data and took the lead in writing the manuscript. All the authors have read and approved the final version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation, and with the Helsinki Declaration of 1975, as revised in 2008. The patient’s legal guardians signed informed consent was obtained for the procedure.